RSV is a serious public health problem, as it is associated with bronchiolitis, pneumonia, and asthma exacerbations. Currently no effective treatment or vaccine is available, and many molecular mechanisms regarding RSV-induced lung disease are still significantly unknown. This project aims to elucidate an important and novel function of a protein, called EPAC2, in RSV replication and innate inflammatory responses. Our results should provide an important insight into the development of new pharmacologic strategies against RSV infection, thereby reducing RSV-associated morbidity and mortality.
KEYWORDS: EPAC, RSV, replication and immune response, EPAC2, inflammation, replication
ABSTRACT
Respiratory syncytial virus (RSV) is the leading cause of respiratory infection in young children and high-risk adults. However, a specific treatment for this viral infection is not currently available. In this study, we discovered that an exchange protein directly activated by cyclic AMP (EPAC) can serve as a potential therapeutic target for RSV. In both lower and upper epithelial cells, treatment with EPAC inhibitor (ESI-09), but not protein kinase A inhibitor (H89), significantly inhibits RSV replication and proinflammatory cytokine/chemokine induction. In addition, RSV-activated transcriptional factors belonging to the NF-κB and IRF families are also suppressed by ESI-09. Through isoform-specific gene knockdown, we found that EPAC2, but not EPAC1, plays a dominant role in controlling RSV replication and virus-induced host responses. Experiments using both EPAC2 knockout and EPAC2-specific inhibitor support such roles of EPAC2. Therefore, EPAC2 is a promising therapeutic target to regulate RSV replication and associated inflammation.
IMPORTANCE RSV is a serious public health problem, as it is associated with bronchiolitis, pneumonia, and asthma exacerbations. Currently no effective treatment or vaccine is available, and many molecular mechanisms regarding RSV-induced lung disease are still significantly unknown. This project aims to elucidate an important and novel function of a protein, called EPAC2, in RSV replication and innate inflammatory responses. Our results should provide an important insight into the development of new pharmacologic strategies against RSV infection, thereby reducing RSV-associated morbidity and mortality.
INTRODUCTION
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract disease in children, the elderly, and immunocompromised patients, representing a huge medical burden globally (1–5). RSV-caused upper respiratory tract infection results in hospitalization and also contributes to outpatient burden (5). To date, there is no vaccine approved to prevent RSV infection or minimize symptoms of infection (6). Palivizumab, a highly potent RSV-neutralizing monoclonal antibody (MAb), was licensed in 1998 for prophylactic use to prevent RSV-associated hospitalizations in high-risk infants. However, it is not very cost-effective and is mainly limited to selected high-risk infants for their first RSV season (7). In addition, there is no specific and effective treatment against RSV, since the host-RSV interaction is still poorly understood. Continuously exploring novel host and viral molecules involved in antiviral responses to RSV and associated pathogenesis is therefore needed for developing effective therapeutic approaches.
Many signaling pathways rely on second messenger molecules for signal transduction. Cyclic AMP (cAMP), the first identified second messenger, plays a critical role in various biological processes, including cell differentiation, proliferation, and fate (8–10). Current research points to two major families of eukaryotic cAMP receptors: protein kinase A (PKA) and the recently identified exchange protein directly activated by cAMP (EPAC) (11, 12). In mammals, there are two isoforms of EPAC, EPAC1 and EPAC2, which are encoded by Rapgef3 and Rapgef4 genes, respectively. The function and significance of EPAC in cellular processes, including pathogen defense, are emerging but have not been thoroughly investigated (13–15).
The role of EPAC in virus infection was first reported for Middle East respiratory syndrome coronavirus (MERS-CoV). Tao et al. demonstrated that EPAC1 protein regulates the replication of MERS-CoV in a cell type-independent manner, but the molecular mechanisms remained unidentified (15). The importance of EPAC in other viral infections has not been explored. Other than those on replication, there have been no studies investigating the role of EPAC in host responses to viral infections.
