The ubiquitous transcription factor specificity protein 1 (SP1) is heavily modified posttranslationally. These modifications are critical for switching its functions and modulation of its transcriptional activity and DNA binding and stability.
KEYWORDS: β-catenin, destruction complex, phosphodegron, SP1, Wnt signaling
ABSTRACT
The ubiquitous transcription factor specificity protein 1 (SP1) is heavily modified posttranslationally. These modifications are critical for switching its functions and modulation of its transcriptional activity and DNA binding and stability. However, the mechanism governing the stability of SP1 by cellular signaling pathways is not well understood. Here, we provide biochemical and functional evidence that SP1 is an integral part of the Wnt signaling pathway. We identified a phosphodegron motif in SP1 that is specific to mammals. In the absence of Wnt signaling, glycogen synthase kinase 3β (GSK3β)-mediated phosphorylation and β-TrCP E3 ubiquitin ligase-mediated ubiquitination are required to induce SP1 degradation. When Wnt signaling is on, SP1 is stabilized in a β-catenin-dependent manner. SP1 directly interacts with β-catenin, and Wnt signaling induces the stabilization of SP1 by impeding its interaction with β-TrCP and axin1, components of the destruction complex. Wnt signaling suppresses ubiquitination and subsequent proteosomal degradation of SP1. Furthermore, SP1 regulates Wnt-dependent stability of β-catenin and their mutual stabilization is critical for target gene expression, suggesting a feedback mechanism. Upon stabilization, SP1 and β-catenin cooccupy the promoters of TCFL2/β-catenin target genes. Collectively, this study uncovers a direct link between SP1 and β-catenin in the Wnt signaling pathway.
INTRODUCTION
Wnt signaling plays an essential role in tissue homeostasis, cell fate determination and proliferation, and embryonic development (1, 2). Mutations in the Wnt signaling pathway are associated with developmental defects and cancers (1, 3–5). The core event in the Wnt pathway is stabilization of β-catenin upon activation of Wnt signaling. The stabilization of β-catenin is regulated by the cytoplasmic destruction complex, consisting of scaffold protein axin1, APC, glycogen synthase kinase 3β (GSK3β), and CSK1α. In the absence of Wnt ligand (Wnt-off condition), β-catenin is engaged by the destruction complex followed by sequential phosphorylation by CSK1α and GSK3β (6, 7). Phosphorylated β-catenin is recognized by the ubiquitin E3 ligase β-TrCP and subsequently degraded by the proteosomal pathway. In the presence of Wnt ligand (Wnt-on condition), β-catenin is disengaged from cytoplasmic destruction complex by multivesicular sequestering of GSK3β (8–10) and other mechanisms. Disengaged β-catenin evades phosphorylation and recognition by β-TrCP and hence degradation by the proteosomal pathway. Dephosphorylated β-catenin translocates inside nucleus and interacts with the T-cell factor/lymphoid enhancer factor (LEF/TCF) transcription factors to drive target gene expression (2, 7). Wnt/β-catenin target genes play a critical role in tumor development and progression. The components of the Wnt signaling cascade play a critical role in tumor development and poor prognosis thereof and hence can be targeted for therapeutic intervention in cancers driven by dysregulation of the Wnt pathway (3).
Specificity protein 1 (SP1), a member of SP family proteins containing a C2H2 zinc finger domain (11), was earlier thought to be ubiquitously expressed and involved in transcriptional regulation of housekeeping genes. However, multiple recent pieces of evidence suggest a tissue-specific role of SP1 in regulating genes considered hallmarks of cancers and genes required during development and differentiation (12, 13). SP1 is heavily decorated by various posttranslational modifications, such as O-linked glycosylation, acetylation, phosphorylation, ubiquitination, and sumoylation, which are critical for switching SP1 functions. A number of signaling kinases have been implicated in SP1 posttranslational modifications that influence SP1's mode of action and DNA binding, stability, and transactivation (13). Considering the role of SP1 in regulating gene expression required during embryonic development and progression of cancers (12–16), it is essential to understand the mechanism regulating SP1 expression. Although phosphorylation-mediated degradation of SP1 at the protein level has been reported (17, 18), how phosphorylation takes place in the cellular context and thereby affects its stability is not fully elucidated.
Aberrant activation of Wnt signaling has been implicated in various cancers mediated by mutations in various regulators of this pathway (19). Recently, cross talk between novel targets and hyperactivation of Wnt signaling have been implicated in regulating the fate of Wnt signaling (20, 21). Our earlier study provided further evidence that such cross talk with novel targets plays a critical role in regulating colorectal tumorigenesis and regulation of Wnt-responsive genes (22). Interestingly, both SP1 and β-catenin possess the regulatory phosphodegron motif and interact with β-TrCP, thereby hinting at a possible overlap of regulation of β-catenin and SP1. Overlap of regulation and function between β-catenin and SP1 has been reported (23). Thus, these studies point to the possibility of SP1 and β-catenin molecular cross talk regulated by a common pathway and hence coordinately regulating the Wnt target genes.
Here we report that SP1 is a downstream target of the Wnt signaling pathway. We show that β-catenin is required for SP1 stability in a GSK3β-dependent manner. We found that the cytoplasmic destruction complex required for β-catenin destabilization is also required for SP1 destabilization. We show that Wnt signaling induces stabilization of SP1 by impeding its interaction with the E3 ubiquitin ligase β-TrCP and axin1. We present a direct link demonstrating that SP1 regulates the stability of β-catenin by impeding its interaction with the cytosolic destruction complex. Further, destabilization of the destruction complex and mutual stabilization of SP1 and β-catenin are required for Wnt/β-catenin signaling-dependent regulation of Wnt-responsive genes. We show that SP1 and β-catenin directly interact with each other to form a complex and this complex binds to the regulatory elements of Wnt/β-catenin target genes.
RESULTS
Wnt signaling promotes SP1 stabilization.
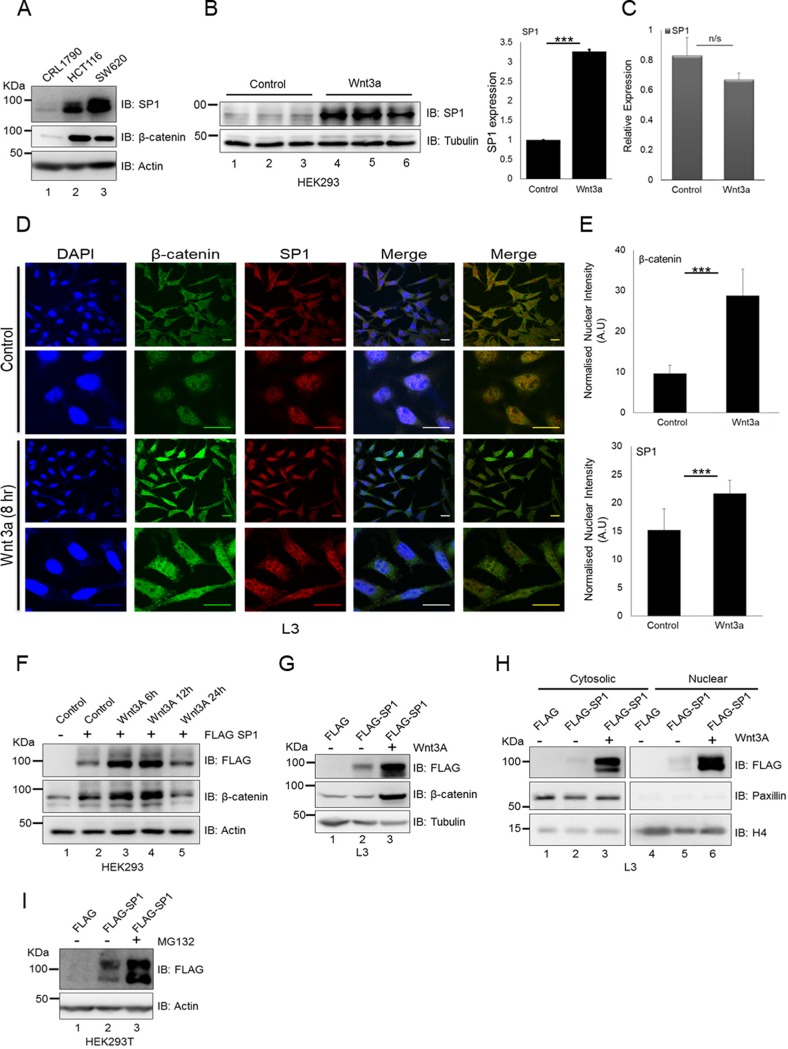
To study the regulation of SP1, we analyzed the expression of SP1 in Wnt signaling-driven colorectal cancer cell lines in comparison with the primary cell line CRL1790. The expression pattern indicated that Wnt-driven cancer cells exhibit elevated levels of SP1 (Fig. 1A). To test whether stimulation of Wnt signaling could be involved in regulation of SP1, we treated HEK293 cells with Wnt3A. The activation of Wnt signaling appeared to induce the expression of SP1 at the protein level (Fig. 1B, left panel; the densitometric quantitation of the same is depicted in Fig. 1B, right panel). Strikingly, Wnt3A treatment did not induce expression of SP1 at the transcript level (Fig. 1C). Similarly, L3 mouse fibroblast cells exhibited significant upregulation of SP1 along with β-catenin upon activation of the Wnt pathway using Wnt-conditioned medium (Fig. 1D and E). Lack of change in transcript levels suggested that Wnt3A stimulation could induce stability of SP1 at the protein level. Since HEK293 cells possess an intact destruction complex and respond to Wnt stimulation, we sought to use these cells to determine whether Wnt stimulation by Wnt ligand Wnt3A can induce stability of SP1 at the protein level. We therefore treated FLAG-SP1-overexpressing HEK293 cells with Wnt3A for 6 to 24 h. Wnt3A stimulation induced the stability of SP1 in a time-dependent manner as determined by the levels of FLAG-tagged protein in comparison with control (Fig. 1F, compare lane 2 with lanes 3 and 4). Wnt stimulation also induced the levels of the known target β-catenin, confirming the activation of the Wnt signaling cascade. Similarly, Wnt3A treatment in L3 cells induced stabilization of ectopically expressed FLAG-SP1 (Fig. 1G, compare lanes 2 and 3). This analysis revealed that Wnt pathway activation is required to induce the stability of SP1 and thus could potentially be the reason that Wnt-driven colorectal cancer cells express higher levels of SP1. Next, to determine if Wnt stimulation induces the stability of SP1 in cytosol and nucleus, we analyzed the expression of FLAG-tagged SP1 in cytosolic and nuclear fractions. Immunoblot analysis revealed that Wnt stimulation induces SP1 in both cytosolic and nuclear fractions (Fig. 1H, compare lanes 2 and 3 and lanes 5 and 6). Next, to investigate whether the stability of SP1 requires inhibition of the proteosomal pathway, we treated FLAG-SP1-expressing HEK293 cells with the proteosome inhibitor MG132. Immunoblot analysis using anti-FLAG antibody indicated that inhibition of the proteosomal pathway induced the stability of SP1 (Fig. 1I, compare lanes 2 and 3). Collectively, these results indicated that Wnt signaling promotes the stabilization of SP1 by averting its degradation through the proteosomal pathway.
FIG 1.