In this study, we demonstrated that an EPAC-specific inhibitor (ESI-09) inhibits RSV replication and RSV-induced inflammatory responses in human upper and lower airway epithelial cells, while a PKA inhibitor (H89) does not have such an effect. By treating cells with EPAC1/2-specific short interfering RNA (siRNA), we found that EPAC2, but not EPAC1, plays a dominant role in promoting viral replication and inflammatory responses. By using an EPAC2-specific inhibitor, ESI-05, and EPAC2-deficient cells, we also confirmed roles of EPAC2 in RSV infection. In summary, this is the very first demonstration of the regulatory functions of EPAC2 in RSV infection. EPAC2 could serve as a novel therapeutic target to control both RSV replication and associated host inflammatory responses.
RESULTS
The effect of EPAC inhibitor ESI-09 on RSV replication.
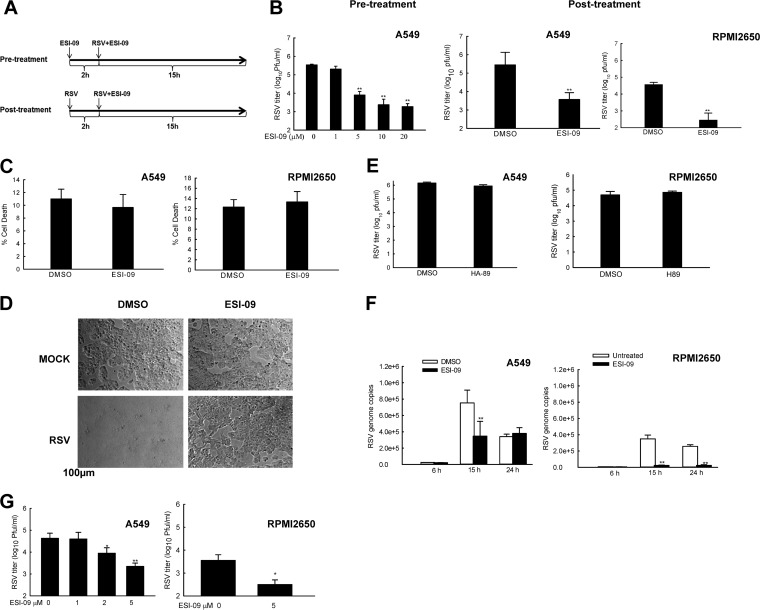
Many studies suggest EPACs are implicated in multiple pathologies, including cancer, chronic pain, and diabetes (16–18). However, the importance and regulatory mechanisms of EPAC in viral infection remain largely unknown, except for a recent report demonstrating its function in controlling MERS-CoV replication, without the mechanism(s) being revealed (15). Both MERS-CoV and RSV are respiratory RNA viruses: MERS-CoV is a positive single-stranded virus, while RSV is a negative single-stranded virus (19, 20). To investigate whether EPAC also plays a general role in RSV replication and/or RSV-induced host responses, A549 cells, from a well-established lower respiratory tract cell model for RSV infection (21), were treated with ESI-09, an EPAC-specific inhibitor suppressing the activity of EPAC1 and EPAC2 with respective apparent 50% inhibitory concentration (IC50) values of 10.8 μM and 4.4 μM. These IC50 values are well below the concentrations shown to induce protein denaturation (22, 23). Cell culture media with the same amount of dimethyl sulfoxide (DMSO) was used as a vehicle control. The overall experimental flow on the infection and treatment with ESI-09 is illustrated in Fig. 1A. Under the ESI-09 pretreatment condition, cells were exposed to ESI-09 for 2 h, followed by RSV infection at a multiplicity of infection (MOI) of 1 for 15 h. The production of infectious progeny virus was measured by immunostaining using RSV-specific antibody, as we previously described (24, 25). We found that pretreatment with ESI-09 significantly decreased virus yields in a dose-dependent manner compared to DMSO treatment during RSV infection (Fig. 1B, left). Since treating cells with ESI-09 at 5 μM already resulted in more than a log decrease in infectious particles than control cells, 5 μM was chosen as a minimal treatment dose throughout our studies. To make the treatment more therapeutically relevant, A549 cells were treated with ESI-09 after 2 h of RSV infection. Posttreatment with 5 μM ESI-09 also led to remarkable reduction of RSV titer (Fig. 1B, middle). RSV infects and replicates in not only lower airway epithelial cells but also upper airway epithelial cells (26). We therefore also examined the effect of ESI-09 on RSV replication in cells from the upper respiratory tract. RPMI 2650 is a human nasal epithelial cell line that has been widely used as an upper respiratory tract cell