Wnt/β-catenin signaling induces SP1 stabilization. (A) Immunoblots for expression of SP1 and β-catenin in CRL1790, HCT116, and SW620. (B) Left, immunoblots for expression of SP1 in HEK293 cells treated with Wnt3A for 6 h in triplicates. Data representative of two independent experiments. Right, densitometric quantitation of SP1 expression upon Wnt3A treatment (two-way analysis of variance [ANOVA], P < 0.0001). (C) Relative transcript levels of SP1 in control and Wnt3A-treated cells. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as an endogenous control. Error bars represent standard deviations (SD) for triplicates. (D) Immunofluorescence assay showing increases in the cytosolic and nuclear levels of SP1 and β-catenin upon Wnt stimulation in L3 cells (bar, 20 μm). The first merged panel indicates merging of all three channels, whereas in the second merged panel the DAPI is removed to better visualize the colocalization of nuclear β-catenin and SP1. (E) Quantification of nuclear intensities of β-catenin and SP1 levels (Mann-Whitney test, two tailed, P < 0.0001). (F) Immunoblots for FLAG and β-catenin in FLAG-SP1-expressing HEK293 cells after treatment with Wnt3A in a time-dependent manner. (G) Immunoblots for FLAG and β-catenin in FLAG-SP1-expressing L3 cells upon treatment with Wnt3A. Tubulin was used as an endogenous control. Data representative of two independent experiments. (H) Immunoblots for FLAG, paxillin, and histone H4 in cytosolic and nuclear fractions of FLAG-SP1-expressing L3 cells upon treatment with Wnt3A. (I) Immunoblots for FLAG-SP1 in control and MG132-treated cells. HEK293T cells were transfected with FLAG-SP1 and treated with dimethyl sulfoxide (DMSO) and proteosome pathway inhibitor MG132 for 4 h.
SP1 interacts with β-catenin in colorectal cancer cells.
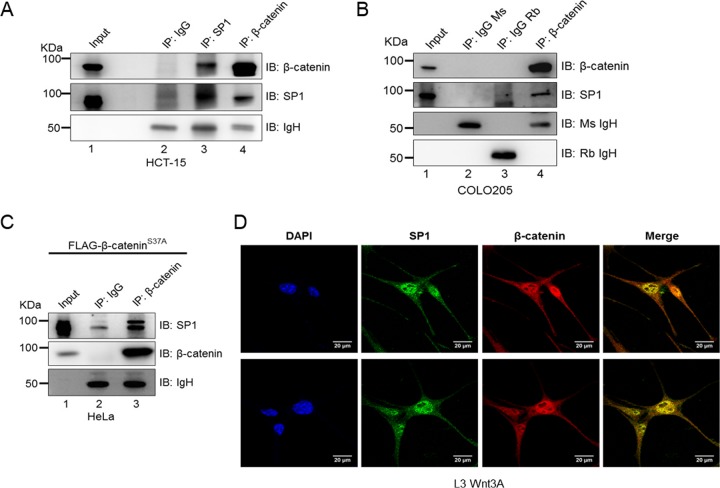
To determine whether SP1 physically interacts with β-catenin in the form of a molecular complex, we performed coimmunoprecipitation (co-IP) assays in HCT-15 colorectal cancer cells. Immunoblot analysis following coimmunoprecipitation revealed that SP1 interacts with β-catenin (Fig. 2A, lane 3 and lane 4). Such interaction was also observed in COLO205 cells, in which immunoprecipitation with anti-β-catenin pulled down SP1 (Fig. 2B). Next, to confirm if such a complex is observed in a different cellular model, we overexpressed the mutant S37A constitutively stabilized form of β-catenin in HeLa cells and performed immunoprecipitation with anti-β-catenin antibody. The coimmunoprecipitation analysis revealed that SP1 physically interacts with β-catenin (Fig. 2C). To further test if SP1 is associated with β-catenin, we monitored their localization in L3 Wnt3A cells that exhibit constitutively active Wnt signaling. Immunofluorescence analysis revealed that SP1 colocalizes with β-catenin (Fig. 2D).
FIG 2.
SP1 interacts with β-catenin in colorectal cancer cells. (A) Immunoblots for endogenous SP1 and β-catenin coimmunoprecipitated with β-catenin and SP1, respectively, from HCT-15 lysates. Immunoprecipitation (IP) was done using antibody against β-catenin and SP1. IP with IgG was used as a negative control. (B) Immunoblots for endogenous SP1 coimmunoprecipitated with endogenous β-catenin from COLO205 cells. IP with the respective IgG isotype was used as a negative control. (C) Immunoblots for endogenous SP1 coimmunoprecipitated with β-catenin from FLAG–S37A β-catenin-expressing HeLa lysate. FLAG–S37A β-catenin was overexpressed in HeLa cells, and IP was performed with anti-β-catenin. IP using specific IgG isotype was used as a negative control. (D) Representative confocal microscopic image showing colocalization of SP1 and β-catenin using SP1 and β-catenin antibodies for immunofluorescence assay in L3 Wnt3A cells.
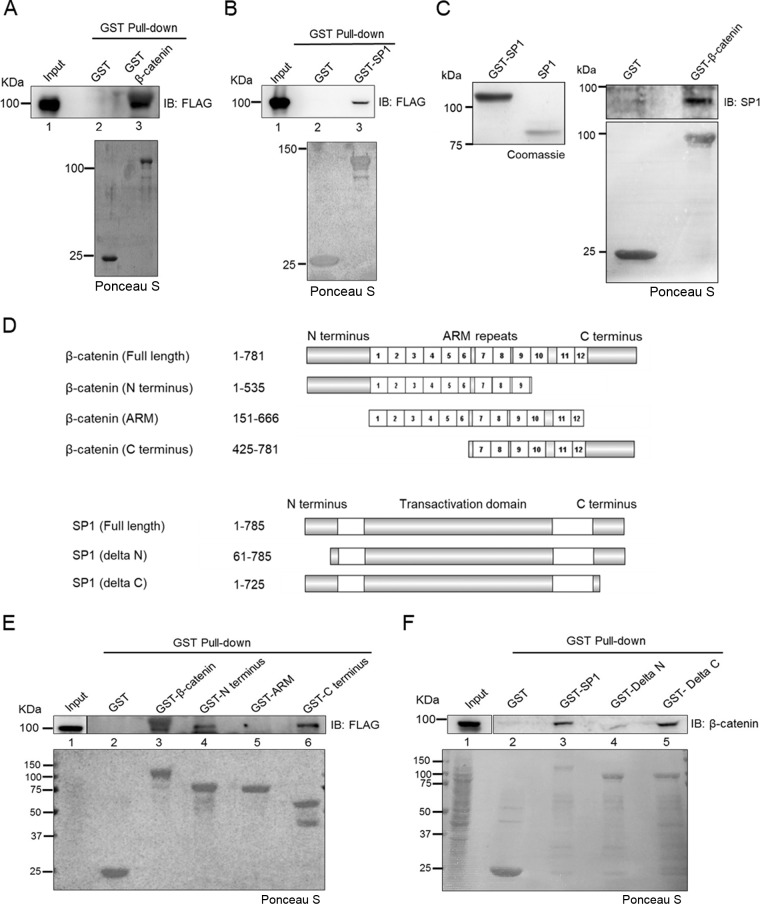
To determine whether SP1 interacts with β-catenin directly, we performed in vitro glutathione S-transferase (GST) pulldown assays using FLAG-SP1-expressing HEK293 cells (Fig. 3A, lane 3). Similar results were observed in a GST pulldown assay with GST-SP1 using lysates of FLAG–β-catenin-expressing HEK293 cells (Fig. 3B, lane 3). Next, we performed an in vitro interaction study using purified recombinant proteins. Bacterially expressed GST-SP1 was affinity purified and cleaved with thrombin to release full-length SP1 from the GST tag (Fig. 3C, left panel). The GST pulldown assay was then performed using GST–β-catenin with purified SP1 and confirmed that SP1 and β-catenin interact directly in vitro (Fig. 3C, right panel). Further, to delineate which domain of β-catenin interacts with SP1, we performed pulldown assays using various GST-tagged domains of β-catenin (schematically depicted in Fig. 3D). GST pulldown assays revealed that both N and C termini of β-catenin interact with SP1 but not the arm domain (Fig. 3E, lane 4 and lane 6). Furthermore, the GST-SP1 pulldown assay revealed that SP1 interacts with β-catenin through its N terminus (Fig. 3F). Collectively, these findings indicated that SP1, stabilized upon Wnt–β-catenin signaling, directly interacts with β-catenin.
FIG 3.
The N terminus of SP1 is required for its interaction with β-catenin. (A) Immunoblot for FLAG after lysate of HEK293T FLAG-SP1-expressing cells was subjected to pulldown by GST–β-catenin immobilized on glutathione resin. GST protein was used as a negative control. Lower panel, Ponceau S-stained gel for GST tag-purified proteins. (B) Immunoblot for FLAG from HEK293T FLAG–β-catenin-expressing cells pulled down by GST SP1 immobilized on glutathione resin. GST protein was used as a negative control. Lower panel, Ponceau S-stained gel for GST tag-purified proteins. (C) Immunoblot for SP1 after in vitro protein interaction assay with GST–β-catenin. GST was used as a negative control (right panel). Lower panel, Ponceau S-stained gel for GST tag-purified proteins. Left panel, cleavage of GST-SP1 with thrombin. (D) Schematic depicting full-length and deletion constructs of β-catenin and SP1 used for in vitro pulldown assay. Amino acid residues included in each deletion are indicated next to each construct. (E) Immunoblot for FLAG from HEK293T FLAG-SP1-expressing cells pulled down by GST–full-length β-catenin, GST-N terminus, GST-ARM repeats, and GST-C terminus immobilized on glutathione resin. GST protein was used as a negative control. (F) Immunoblot for FLAG from HEK293T FLAG–β-catenin-expressing cells pulled down by GST–full-length SP1, GST-delta N terminus, and GST-delta C terminus immobilized on glutathione resin. GST was used as a negative control.
Stabilization of β-catenin is required for Wnt signaling-induced stabilization of SP1.
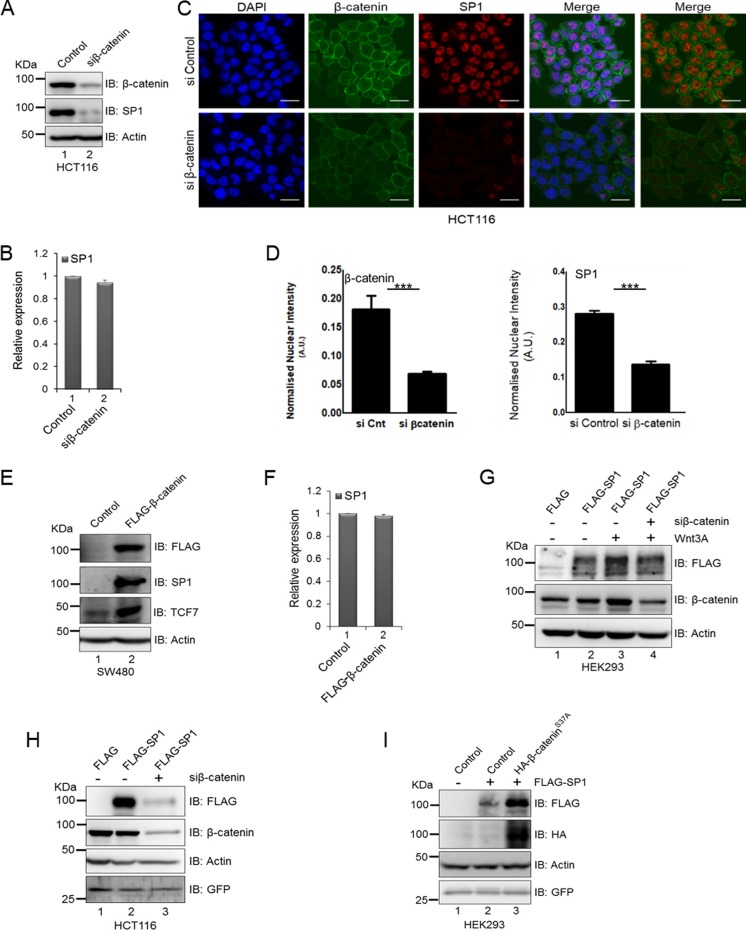
The coimmunoprecipitation assay indicated that SP1 interacts with β-catenin in Wnt signaling-driven colorectal cancer cells (Fig. 2A and B); this prompted us to investigate whether β-catenin is required for SP1 stabilization. To test this, we analyzed the expression of SP1 under β-catenin knockdown in β-catenin mutant HCT116 cells. Knockdown of β-catenin using RNA interference resulted in a drastic decrease in SP1 expression at the protein level (Fig. 4A); however, no change was observed at the transcript level (Fig. 4B). Immunofluorescence localization of both proteins in HCT116 cells confirmed the changes at the protein level (Fig. 4C). Quantification of nuclear intensities revealed a significant reduction in SP1 levels upon silencing of β-catenin (Fig. 4D). Furthermore, overexpression of β-catenin in SW480 cells resulted in upregulation of SP1 at the protein level (Fig. 4E) but not at the transcript level (Fig. 4F), suggesting that β-catenin is required for stability of SP1 at the protein level. Next, to determine whether Wnt signaling-induced SP1 stability requires β-catenin, we stimulated the FLAG-SP1-expressing HEK293 cells with Wnt3A and depleted β-catenin. Activation of Wnt signaling by Wnt3A treatment induced the stability of ectopically expressed FLAG-tagged SP1 (Fig. 4G). Small interfering RNA (siRNA)-mediated silencing of β-catenin in FLAG-SP1-expressing cells reduced the stability of SP1 even after stimulation by Wnt3A (Fig. 4G, compare lanes 3 and 4). Collectively, these results suggested that β-catenin is required for Wnt signaling-induced SP1 stabilization. To further corroborate the role of β-catenin in stabilization of SP1, we overexpressed FLAG-SP1 HCT116 cells and β-catenin was simultaneously depleted through siRNA-mediated RNA interference. Silencing of β-catenin in FLAG-SP1-expressing HCT116 cells drastically reduced the levels of ectopically expressed FLAG-SP1 in comparison with siLuciferase FLAG-SP1-expressing cells (Fig. 4H, compare lanes 2 and 3). Green fluorescent protein (GFP) was used as a transfection control. Conversely, to investigate whether β-catenin stabilization is sufficient to induce SP1 stability, we overexpressed FLAG-SP1 in control HEK293 cells and in HA–S37A β-catenin-expressing cells. The phosphorylation-defective S37A mutant β-catenin is nonresponsive to GSK3β-mediated regulation and therefore remains constitutively active. The overexpression of β-catenin robustly induced the stability of SP1 in comparison with vector control FLAG-SP1-expressing cells (Fig. 4I, compare lanes 2 and 3). Thus, β-catenin stabilization is sufficient to induce SP1 stability.
FIG 4.
Expression of β-catenin is required for Wnt signaling-induced stabilization of SP1. (A) Immunoblots for expression of β-catenin and SP1 in siLuciferase (control) and siβ-catenin HCT116 cells. (B) Relative transcript levels of SP1 in siControl and siβ-catenin-silenced cells. GAPDH was used as an endogenous control. Error bars represent SD for triplicates. (C) Immunofluorescence analysis for expression of β-catenin and SP1 in siControl and siβ-catenin-transfected HCT116 cells (bars, 20 μm). (D) Quantification of nuclear intensities of SP1 and β-catenin (Mann-Whitney test, two tailed, P < 0.0001); A.U., arbitrary units. (E) Immunoblots for expression of SP1, TCF7, and FLAG–β-catenin in control and FLAG β-catenin SW480 cells. (F) Relative transcript levels of SP1 in control and FLAG–β-catenin SW480 cells. GAPDH was used as an endogenous control. Error bars represent SD for triplicates. (G) Immunoblots for FLAG and β-catenin in FLAG-SP1 siLuciferase and FLAG-SP1 Wnt3A-treated and siβ-catenin FLAG-SP1 Wnt3A-treated HEK293 cells. (H) Immunoblots for FLAG and β-catenin in siLuciferase-, FLAG-SP1-, and siβ-catenin-transfected FLAG-SP1-overexpressing HCT116 cells. GFP was used as a transfection control (immunoblots are representative of two independent experiments). (I) Immunoblots for FLAG and HA in control and HA–β-cateninS37A-expressing HEK293 cells. GFP was used as a transfection control.
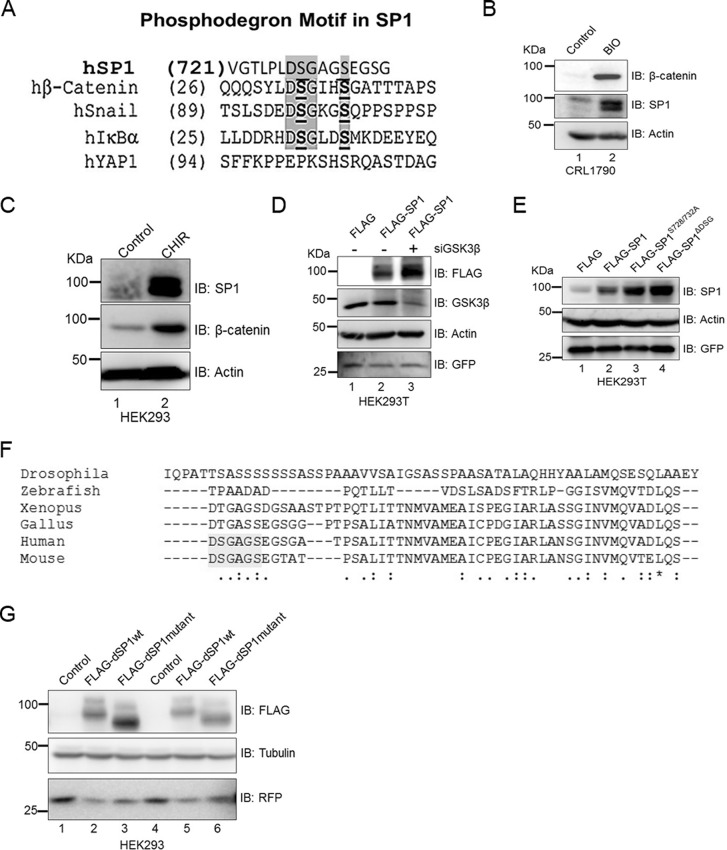
GSK3β is required for SP1 degradation via phosphorylation of serines in its phosphodegron motif.
Next, we sought to determine the mechanism by which the Wnt pathway induces stability of SP1. Interestingly, search for putative motifs using in silico analysis revealed that the C terminus of SP1 harbors a canonical phosphodegron motif that is known to be recognized by GSK3β (8). Such a motif occurs in multiple human proteins, including β-catenin, in snail IκBα, and in YAP1 (Fig. 5A). To determine whether GSK3β could be involved in degradation of SP1 in the Wnt-off state, we monitored the status of SP1 in CRL1790 primary colorectal cells treated with BIO (6-bromoindirubin-3′-oxime), a GSK3β inhibitor. Inhibition of GSK3β mimicked the changes observed upon activation of Wnt signaling and appeared to robustly induce the expression of SP1 and β-catenin (Fig. 5B). Treatment with GSK3β inhibitor CHIR also resulted in similar increases in the levels of these two proteins in HEK293 cells (Fig. 5C). Next, to test whether stimulation of the Wnt signaling cascade in HEK293T cells can induce SP1 stabilization at the protein level, we depleted GSK3β in FLAG-SP1-expressing HEK293T cells. siRNA-mediated silencing of GSK3β increased the level of FLAG-tagged SP1 (Fig. 5D, compare lanes 2 and 3), thus demonstrating that activity of GSK3β is critical in regulating the stability of SP1. GSK3β regulates the stability of proteins through phosphorylation of the phosphodegron motif DSGXXS (8). We then argued that if such a mechanism contributed to SP1 stability, mutation of the two serine residues within the phosphodegron motif at the C terminus should increase the stability of SP1 even in the Wnt-off state. We mutated both serine residues to alanine (SP1S726/732A) and also deleted the phosphodegron-containing C terminus of SP1 (SP1ΔDSG). Strikingly, mutation and deletion of the canonical phosphodegron motif dramatically increased the levels of ectopically expressed FLAG-SP1 in HEK293 cells, phenocopying the effect of Wnt stimulation and GSK3β inhibition (Fig. 5E). To further understand the importance of the phosphodegron motif, we analyzed whether SP1 from lower vertebrates such as zebrafish, Xenopus, and chicken harbors the phosphodegron motif. Multiple-sequence alignment revealed that the phosphodegron motif in SP1 is specific to mammals (Fig. 5F). To investigate whether phosphodegron is critical for stabilization of SP1, we introduced the phosphodegron motif in Drosophila SP1 (dSP1) and monitored the stability of such mutant dSP1. Interestingly, the stability of the mutant was reduced in comparison with wild-type dSP1 (Fig. 5G).
FIG 5.
The presence of phosphodegron is essential for stabilization of SP1. (A) Amino acid homology analysis depicting the presence of the phosphodegron motif at the C terminus of SP1. (B) Immunoblots for expression of SP1 and β-catenin in control and GSK3β inhibitor and Wnt agonist BIO-treated CRL1790 primary cells. (C) Immunoblots for SP1 and β-catenin in control and CHIR-treated HEK293 cells. Treatment of CHIR increases the expression of SP1 and β-catenin in comparison with control. Actin was used as an endogenous control. (D) Immunoblots for FLAG-SP1, GSK3β in siLuciferase- and in siGSK3β-transfected HEK293 cells. Actin and GFP were used as controls for protein and transfection, respectively. (E) Mutation of serine residues or deletion of DSG motif induces stabilization of SP1. Shown are immunoblots for FLAG in FLAG-SP1, FLAG-SP1 serine-alanine double mutant (S726/732A), and FLAG-SP1 delta phosphodegron (ΔDSG)-expressing HEK293 cells. GFP was used as a transfection control. (F) Clustal multiple-sequence alignment analysis to indicate the presence of phosphodegron motif DSG in human and mouse SP1. Such a motif is absent in SP1 from Xenopus, zebrafish, and Drosophila. (G) Incorporation of the DSG motif in Drosophila SP1 induces its degradation. Shown are immunoblots for FLAG in Drosophila wild-type FLAG-SP1 and Drosophila mutant FLAG-SP1-expressing HEK293 cells. Red fluorescent protein (RFP) was used as a transfection control. Tubulin was used as an endogenous control.