model for RSV infection (27, 28). Consistent with the inhibitory effect of ESI-09 on RSV infection in A549, RPMI 2650 cells with ESI-09 posttreatment had considerably decreased replication compared to that of control cells (Fig. 1B, right). To exclude the possibility that ESI-09 induces cell toxicity, leading to a less favored environment for RSV replication, we compared the lactate dehydrogenase (LDH) release from cells treated with ESI-09 or its control. We found that ESI-09 did not affect cell viability (Fig. 1C). The nontoxicity of ESI-09 on cells was also supported by its inhibitory effect on cytopathic effect (CPE) formation in RPMI 2650 cells. Unlike A549 cells, RPMI 2650 cells had apparent and significant cell-cell fusion at 15 h postinfection (p.i.). (Fig. 1D, lower left). Many fewer syncytia were formed in response to the posttreatment with ESI-09 compared to those of infected cells without ESI-09 treatment (Fig. 1D, lower right). PKA and EPAC are known as two main downstream effectors of cAMP signaling (29). To explore whether cAMP/PKA signaling is also involved in the control of RSV production, cells were treated with a PKA-specific inhibitor (H89). We found that treatment with 10 μM H89 did not affect RSV production in either A549 or RPMI 2650 cells (Fig. 1E), suggesting that anti-RSV effect is controlled by the EPAC-mediated pathway. Furthermore, the anti-RSV effect of ESI-09 was confirmed by examining viral genome copies using quantitative reverse transcription-PCR (qRT-PCR). As shown in Fig. 1F, in response to RSV infection, ESI-09 treatment led to fewer viral genome copies at 15 h p.i. (left, column 4 versus column 3). Infected control cells also had a significant drop in genome copies at 24 h p.i., compared with control cells at 15 h p.i. (left panel, column 3 versus column 5). This drop was possibly due to RSV-induced pathogenic environment, which was unfavorable for RSV replication, as many cells detached and became unhealthy at 24 h p.i. We also found that the number of genome copies in ESI-09-treated RPMI 2650 cells were significantly lower than that in control cells, both at 15 and 24 h p.i. (right). The inhibitory effect of ESI-09 on genome replication in RPMI 2650 cells was more potent than that in A549 cells (right versus left), although RSV progeny suppression by ESI-09 was similar between A549 and RPMI 2650 cells (Fig. 1B). The mechanisms underlying the loose correlation between genome copies and RSV progeny are not currently known. It is possible that significant impairment in viral progeny production can be achieved by genome copy deficiency at certain thresholds, but deficiency exceeding the threshold results in no or minimal suppression of viral replication and increased damage in the generation of infectious particles. In this study, we also investigated whether ESI-09 has long-lasting efficacy. We used RSV at a much lower MOI to infect A549 cells in order to avoid significant pathogenic effects at late time points postinfection. As shown in Fig. 1G, ESI-09 treatment resulted in a log10 reduction in infectious particles at 48 h p.i., suggesting the long-lasting efficacy of ESI-09. In summary, these results demonstrate that ESI-09 has an anti-RSV effect in both lower and upper airway epithelial cells.
FIG 1.