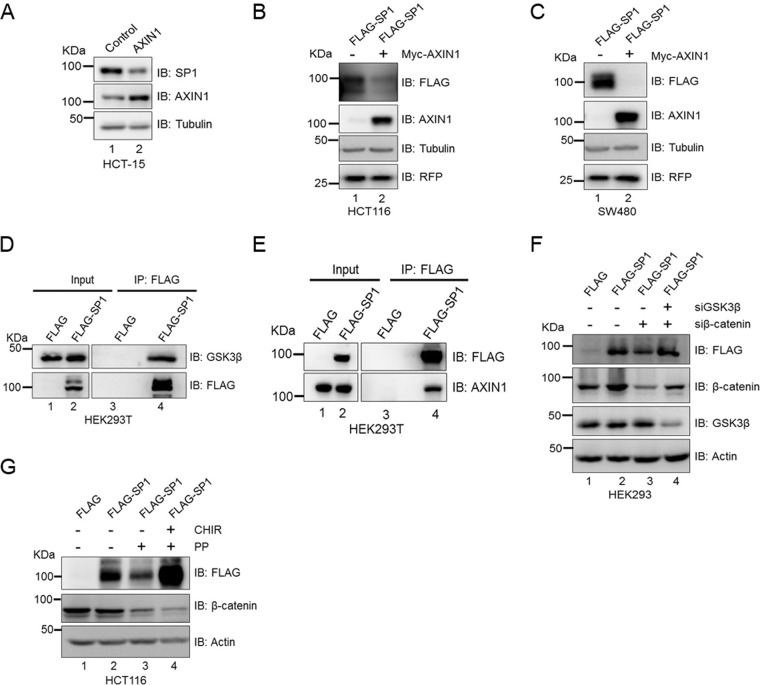
Next, because axin1 is a negative regulator of Wnt signaling and a component of the cytoplasmic destruction complex, we investigated whether axin1 contributes to the degradation of SP1. To test this, we overexpressed axin1 in HCT-15 cells and monitored the expression of SP1. axin1 overexpression reduced the levels of SP1 (Fig. 6A). Further, to understand how SP1 protein levels are reduced in response to overexpression of axin1, we overexpressed Myc-tagged axin1 in FLAG-SP1-expressing HCT116 and SW480 colorectal cancer cells. The overexpression of axin1 reduced the ectopically expressed FLAG-SP1 in comparison with control HCT116 cells (Fig. 6B) and control SW480 cells (Fig. 6C). Since SP1 harbors a canonical phosphodegron motif and GSK3β induces the degradation of SP1 under physiological conditions and is dependent on the Wnt signaling pathway for its stabilization, similar to β-catenin, we investigated whether SP1 interacts with GSK3β in the Wnt-off state. We overexpressed FLAG-SP1 in HEK293T cells and performed an immunoprecipitation assay using anti-FLAG antibody. The results of the coimmunoprecipitation assay suggest that SP1 physically interacts with GSK3β in the Wnt-off state (Fig. 6D). We then sought to determine whether SP1 interacts with axin1 and is part of the destruction complex in the Wnt-off state. Coimmunoprecipitation analysis using HEK293T cells overexpressing FLAG-SP1 revealed that SP1 physically interacts with axin1 in the Wnt-off state (Fig. 6E). Taken together, these results suggest that SP1 is dependent on the Wnt signaling pathway for its stabilization and interacts with components of the cytoplasmic destruction complex.
FIG 6.
GSK3β is essential for degradation of SP1. (A) Immunoblots for expression of SP1 and axin1 in control and Myc-tagged axin1-expressing HCT-15 cells. Tubulin was used as an endogenous control. (B) Immunoblots for FLAG and axin1 in FLAG-SP1-expressing HCT116 cells upon axin1 overexpression. RFP was used as a transfection control. (C) Immunoblots for FLAG-SP1 and Myc-axin1 in SW480 cells upon axin1 overexpression. RFP was used as a transfection control. Tubulin was used as an endogenous control in all panels. Data representative of two independent experiments. (D) Immunoblot for FLAG and GSK3β in FLAG-SP1-expressing HEK293T cells for coimmunoprecipitation of FLAG-SP1 with endogenous GSK3β. (E) Immunoblot for FLAG and axin1 in FLAG-SP1-expressing HEK293T cells for coimmunoprecipitation of FLAG-SP1 with endogenous axin1. Immunoprecipitation was performed using anti-FLAG antibody. Data representative of two independent experiments. (F) Immunoblot analysis in FLAG-SP1-expressing HEK293 cells transfected with siβ-catenin alone (lane 3) and with siGSK3β (lane 4). Actin was used as an endogenous control. (G) Immunoblots for FLAG and β-catenin in control FLAG-SP1 HCT116 cells, pyrvinium pamoate (PP)-treated FLAG-SP1-expressing HCT116 cells, and FLAG-SP1-expressing HCT116 cells treated with both PP and CHIR. Actin was used as an endogenous control.
We observed that GSK3β induces SP1 degradation in the Wnt-off state and also that overexpression of β-catenin reverses the effect of GSK3β by stabilization of SP1 even in the Wnt-off state. This prompted us to investigate whether knockdown of β-catenin induces degradation of SP1 via GSK3β. Toward this, we monitored SP1 stability in HEK293 cells in which FLAG-SP1 was overexpressed and β-catenin was silenced or, alternatively, both GSK3β and β-catenin were silenced simultaneously using RNA interference. Knockdown of β-catenin reduced the levels of ectopically expressed FLAG-SP1 in comparison with siControl (Fig. 6F, compare lanes 2 and 3). In contrast, silencing of GSK3β in β-catenin-depleted FLAG-SP1-expressing HEK293 cells rescued the ectopic levels of FLAG-SP1 (Fig. 6F, compare lane 3 with lane 4). Further, to understand this cross talk, we monitored the stability of SP1 upon β-catenin degradation induced by pyrvinium pamoate (PP), a known inhibitor of Wnt signaling (24), and also inhibited GSK3β activity in PP-treated FLAG-SP1-expressing cells using CHIR. PP treatment induced the degradation of β-catenin and also that of FLAG-tagged SP1 (Fig. 6G, compare lanes 2 and 3), whereas inhibition of GSK3β by CHIR restored the levels of FLAG-SP1 (Fig. 6G, compare lanes 3 and 4). These results demonstrate that β-catenin is essential to prevent the degradation of SP1 mediated by GSK3β activity. Interestingly, HCT116 cells harbor a functional destruction complex; however, they show a mutation in β-catenin (serine 45) to evade the effect of the destruction complex. Thus, higher levels of β-catenin are maintained in these cells, presumably explaining why SP1 levels are higher in HCT116 cells even after having active GSK3β and a functional destruction complex. These findings therefore establish a mechanistic link for the requirement of β-catenin to maintain the stabilization of SP1.
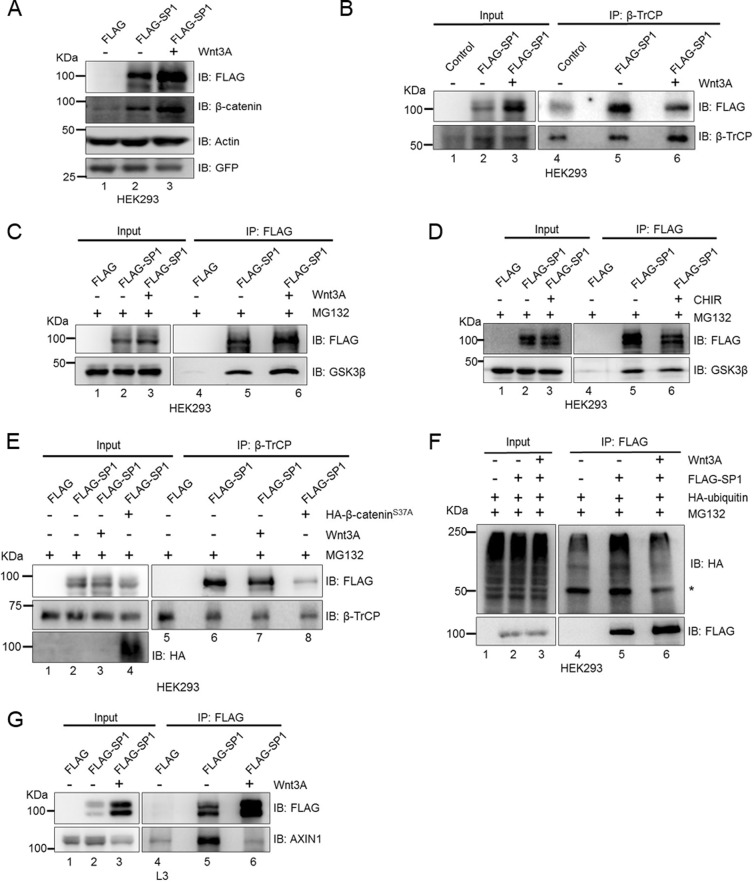
Active Wnt signaling impedes interaction of SP1 with β-TrCP and not with GSK3β.
GSK3β-regulated proteins like β-catenin undergo phosphorylation of serine residues within the phosphodegron motif that is recognized and ubiquitinated by the F box-containing protein β-TrCP ubiquitin E3 ligase followed by degradation through the proteosomal degradation pathway. We therefore sought to determine whether Wnt signaling impedes the interaction of SP1 with GSK3β and β-TrCP in HEK293 cells. To test this, we treated FLAG-SP1 expressing cells with Wnt3A. Treatment with soluble Wnt3A ligand robustly stabilized FLAG-SP1 in HEK293 cells (Fig. 7A, compare lanes 2 and 3). Notably, overexpression of SP1 also increased the stabilization of β-catenin (Fig. 7A, compare lanes 1 and 2). Similarly, Wnt3A treatment further increased β-catenin levels, confirming the activation of Wnt signaling (Fig. 7A, compare lanes 2 and 3). Next, we immunoprecipitated β-TrCP in FLAG-SP1-expressing cells subjected to Wnt stimulation. The coimmunoprecipitation analysis revealed that Wnt stimulation reduces the interaction of SP1 with β-TrCP (Fig. 7B, compare lane 5 and lane 6 in the IP blot). To determine whether Wnt stimulation also affected the interaction of SP1 with GSK3β, we pulled down FLAG-SP1 with anti-FLAG antibody in control and Wnt3A-treated cells and performed coimmunoprecipitation analysis. We observed that Wnt stimulation did not impede the interaction of SP1 with GSK3β (Fig. 7C, compare lanes 5 and 6). These results indicated that Wnt stimulation affects the interaction of SP1 with β-TrCP only and not GSK3β, thereby evading the recognition by the proteosomal pathway following ubiquitination by β-TrCP. To further corroborate the role of aberrant activation of Wnt signaling in SP1 stabilization, we inactivated GSK3β by CHIR treatment, and cells were also treated with MG132 and analyzed for the interaction of GSK3β with SP1. Coimmunoprecipitation analysis revealed that although CHIR treatment results in a robust increase in SP1 stabilization, it did not impede the interaction of SP1 with GSK3β (Fig. 7D, compare lanes 5 and 6). These results indicate that SP1 remains bound to GSK3β even after inactivation of GSK3β activity but presumably loses the interaction with β-TrCP. Since β-catenin induces stabilization of SP1 and mimics the stabilization induced upon Wnt stimulation, we monitored the interaction of SP1 with β-TrCP upon Wnt stimulation and mutant β-catenin overexpression. Immunoblot analysis revealed that both Wnt stimulation and β-catenin overexpression resulted in reduction in the interaction of SP1 with β-TrCP, thereby inducing stabilization of SP1 (Fig. 7E). Wnt stimulation, via affecting the interaction of SP1 with β-TrCP, presumably prevents its ubiquitination and subsequent proteosomal degradation. To investigate this, we analyzed the in vivo ubiquitination of SP1 upon Wnt stimulation. We overexpressed FLAG-SP1 in hemagglutinin (HA)-ubiquitin-expressing HEK293 control cells and Wnt3A-treated cells. Coimmunoprecipitation analysis revealed reduction of SP1 ubiquitination upon Wnt stimulation (Fig. 7F). To extend the role of Wnt signaling in stabilization of SP1, we monitored the interaction SP1 with axin1 upon Wnt stimulation. Coimmunoprecipitation analysis revealed that Wnt stimulation impedes the interaction of SP1 with axin1 despite increased stabilization of SP1 (Fig. 7G, compare lanes 5 and 6). These results provide a novel indication that in Wnt-on cells, Wnt stimulation prevents interaction of axin1 and β-TrCP with SP1, thereby preventing ubiquitination and inducing its stability. In contrast, in the Wnt-off state, GSK3β-mediated phosphorylation of serine residues within the phosphodegron motif allows recognition of SP1 by β-TrCP and subsequent degradation via the proteosomal pathway.
FIG 7.
Wnt signaling stimulation abrogates interaction of SP1 with β-TrCP and not with GSK3β. (A) Immunoblots using anti-FLAG antibody in FLAG control, FLAG-SP1, and FLAG-SP1 Wnt3A-treated HEK293 cells. HEK293 cells were treated with Wnt3A for 6 h. Actin was used as an endogenous control. GFP was used as a transfection control. (B) Immunoblots for FLAG and β-TrCP for coimmunoprecipitation of FLAG-SP1 with endogenous β-TrCP from FLAG, FLAG-SP1, and FLAG-SP1 Wnt3A-treated HEK293 cells. IP with β-TrCP was performed in HEK293 cells overexpressing FLAG, FLAG-SP1 control, and FLAG-SP1 plus Wnt3A treatment. (C) Immunoblots for endogenous GSK3β coimmunoprecipitation with FLAG-SP1 from FLAG and FLAG-SP1 HEK293 cells. IP was performed using anti-FLAG antibody. HEK293 cells were treated with MG132 along with Wnt3A for 6 h. (D) Immunoblots for endogenous GSK3β coimmunoprecipitation with FLAG-SP1 from FLAG, FLAG-SP1, and FLAG-SP1 CHIR-treated HEK293 cells. IP was performed using anti-FLAG antibody. HEK293 cells were treated with MG132 along with CHIR for 6 h. (E) Immunoblots for FLAG, β-TrCP, and HA for coimmunoprecipitation of FLAG-SP1 with endogenous β-TrCP in FLAG, FLAG-SP1 control, FLAG-SP1 Wnt3A-treated HEK293 cells and FLAG-SP1 plus S37A β-catenin-overexpressing HEK293 cells. IP was performed using anti-β-TrCP antibody. (F) Immunoblots for HA and FLAG upon coimmunoprecipitation of FLAG-SP1 with HA-ubiquitin in HA-ubiquitin-expressing FLAG, FLAG-SP1 control, and FLAG-SP1 Wnt3A-treated HEK293 cells. The asterisk denotes IgG heavy chain. (G) Immunoblots for FLAG and axin1 upon coimmunoprecipitation of FLAG-SP1 with endogenous axin1 in FLAG, FLAG-SP1 control, and FLAG-SP1 Wnt3A conditioned medium-treated L3 cells.
SP1 is required for Wnt signaling-dependent β-catenin stabilization and regulation of Wnt-responsive genes.
The common pathway regulating SP1 and β-catenin prompted us to investigate whether SP1 regulates β-catenin. To test this, we silenced SP1 using RNA interference in HCT-116 cells. Immunofluorescence analysis in these cells revealed significant reduction in β-catenin levels upon SP1 knockdown (Fig. 8A). Quantification of nuclear intensities confirmed a significant reduction in β-catenin levels upon SP1 knockdown (Fig. 8B). These observations further prompted us to investigate whether SP1 is required to stabilize β-catenin. To investigate this, we treated siControl-transfected HEK293 cells and siSP1-transfected HEK293 cells with Wnt3A for 6 h. Wnt stimulation induced the stability of both SP1 and β-catenin (Fig. 8C, compare lane 1 with lane 2), whereas SP1 knockdown in Wnt-stimulated HEK293 cells was sufficient to reduce the levels of β-catenin and also the expression of the known Wnt-responsive gene TCF7 (Fig. 8C, compare lanes 2 and 3). Further, CHIR-induced expression of β-catenin in CRL1790 was reduced upon knockdown of SP1 in comparison with control (Fig. 8D, compare lanes 2 and 3). To test whether SP1 is required to stabilize β-catenin at the protein level, we investigated the stability of ectopically expressed FLAG–β-catenin in HEK293 cells upon SP1 knockdown. We overexpressed FLAG–β-catenin in siLuciferase- and siSP1-transfected cells. Overexpression of β-catenin resulted in a robust increase in SP1 expression (Fig. 8E, compare lanes 1 and 2), whereas knockdown of SP1 reduced the levels of ectopically expressed FLAG–β-catenin (Fig. 8E, compare lanes 2 and 3). Next, to investigate whether SP1 is sufficient to stabilize β-catenin, we overexpressed Myc-tagged β-catenin in control and phosphodegron mutant SP1-expressing HEK293 cells. The overexpression of mutant SP1 induced the levels of ectopically expressed Myc–β-catenin in comparison with control (Fig. 8F, compare lanes 2, 3, and 4). The requirement of SP1 for β-catenin stabilization prompted us to investigate whether SP1 abolishes the interaction of β-catenin with components of the destruction complex and thereby β-TrCP-mediated ubiquitination. To investigate this hypothesis, we overexpressed mutant SP1 in HA ubiquitin-expressing HEK293T cells and performed in vivo ubiquitination followed by coimmunoprecipitation assays. Overexpression of mutant SP1 abolished the interaction of β-catenin with axin1, GSK3β, and β-TrCP (Fig. 8G, compare lane 5 and lane 6) and abolished β-TrCP-mediated ubiquitination (Fig. 8G, compare lanes 5 and 6). Additionally, coimmunoprecipitation analysis established that stabilized SP1 impedes the recruitment of GSK3β to axin1, thereby inducing the destabilization of the destruction complex (Fig. 8H, compare lanes 5 and 6). To further understand if SP1 is required for β-catenin stabilization, we generated a stable β-catenin-overexpressing HEK293 cell line and induced the stabilization of β-catenin by CHIR treatment. Inactivation of GSK3β induced the stability of FLAG–β-catenin as well as that of SP1 (Fig. 8I, compare lanes 1 and 2), whereas SP1 knockdown in FLAG–β-catenin-expressing cells reduced the levels of ectopically expressed FLAG–β-catenin (Fig. 8I, compare lanes 2 and 3). The activation of Wnt signaling resulted in upregulation of the Wnt-responsive axin2 and TCF7 genes, whereas SP1 knockdown was sufficient to reduce their expression significantly. To test whether SP1 knockdown-mediated degradation of FLAG-tagged β-catenin at the protein level occurs via the proteosomal pathway, we treated CHIR-treated FLAG–β-catenin SP1-depleted cells with MG132 (Fig. 8I, lane 4). MG132 treatment rescued the stabilization of FLAG–β-catenin (Fig. 8I, compare lanes 3 and 4). Interestingly, the rescue of β-catenin stabilization did not reinduce the expression of Wnt-responsive genes, suggesting the role of SP1 in regulation of Wnt-responsive genes. This was further confirmed by the Wnt-specific TOP/FOP reporter activity in CHIR-treated SP1-depleted cells. CHIR treatment induced the reporter activity and SP1 expression (Fig. 8J, compare lanes 1 and 2). The knockdown of SP1 in CHIR-treated cells was sufficient to reduce the TOP/FOP reporter activity significantly (Fig. 8J, compare bars 2 and 3). To further establish the dual role of SP1 in β-catenin stabilization and regulation of Wnt-responsive genes, we overexpressed mutant β-catenin in a dose-dependent manner in SP1-silenced HEK293 cells to overcome the effect of SP1-dependent β-catenin stabilization. Overexpression of β-catenin induced the stability of SP1 as well as the expression of Wnt-responsive axin2 and TCF7 genes, whereas knockdown of SP1 reduced the levels of Wnt-responsive genes even after rescuing the levels of β-catenin (Fig. 8K). TCF7L2 is a major transcription factor driving the expression of Wnt-responsive genes (25). The lack of any noticeable change in TCF7L2 expression in SP1-depleted cells rules out the possibility that the decrease in expression of Wnt-responsive genes upon SP1 knockdown could be through regulation of TCF7L2.
FIG 8.
SP1 is required for Wnt signaling-dependent β-catenin stabilization and regulation of Wnt-responsive genes. (A) Immunofluorescence analysis for expression of β-catenin and SP1 in siControl and siSP1-transfected HCT116 cells (bars, 20 μm). (B) Quantification of nuclear intensities of SP1 and β-catenin (Mann-Whitney test, two tailed, P < 0.0001). (C) Immunoblots for SP1, β-catenin, and TCF7 upon Wnt3A treatment in control and SP1-depleted HEK293 cells. HEK293 cells were transfected with siGFP and siSP1 and treated with Wnt3A after 42 h for 6 h. (D) Immunoblots for SP1 and β-catenin upon CHIR treatment in control and SP1-depleted CRL-1790 cells. CRL-1790 cells were transfected with siGFP and siSP1 and treated with CHIR for 48 h. (E) Immunoblots for FLAG and for SP1 in FLAG–β-catenin-expressing siLuciferase-transfected HEK293 cells and FLAG–β-catenin-expressing siSP1-transfected cells. Data representative of three independent experiments. (F) Immunoblots for MYC and FLAG using FLAG in MYC–β-catenin control and MYC–β-catenin FLAG-SP1 mutant HEK293 cells. RFP was used for transfection control. (G) Immunoblots for HA, axin1, GSK3β, β-TrCP, and β-catenin in control HA-ubiquitin and HA-ubiquitin-FLAG–mutant SP1 HEK293 upon coimmunoprecipitation of β-catenin with ubiquitin, axin1, GSK3β, and β-TrCP. Mouse IgG was used as a negative control. The asterisk denotes IgG heavy chain. (H) HEK293 cells were transfected with FLAG and FLAG mutant SP1. Lysates were subjected to IP using anti-axin1 antibody and rabbit IgG. Coimmunoprecipitated proteins were analyzed by Western blotting. Overexpression of mutant SP1 impedes the recruitment of GSK3β to the destruction complex. (I) Immunoblots for FLAG, axin2, TCF7, and SP1. Stable FLAG–β-catenin HEK293 cells were treated with CHIR in siControl, siSP1, and siSP1 MG132 HEK293 cells. RFP was used as a transfection control. (J) Graphical representation of TOP/FOP reporter activity under CHIR treatment in siControl and siSP1 HEK293 cells. The experiment was performed in triplicates and repeated twice. The data show a significant increase in TOP/FOP reporter activity upon CHIR treatment (P < 0.0001). Knockdown of SP1 significantly reduces TOP/FOP reporter activity (two-way ANOVA, P < 0.0001). Immunoblots of SP1 for the reporter assay are presented below the graph. The actin blot serves as endogenous control. (K) Immunoblots for HA, SP1, axin2, and TCF7 in control and in SP1-depleted HEK293 cells upon S37A β-catenin overexpression in a dose-dependent manner. Tubulin was used as an endogenous control for all immunoblots.