ESI-09 inhibits RSV replication. (A) The experimental flow of ESI-09 treatment. (B) The effect of ESI-09 on RSV replication. A549 cells in a 6-well plate were pre- or posttreated with ESI-09 at the indicated concentrations and infected with RSV at an MOI of 1. At 15 h p.i., total viruses were harvested and titers determined (shown at the left and middle, respectively). DMSO was used as a vehicle control for ESI-09. The effect of ESI-09 on RSV replication was also done for RPMI 2650 cells (right, posttreatment only). Data shown are representative of three independent experiments. **, P < 0.01 relative to the DMSO-treated group. (C) The cytotoxicity of ESI-09. A549 or RPMI 2650 cells in triplicate were treated with 5 μM ESI-09 for 24 h and harvested for the lactate dehydrogenase assay to measure the cytotoxicity of ESI-09. DMSO was used as a vehicle control. (D) The impact of ESI-09 on syncytium formation. RPMI 2650 cells were mock infected or infected with RSV, followed by ESI-09 treatment. At 15 h posttreatment, cells were observed using a phase-contrast microscope. (E) The influence of a PKA inhibitor, H89, on RSV replication. A549 or RPMI 2650 cells were infected with RSV, followed by treatment with 10 μM H89. At 15 h posttreatment, viruses were harvested for titration. Data shown are representative of three independent experiments. (F) The role of ESI-09 in regulating viral genome. A549 or RPMI 2650 cells were infected with RSV at an MOI of 1, followed by treatment with 5 μM ESI-09. At 6, 15, or 24 h posttreatment, total RNA was extracted and subjected to qRT-PCR to measure RSV genome copies. (G) Efficacy lasting of ESI-09. A549 or RPMI 2650 cells were mock infected or infected with RSV at an MOI of 0.01. At 48 h p.i., total viruses were harvested for titration. Single and double asterisks represent P values of <0.05 and <0.01, respectively. Data shown are representative of three independent experiments. Data are means ± SE. **, P < 0.01 relative to the DMSO-treated group.
The impact of ESI-09 on RSV-induced proinflammatory response.
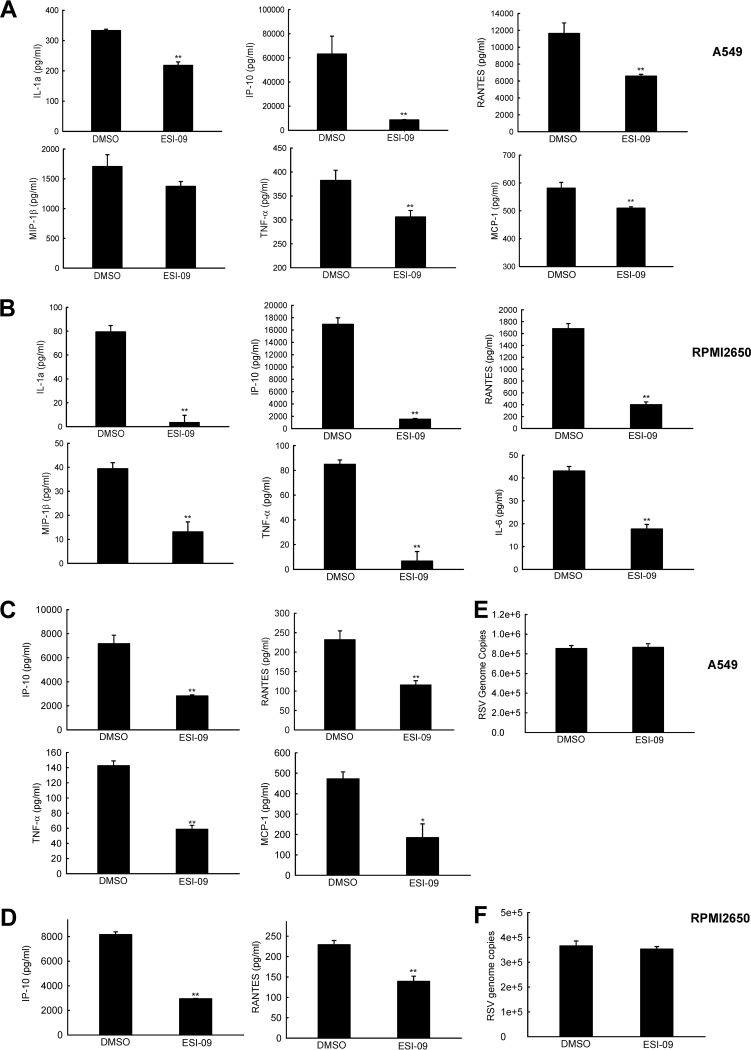
By comparing the cytokines/chemokines from the samples described in Fig. 1, we found that RSV-induced interleukin-1α (IL-1α), IP-10, RANTES, MIP-1β, and tumor necrosis factor alpha (TNF-α) were significantly decreased in both A549 and nasal RPMI 2650 cells by ESI-09 (Fig. 2A and B). ESI-09 especially suppressed induction of MCP-1 and IL-6 in A549 and RPMI 2650 cells, respectively. These results suggested that, in addition to the effect of EPAC on RSV replication, EPAC regulated proinflammatory responses to RSV infection as well.