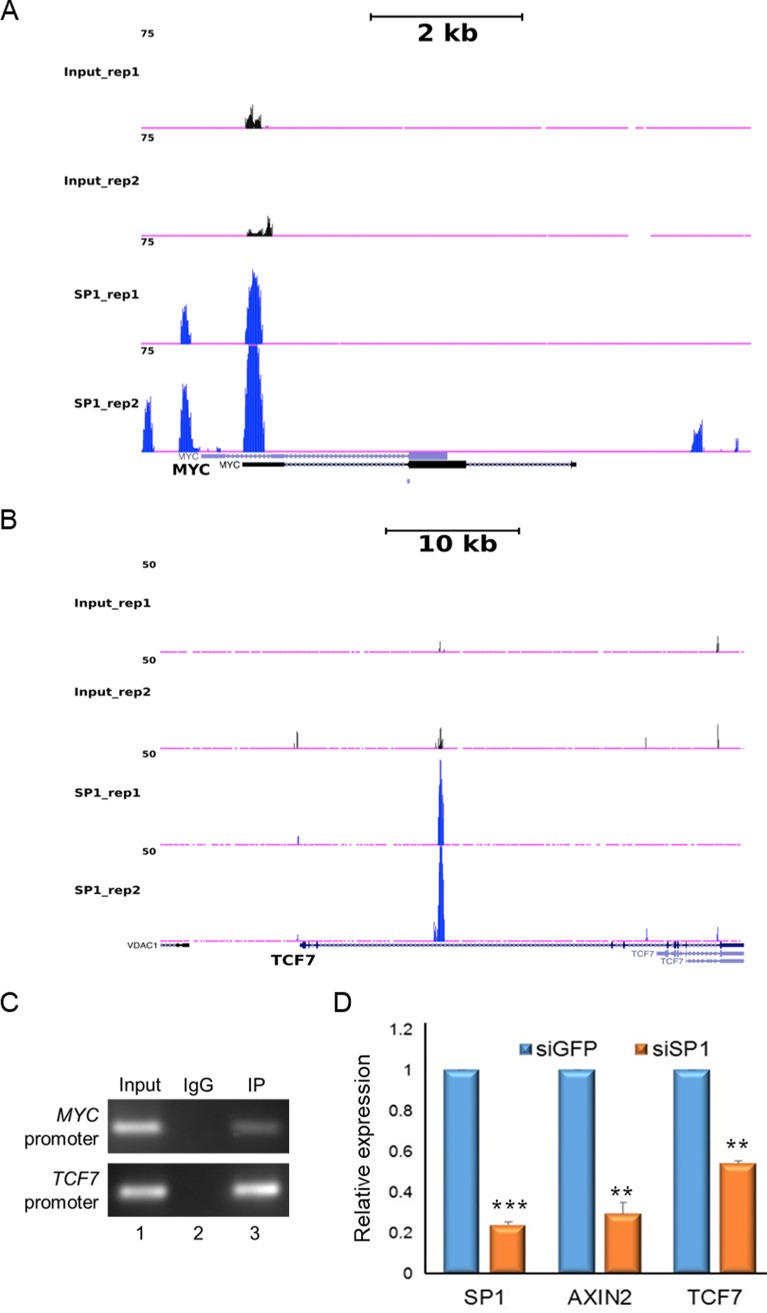
SP1 binds to promoters of TCF7L2/β-catenin target genes.
To elucidate how stability of SP1 could be involved in regulating Wnt-responsive genes, we analyzed the chromatin immunoprecipitation sequencing (ChIP-seq) data for genome-wide occupancy of SP1 in HCT116 colorectal cancer cells from the ENCODE consortium. We identified SP1 binding peaks on promoters of two TCF7L2/β-catenin target genes, namely, c-Myc and TCF7 (Fig. 9A and B). Similar occupancy profiles were seen on regulatory sequences of multiple TCF7L2/β-catenin target genes, including AXIN2 (data not shown). To directly demonstrate the role of the SP1/β-catenin complex in regulation of Wnt-responsive genes, we performed sequential ChIP in HCT116 cells. Chromatin was subjected to one round of immunoprecipitation with anti-SP1 antibody followed by another round of immunoprecipitation using anti-β-catenin antibody. The immunoprecipitated DNA was then subjected to PCR amplification using specific primers for the Wnt target genes Myc and TCF7. The sequential ChIP analysis demonstrated cooccupancy of SP1 and β-catenin on promoters of Myc and TCF7 (Fig. 9C). Quantitative gene expression profiling revealed that SP1 knockdown robustly reduced the expression of Wnt-responsive genes AXIN2 and TCF7 (Fig. 9D). These findings suggest that the SP1 and β-catenin complex presumably occupies the promoters of Wnt responsive genes and regulates their expression. These results further establish the direct mechanistic role of SP1 in regulating β-catenin stabilization and regulation of Wnt-responsive genes in a β-catenin-dependent manner. Collectively, these results strongly argue for a mechanism wherein SP1 and β-catenin are required for their mutual stabilization in a Wnt signaling-dependent manner and for coordinated regulation of Wnt-responsive genes (Fig. 10).
FIG 9.
SP1/β-catenin directly binds to the promoters of Wnt-responsive genes. (A and B) UCSC genome browser view showing occupancy of SP1 in two independent replicates on c-Myc (A) and Tcf7 (B) promoters. ChIP-seq data were analyzed as described in Materials and Methods. (C) Sequential chromatin immunoprecipitation (ChIP-reChIP) performed as described in Materials and Methods showing cooccupancy of SP1 and β-catenin on promoters of Myc and TCF7. (D) Quantitative RT-PCR analysis to monitor expression of Wnt-responsive genes upon SP1 knockdown in HCT-116 cells. The data are representative of two independent experiments. Error bars represent standard deviations for replicates; ***, P < 0.0001; **, P < 0.005; n = 2.
FIG 10.
Model depicting Wnt signaling-mediated stabilization of SP1 and mutual stabilization of SP1 and β-catenin. In the Wnt-off state, SP1 and β-catenin are degraded in a GSK3β-dependent manner. Reduced expression of SP1 and β-catenin is reflected in the decreased expression of Wnt-responsive genes. In the Wnt-on state, both SP1 and β-catenin are stabilized. Increased expression of SP1 and β-catenin induces the expression of Wnt-responsive genes. In the Wnt-on state, knockdown of β-catenin induces the degradation of SP1 and knockdown of SP1 induces the degradation of β-catenin, thereby reversing the Wnt-induced cellular phenotype of increased expression of Wnt-responsive genes. Knockdown resulting in lowering of protein level is depicted in the form of lightly shaded molecules.
DISCUSSION
Here, we present a novel mode of regulation of Wnt signaling involving cross talk of SP1 and β-catenin stabilization as an integral part of the Wnt signaling cascade. We show that Wnt stimulation induces the simultaneous stabilization of SP1 and β-catenin. Further, using various biochemical inhibitors of downstream effectors, we demonstrate that Wnt signaling induces SP1 stabilization. After demonstrating that SP1 and β-catenin follow similar events upon activation of canonical Wnt signaling, we investigated whether SP1 is a component of the β-catenin destruction complex. We show that SP1 interacts with axin1 and GSK3β in the Wnt-off state. In colorectal cancer cells characterized by constitutively active Wnt signaling, SP1 interacts with β-catenin and is part of the destruction complex. Further, the SP1/β-catenin complex directly binds to the regulatory elements of Wnt targets and regulates their expression.
β-Catenin promotes Wnt signaling-dependent stabilization of SP1.
The multiple lines of evidence presented here strongly argue in favor of a novel regulatory complex in Wnt signaling wherein not only is β-catenin stabilized but the ubiquitous transcription factor SP1 also follows the same route to stabilization. This study sheds light on the hitherto-uncharacterized role of Wnt signaling in stabilization of SP1. We show that in the absence of Wnt ligand (Wnt-off state), SP1 interacts with components of the destruction complex, suggesting that the destruction complex is critical for SP1 destabilization. Stimulation by Wnt ligand (Wnt-on state) leads to loss of interaction of SP1 with β-TrCP and axin1 but not with GSK3β, leading to an increase in levels of SP1 in cytosol and nucleus. This change in SP1 stabilization is similar to β-catenin stabilization, whereby Wnt stimulation impedes the interaction of β-catenin with β-TrCP but not with GSK3β (26).
After understanding the importance of Wnt signaling and the destruction complex, we focused on understanding the role of β-catenin in stabilization of SP1. Inhibition of the cytoplasmic destruction complex leads to stabilization and nuclear translocation of β-catenin. Here we report the role of β-catenin in the Wnt-off and Wnt-on states. We provide evidence that in the Wnt-on state, stabilization of β-catenin results in an increase in SP1 at the protein level but the transcript levels are unaffected. We show that stabilization of SP1 is dependent on that of β-catenin. In the Wnt-off state, the stabilization of β-catenin mimics the changes acquired upon Wnt stimulation by impeding the interaction of SP1 with the E3 ubiquitin ligase β-TrCP. These results suggest that both SP1 and β-catenin form a complex and that release of β-catenin from the destruction complex upon Wnt stimulation prevents recruitment of SP1 to the destruction complex (Fig. 10). The dependence of SP1 stabilization on β-catenin could be very critical for therapeutic intervention in cancers driven by SP1 hyperexpression. Small molecules that are used to target Wnt signaling-driven cancers can presumably be used to target SP1-driven cancers. Furthermore, SP1 has been shown to be associated with cancers driven by aberrant activation of Wnt signaling (26–28). Thus, it is of immediate interest to understand how this novel cross talk of SP1 and β-catenin could be critical for targeted drug therapy in cancers driven by SP1/β-catenin signaling. It would be important to elucidate whether SP1 can modulate the function of novel players such as YAP and SMADs, which were recently shown to exhibit cross talk with Wnt signaling (29–31).
GSK3β-mediated SP1 degradation and role of the phosphodegron motif.
GSK3β is critical for regulating the stabilization of proteins containing the DSGXXS motif, also referred to as the phosphodegron motif (2, 30, 31). Wnt signaling induces sequestration of GSK3β into multivesicular bodies, thereby reducing its kinase activity. These changes are associated with increasing the half-life of Wnt signaling-regulated proteins (8). Phosphorylation of serine residues in this DSG motif is the recognition tag for β-TrCP to ubiquitinate, followed by proteosomal degradation (7). We observed that GSK3β-mediated destabilization of SP1 requires loss of β-catenin. We provide evidence that SP1 interacts with both GSK3β and β-catenin. Further, stabilization of β-catenin impedes interaction of SP1 with components of the destruction complex and destabilization of SP1 upon β-catenin knockdown can be rescued by depleting or inhibiting GSK3β. Thus, availability of β-catenin is essential for SP1 stabilization and GSK3β can promote SP1 degradation only in the absence of β-catenin. Further, in the Wnt-off state, availability of GSK3β is critical to induce SP1 degradation and knockdown of GSK3β mimics the changes observed upon stabilization of β-catenin and/or upon Wnt stimulation. The degradation of SP1 upon β-catenin knockdown can be reversed by depleting GSK3β or inactivating its kinase activity. We propose that in the absence of β-catenin or in the Wnt-off state, GSK3β engages SP1 for phosphorylation of its phosphodegron motif, which is subsequently ubiquitinated by E3 ubiquitin ligase followed by proteasome-mediated degradation. The importance of phosphodegron in SP1 is also underscored by the fact that it is specific only to mammals. The introduction of mammalian phosphodegron in Drosophila SP1 resulted in its destabilization. Thus, SP1 might have been adopted into the Wnt signaling pathway much later during the evolution of vertebrates.
Earlier studies have shown that thiazolidinedione drugs induce expression of β-TrCP, thereby destabilizing SP1 in prostate cancers (32). Further, thiazolidinediones have been shown to induce degradation of β-catenin in a GSK3β-dependent manner (32), thus hinting at the common route to stabilization. Here we demonstrate a connecting link that coregulates SP1 and β-catenin. We report that β-catenin stability is essential for SP1 stability and function of GSK3β is critical for this regulation. Further, we show that the N and C termini of β-catenin interact with the N terminus of SP1, leaving the C terminus containing the phosphodegron motif available for interaction with GSK3β. Wnt stimulation negatively affects the interaction of SP1 with β-TrCP and axin1. These results therefore indicate that availability of β-catenin functions as a strong molecular cue regulating SP1 stabilization. Further investigation is required to delineate the molecular mechanism governing recruitment of β-TrCP to SP1. A recent study revealed that YAP is required for recruitment of β-TrCP to β-catenin in the Wnt-off state, hence inducing β-catenin degradation (30). It will be interesting to test whether YAP is a common mediator for β-catenin and SP1 regulation in the Wnt-off state.
SP1 promotes β-catenin stabilization.
We further established that SP1 could mimic Wnt signaling-associated stabilization of β-catenin. We provide evidence that Wnt/GSKβ signaling-induced stabilization of β-catenin is attenuated in the absence of SP1. The common route of SP1 and β-catenin stabilization by Wnt stimulation seems to be critical for their mutual stabilization. Further, we found that overexpression of the stabilized phosphodegron mutant SP1 in the Wnt-off state leads to stabilization of β-catenin by impeding its recruitment to the cytoplasmic destruction complex. The stabilization of SP1 seems to be critical for regulating stabilization of the destruction complex such that it impedes the interaction of GSK3β to axin1. The requirement of SP1 in the Wnt signaling cascade might be critical for stringent regulation during embryonic development and for progression of Wnt-driven cancers. Hence, understanding the molecular mechanism of how SP1 regulates the interaction of β-catenin with components of the destruction complex will be useful for targeting drugs against developmental defects and cancers. SP1 and β-catenin are required for reciprocal stabilization, wherein stabilization of one mediates the changes required to induce stabilization of the other, thereby exerting a regulatory feedback (schematically presented in Fig. 10). The mutual sequestration targeting the SP1 and β-catenin complex could be important for therapeutic intervention in future. Understanding the interaction/cross talk of the SP1/β-catenin signaling axis with other signaling pathways could provide further mechanistic insights into tumorigenesis.
Stability of SP1 affects the outcome of Wnt signaling.
We provide evidence that SP1 is not only required to stabilize β-catenin but also essential to regulate the expression of Wnt-responsive genes. There are two changes associated with SP1 expression. In the first scenario, SP1 interacts with β-catenin and is required for its Wnt-dependent regulation of stabilization. In the second scenario, the role of SP1 is critical in the nucleus, where it forms a complex with β-catenin and cooccupies the promoters of Wnt-responsive genes and regulates their expression. The importance of SP1 expression can be underscored by the fact that even rescuing the levels of β-catenin in SP1-depleted cells does not reinduce the expression of Wnt-responsive genes. These molecular changes that govern the expression of SP1/β-catenin signaling-dependent regulation of Wnt-responsive genes are independent of TCF7L2 expression. Taken together, these findings suggest that SP1 acts as a double-edged sword that is required for the stabilization of β-catenin and for the regulation of Wnt-responsive genes and is therefore a critical determinant of the molecular events associated with the Wnt signaling pathway.
SP1 induces transcriptional superactivation by tetramerization through its D domain (33–36). This mode of SP1 organization mediates synergistic activation or repression of genes. Further, tetrameric aggregation of SP1 has been shown to induce DNA looping between enhancer and promoter regions of genes (36). The tetrameric assembly of SP1 on DNA provides docking site for other transcription factors, transcriptional regulators, and chromatin remodelers (36), thus providing an essential platform to activate or repress gene expression. Hence, it will be interesting to understand whether interaction of SP1 with β-catenin influences such an arrangement on promoters of Wnt-responsive genes along with the TCF7L2/β-catenin complex to superactivate the expression of target genes upon Wnt stimulation. Further, it will be interesting to understand whether differential gene expression regulated by SP1 depends on β-catenin cooccupancy on target genes. Genome-wide approaches would reveal the differential role of SP1 and the SP1/β-catenin complex for regulation of gene expression and thereby influencing the different developmental paradigms and progression of cancers.
MATERIALS AND METHODS
Cell cultures, transfections, and Western blotting.
SW480, SW620, and HCT116 cell lines were grown in Dulbecco's modified Eagle medium (DMEM) with 10% fetal calf serum (FCS). CRL1790 cells were grown in MEM with 10% FCS. CRL1790, SW480, SW620, and HeLa cell lines were obtained from American Type Culture Collection (ATCC). HCT116 was obtained from European Collection of Authenticated Cell Cultures (ECACC, through Sigma). For transfection to achieve siRNA-mediated knockdown, cells were seeded and transfected after 24 h with the indicated siRNA and then harvested for immunoblotting and RNA extraction after another 48 h. The sequences of short hairpin RNA (shRNA) and siRNA used are listed in Table S1 in the supplemental material.
Treatments with inhibitors.
To activate Wnt signaling, CRL1790 cells were treated with CHIR (3 μM) and BIO (1 μM) for 48 h and harvested for protein and RNA. For stability experiments, cells were first transfected with siRNA and after 24 h transfected with plasmids and/or treated with Wnt3A (100 ng/ml) or CHIR (3 μM). Similarly, pyrvinium pamoate (PP) was used at a concentration of 100 nM and cells were harvested after 48 h. For knockdown of SP1 and β-catenin under CHIR and Wnt3A, cells were first transfected with control siRNA, siSP1, and siβ-catenin and after 12 h treated with CHIR for 48 h in the case of CRL1790 cells, whereas other cells were treated with CHIR and Wnt3a for 6 h after 42 h of transfection. ALLN (N-acetyl-leucinyl-leucinyl-norleucinal) was used at 25 μg/ml. MG132 was used at 10 μM for 6 h.
Immunofluorescence analysis and quantification.
Cells were cultured on fibronectin-coated glass coverslips and fixed using 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 15 min at room temperature (RT). Permeabilization was performed using 0.25% Triton X-100 for 5 min, followed by blocking in 10% bovine serum albumin (BSA) in PBS for 1 h at room temperature. The cells were incubated in primary antibody (diluted in 5% BSA in PBS) for 16 h at 4°C followed by four washes in PBS at room temperature, incubation in secondary antibody (diluted in 2% BSA) for 1 h, and four washes in PBS. Immunofluorescence images were obtained using a Zeiss 710 LSM confocal microscope. Nuclear intensity was quantified using ImageJ software. The nuclear area within each cell was selected using 4′,6-diamidino-2-phenylindole (DAPI) staining as the region of interest (ROI). Quantified nuclear intensity was normalized with the nuclear area and DAPI. At least 300 cells were used for quantification in each set.
ChIP-seq data analysis.
Genome-wide occupancy data sets of SP1 in HCT116 cells were obtained from the publicly available database from the ENCODE consortium with experiment IDs ENCSR000BVT and ENCSR000BSF for input and SP1 ChIP samples, respectively. Briefly, reads were aligned to human genome (hg19) with bowtie2 using the “very-sensitive” mode. Peaks were called using MACS14, and wiggle files were uploaded on the UCSC genome browser for visualization.
Antibodies, reagents, and plasmids.
SATB1, TCF7L2, TCF7, SP1, GSK3β, β-TrCP, axin1, and axin2 antibodies were obtained from Cell Signaling Technology. Anti-β-catenin antibody was obtained from BD Transduction Laboratories. β-Catenin for IP in HeLa cells was used (from Santa Cruz). Actin and gamma-tubulin antibodies were obtained from Sigma. Secondary horseradish peroxidase (HRP)-conjugated antibodies were obtained from Bio-Rad. Recombinant Wnt3A protein was obtained from R&D systems. Pyrvinium pamoate was obtained from Sigma. SP1 was cloned in pCMV10-3XFLAG vector (Sigma). ALLN was from Calbiochem, and MG132 was from Sigma.
Mutant phosphodegron was generated by site-directed mutagenesis, and primers were designed as described in the QuikChange primer designer tool. Mutations of the phosphodegron were verified by sequencing of the constructs. Phosphodegron mutant and deleted versions were cloned in pCMV10-3×FLAG vector (Sigma). 3×FLAG–β-catenin, HA–S37A β-catenin, GST-N terminus, GST-ARM repeats, and GST–C terminus β-catenin were used as described by Notani et al. (39). The siRNA sequences for shSP1 and shβ-catenin were designed using the Dharmacon design center. The siRNAs for silencing SP1, β-catenin, and GSK3β were procured from Dharmacon. All shRNAs were cloned in pSUPER Puro vector (Oligo Engine). The sequences of siRNAs and shRNAs used in this study are listed in Table S1.
RNA isolation and RT-PCR.
RNA was isolated using TRIzol reagent (Invitrogen). Two micrograms of RNA was used for first-strand cDNA synthesis using Superscript III (Invitrogen). The cDNA was then used for quantitative PCR analysis in triplicates using an ABI 7500 Fast real-time PCR system (Applied Biosystems) as described previously (2). The sequences of oligonucleotide primers used for real-time quantitative PCR analysis are listed in Table S2 in the supplemental material.
Sequential chromatin immunoprecipitation assay.
The ChIP assay was performed as described previously (3). Briefly, cells were cross-linked by addition of formaldehyde to 1% final concentration in medium and incubated at room temperature for 10 min, neutralized with 125 mM glycine. Cells were then subjected to sonication using a Covaris sonicator to fragment chromatin to obtain 200- to 500-bp fragments. Sonicated chromatin was precleared with nonsaturated beads. Precleared chromatin was incubated with specific antibodies, and the respective IgG types were used as isotype controls. The next day, beads saturated with tRNA and BSA were added (40 μl packed beads), incubated for 4 h on a rocker to pull down the antibody-bound chromatin, and subjected to elution using buffer containing SDS and sodium bicarbonate. Eluted chromatin was de-cross-linked, and protein was removed by treating with proteinase K. Purified immunoprecipitated chromatin was subjected to PCR amplification using specific primers. Input chromatin was used as a control. The first round of immunoprecipitation was performed using 200 μg of chromatin and 20 μg of anti-SP1 antibody (Cell Signaling Technology), followed by 2 washes with low-salt buffer and 1 wash with high-salt buffer. Chromatin immunoprecipitated DNA was eluted using ChIP elution buffer at 37°C. Eluted chromatin was diluted 10 times with ChIP dilution buffer (16.7 mM Tris-Cl, 167 mM NaCl, 1.2 mM EDTA, 0.01% SDS) and subjected to a second immunoprecipitation using 20 μg of anti-β-catenin antibody (Santa Cruz Biotechnology). An equal amount of rabbit IgG was used for a negative-control reaction. Chromatin-antibody complexes were pulled down using 100 μl of M280 sheep anti-rabbit IgG magnetic beads (Invitrogen) and further washed 3 times each with low-salt buffer, high-salt buffer, LiCl wash buffer, and Tris-EDTA (TE) buffer before eluting with elution buffer at 65°C. The eluted fraction was reverse cross-linked and purified using a PCR microkit (Invitrogen). ChIP primer details are listed in Table S2.
Immunoprecipitation, coimmunoprecipitation, and ubiquitination.
Immunoprecipitation and coimmunoprecipitation were done essentially as mentioned in reference 4. Briefly, cells were harvested and lysed in lysis buffer, consisting of 20 mM HEPES (pH 7.8), 400 mM KCl, 5% glycerol, 5 mM EDTA, 0.4% NP-40, phosphatase, and protease inhibitors (Roche). Lysates were sonicated and cleared by centrifugation. Before immunoprecipitation, lysates were diluted in 20 mM HEPES (pH 7.8), 50 mM KCl, 5% glycerol, 2.5 mM MgCl2, and 0.05% NP-40 and incubated with the appropriate protein G-Dyna bead-bound antibodies for 4 h at 4°C (1/8 to the lysis buffer). After three washes in binding buffer, copurified proteins were analyzed by immunoblotting by using the ExactaCruz reagents (Santa Cruz Biotechnology) as secondary antibodies to reduce the background from IgG. Beads used for IP were precoated with antibody and then washed twice with binding buffer. In vivo ubiquitination was performed as described in Jeong et al. (37). Briefly, cells were harvested and lysed in co-IP lysis buffer containing phosphatase, protease, and deubiquitinase inhibitor NEM (N-ethylmaleimide; 10 mM), and IP and co-IP were performed as described above.
GST pulldown.
For GST pulldown, beads with purified proteins GST and GST–β-catenin were incubated with lysate in binding buffer (25 mM HEPES [pH 7.9], 0.4 M KCl, 0.4% NP-40, 5 mM EDTA, 1 mM dithiothreitol [DTT], 10% glycerol with protease inhibitors) for 1 h. After four washes with binding buffer, interaction of endogenous protein from cell lysate with purified proteins was determined by immunoblotting. For in vitro protein interaction, GST-SP1 was cleaved with thrombin (GE Healthcare) for 16 h at 25°C to release SP1 from the GST tag. The cleaved protein was purified using Microcon YM30 columns (Millipore). Purified protein was incubated with GST–β-catenin on glutathione resin for 1 h at 4°C in binding buffer, followed by four washes with binding buffer. For GST pulldown assays with purified protein FLAG-SP1-expressing cells, FLAG-SP1 was first immunoprecipitated using anti-FLAG antibody. After immunoprecipitation, FLAG-SP1 was eluted from beads using the FLAG peptide, followed by incubation with GST and GST–β-catenin in binding buffer for 1 h. After four washes with binding buffer, interaction complexes were resolved by SDS-PAGE and determined by immunoblotting. Domain Graph version 1.0 was used to prepare protein domain structures (38).
Cellular fractionation.
For cytosolic and nuclear fractionation, cells were washed in ice-cold PBS. Cell pellets were then resuspended in hypotonic lysis buffer (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT) containing protease inhibitor and phosphatase inhibitor cocktail (Roche). Cell suspensions were incubated on ice for 30 min to swell followed by addition of 25 μl of 10% NP-40. Nuclear proteins, including the unlysed cells, were pelleted by spinning at 2,000 rpm for 2 min at 4°C. The supernatant containing cytoplasm proteins was collected. The pellet was then washed twice with hypotonic lysis buffer and then resuspended in lysis buffer (20 mM HEPES [pH 7.9], 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT) containing protease inhibitor and phosphatase inhibitor cocktail. The lysate was then centrifuged at 14,000 rpm for 30 min at 4°C for nuclear fractions and analyzed further by SDS-PAGE followed by Western blotting.
Supplementary Material
ACKNOWLEDGMENTS
We thank Randall T. Moon for the gift of TOP/FOP constructs and Kang-Yell Choi for the gift of the HA-ubiquitin construct.
This work was supported by grants from the Centre of Excellence in Epigenetics program of the Department of Biotechnology, the Swarnajayanti Fellowship from the Department of Science and Technology, Government of India, and institutional funding from IISER Pune to S.G. R.M. and A.S. are supported by fellowships from the University Grants Commission, India. S.J.P. is supported by fellowship from the Council of Scientific and Industrial Research, Government of India.
Author contributions were as follows. R.M. and S.G. conceived the project. R.M., A.S., and S.G. designed experiments and interpreted results. Experiments were performed by R.M., A.S., and S.J.P. S.J.P. performed ChIP-seq data analysis. The manuscript was written by R.M. and S.G.
We declare that we have no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/MCB.00188-18.
REFERENCES
- 1.Clevers H. 2006. Wnt/β-catenin signaling in development and disease. Cell 127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Valenta T, Hausmann G, Basler K. 2012. The many faces and functions of β-catenin. EMBO J 31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anastas JN, Moon RT. 2013. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 4.Najdi R, Holcombe RF, Waterman ML. 2011. Wnt signaling and colon carcinogenesis: beyond APC. J Carcinog 10:5. doi: 10.4103/1477-3163.78111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schepers A, Clevers H. 2012. Wnt signaling, stem cells, and cancer of the gastrointestinal tract. Cold Spring Harb Perspect Biol 4:a007989. doi: 10.1101/cshperspect.a007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagotto F. 2013. Looking beyond the Wnt pathway for the deep nature of β-catenin. EMBO Rep 14:422–433. doi: 10.1038/embor.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacDonald BT, Tamai K, He X. 2009. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taelman VF, Dobrowolski R, Plouhinec J-L, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM. 2010. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinyoles M, Del Valle-Pérez B, Curto J, Viñas-Castells R, Alba-Castellón L, García de Herreros A, Duñach M. 2014. Multivesicular GSK3 sequestration upon Wnt signaling is controlled by p120-catenin/cadherin interaction with LRP5/6. Mol Cell 53:444–457. doi: 10.1016/j.molcel.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Kim HV, Hedtke P, Ploper J, De Robertis D, Edward M. 2015. Wnt signaling translocates Lys48-linked polyubiquitinated proteins to the lysosomal pathway. Cell Rep 11:1151–1159. doi: 10.1016/j.celrep.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 11.Black AR, Black JD, Azizkhan-Clifford J. 2001. Sp1 and krüppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol 188:143–160. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 12.Gilmour J, Assi S, Jaegle U, Kulu D, van de Werken H, Clarke D, Westhead DR, Philipsen S, Bonifer C. 2014. A crucial role for the ubiquitously expressed transcription factor Sp1 at early stages of hematopoietic specification. Development 141:1–11. doi: 10.1242/dev.106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beishline K, Azizkhan-Clifford J. 2015. Sp1 and the ‘hallmarks of cancer.’ FEBS J 282:224–258. doi: 10.1111/febs.13148. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Wei D, Huang S, Peng Z, Le X, Wu TT, Yao J, Ajani J, Xie K. 2003. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res 9:6371–6380. [PubMed] [Google Scholar]
- 15.Wang X-B, Peng W-Q, Yi Z-J, Zhu S-L, Gan Q-H. 2007. Expression and prognostic value of transcriptional factor sp1 in breast cancer. Ai Zheng 26:996–1000. [PubMed] [Google Scholar]
- 16.Hsu T-I, Wang MC, Chen SY, Yeh YM, Su WC, Chang WC, Hung J-J. 2012. Sp1 expression regulates lung tumor progression. Oncogene 31:3973–3988. doi: 10.1038/onc.2011.568.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spengler ML, Guo LW, Brattain MG. 2008. Phosphorylation mediates Sp1 coupled activities of proteolytic processing, desumoylation and degradation. Cell Cycle 7:623–630. doi: 10.4161/cc.7.5.5402. [DOI] [PubMed] [Google Scholar]
- 18.Wei S, Chuang H-C, Tsai W-C, Yang H-C, Ho S-R, Paterson AJ, Kulp SK, Chen C-S. 2009. Thiazolidinediones mimic glucose starvation in facilitating Sp1 degradation through the up-regulation of beta-transducin repeat-containing protein. Mol Pharmacol 76:47–57. doi: 10.1124/mol.109.055376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Genome Atlas Network. 2012. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stemmer V, de Craene B, Berx G, Behrens J. 2008. Snail promotes Wnt target gene expression and interacts with beta-catenin. Oncogene 27:5075–5080. doi: 10.1038/onc.2008.140. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Bavarva JH, Wang Z, Guo J, Qian C, Thibodeau SN, Golemis Ea, Liu W. 2011. HEF1, a novel target of Wnt signaling, promotes colonic cell migration and cancer progression. Oncogene 30:2633–2643. doi: 10.1038/onc.2010.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mir R, Pradhan SJ, Patil P, Mulherkar R, Galande S. 2015. Wnt/β-catenin signaling regulated SATB1 promotes colorectal cancer tumorigenesis and progression. Oncogene 35:1679–1691. doi: 10.1038/onc.2015.232. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Lam JBB, Chow KHM, Xu A, Lam KSL, Moon RT, Wang Y. 2008. Adiponectin stimulates Wnt inhibitory factor-1 expression through epigenetic regulations involving the transcription factor specificity protein 1. Carcinogenesis 29:2195–2202. doi: 10.1093/carcin/bgn194. [DOI] [PubMed] [Google Scholar]
- 24.Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, Kim K, Melancon B, Ghidu VP, Sulikowski GA, LaFleur B, Salic A, Lee LA, Miller DM III, Lee E. 2010. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat Chem Biol 6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatzis P, van der Flier LG, van Driel MA, Guryev V, Nielsen F, Denissov S, Nijman IJ, Koster J, Santo EE, Welboren W, Versteeg R, Cuppen E, van de Wetering M, Clevers H, Stunnenberg HG. 2008. Genome-wide pattern of TCF7L2/TCF4 chromatin occupancy in colorectal cancer cells. Mol Cell Biol 28:2732–2744. doi: 10.1128/MCB.02175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li VSW, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, Mohammed S, Heck AJR, Maurice MM, Mahmoudi T, Clevers H. 2012. Wnt Signaling through inhibition of β-catenin degradation in an intact axin1 complex. Cell 149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Waby JS, Chirakkal H, Yu C, Griffiths GJ, Benson RSP, Bingle CD, Corfe BM. 2010. Sp1 acetylation is associated with loss of DNA binding at promoters associated with cell cycle arrest and cell death in a colon cell line. Mol Cancer 9:275. doi: 10.1186/1476-4598-9-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y, Zhang W, Guo Z, Ma F, Wu Y, Bai Y, Gong W, Chen Y, Cheng T, Zhi F, Zhang Y, Wang J, Jiang B. 2013. Inhibition of the transcription factor Sp1 suppresses colon cancer stem cell growth and induces apoptosis in vitro and in nude mouse xenografts. Oncol Rep 30:1782–1792. doi: 10.3892/or.2013.2627. [DOI] [PubMed] [Google Scholar]
- 29.Yu M-H, Zhang W. 2016. TEAD1 enhances proliferation via activating SP1 in colorectal cancer. Biomed Pharmacother 83:496–501. doi: 10.1016/j.biopha.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 30.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, Piccolo S. 2014. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Demagny H, Araki T, De Robertis EM. 2014. The tumor suppressor Smad4/DPC4 is regulated by phosphorylations that integrate FGF, Wnt, and TGF-β signaling. Cell Rep 9:688–700. doi: 10.1016/j.celrep.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Wei S, Lin L-F, Yang C-C, Wang Y-C, Chang G-D, Chen H, Chen C-S. 2007. Thiazolidinediones modulate the expression of beta-catenin and other cell-cycle regulatory proteins by targeting the F-box proteins of Skp1-Cul1-F-box protein E3 ubiquitin ligase independently of peroxisome proliferator-activated receptor gamma. Mol Pharmacol 72:725–733. doi: 10.1124/mol.107.035287. [DOI] [PubMed] [Google Scholar]
- 33.Porter W, Wang F, Wang W, Duan R, Safe S. 1996. Role of estrogen receptor/Sp1 complexes in estrogen-induced heat shock protein 27 gene expression. Mol Endocrinol 10:1371–1378. [DOI] [PubMed] [Google Scholar]
- 34.Doetzlhofer A, Rotheneder H, Lagger G, Koranda M, Kurtev V, Brosch G, Wintersberger E, Seiser C. 1999. Histone deacetylase 1 can repress transcription by binding to Sp1. Mol Cell Biol 19:5504–5511. doi: 10.1128/MCB.19.8.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L, He S, Sun J-M, Davie JR. 2004. Gene regulation by Sp1 and Sp3. Biochem Cell Biol 82:460–471. doi: 10.1139/o04-045. [DOI] [PubMed] [Google Scholar]
- 36.Deshane J, Kim J, Bolisetty S, Hock TD, Hill-Kapturczak N, Agarwal A. 2010. Sp1 regulates chromatin looping between an intronic enhancer and distal promoter of the human heme oxygenase-1 gene in renal cells. J Biol Chem 285:16476–16486. doi: 10.1074/jbc.M109.058586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeong WJ, Yoon J, Park JC, Lee SH, Lee SH, Kaduwal S, Kim H, Yoon JB, Choi KY. 2012. Ras stabilization through aberrant activation of Wnt/beta-catenin signaling promotes intestinal tumorigenesis. Sci Signal 5:ra30. doi: 10.1126/scisignal.2002242. [DOI] [PubMed] [Google Scholar]
- 38.Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X. 2009. DOG 1.0: illustrator of protein domain structures. Cell Res 19:271–273. doi: 10.1038/cr.2009.6. [DOI] [PubMed] [Google Scholar]
- 39.Notani D, Gottimukkala KP, Jayani RS, Limaye AS, Damle MV, Mehta S, Purbey PK, Joseph J, Galande S. 2010. Global regulator SATB1 recruits β-catenin and regulates TH2 differentiation in Wnt-dependent manner. PLoS Biol 8:e1000296. doi: 10.1371/journal.pbio.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.