FIG 2.
ESI-09 inhibits RSV-induced cytokines and chemokines in A549 and RPMI 2650 cells. (A and B) A549 (A) or RPMI 2650 (B) cells were infected with RSV at an MOI of 1. At 2 h p.i., the whole medium replaced with fresh medium containing 5 μM ESI-09. DMSO was used as a vehicle control. At 15 h posttreatment, supernatant was collected and the level of cytokines/chemokines measured by Bio-Plex. (C and D) A549 (C) or RPMI 2650 (D) cells were infected and treated with ESI-09 as described for panels A and B, except we used a higher dose of infection (MOI of 5) and harvested supernatants for Bio-Plex as early as 4 h p.i. to investigate the effect of ESI-09 on inflammatory represses at the early infection window. (E and F) RSV genomic copies were also measured by RT-PCR for samples from panels C and D. Net induction data shown in panels A to D and genome copies shown in panels E and F are representative of three independent experiments and means ± SE. **, P < 0.01 relative to the DMSO-treated group.
In response to viral infection, the induction of some proinflammatory mediators, such as RANTES, is heavily dependent on virus replication (30, 31). Therefore, it is possible that decreased induction of cytokines/chemokines by ESI-09 is an indirect consequence of ESI-09-suppressed RSV replication. However, we cannot exclude the possibility that ESI-09 is able to affect inflammatory response directly. To investigate that, A549 or RPMI 2650 cells were infected with RSV at a much higher dose (MOI of 5), so that detectable cytokines/chemokines can be induced at early time points p.i., when genome copies are comparable in ESI-09-untreated and -treated cells, possibly due to significant virus genome replication not having started. As shown in Fig. 2C and D, some cytokines/chemokines became detectable in A549 and RPMI 2650 cells at 4 h p.i., and the induced mediators were significantly inhibited by ESI-09. In the meantime, no significant difference in RSV genome copies was observed in response to ESI-09 treatment (Fig. 2E and F), demonstrating EPAC could directly regulate proinflammatory responses to RSV.
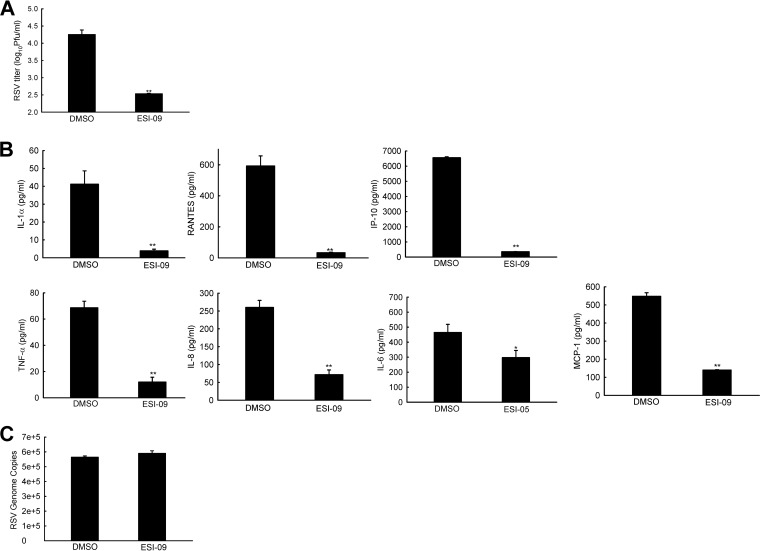
We also validated the inhibitory effect of ESI-09 on RSV replication and cellular responses in primary small alveolar epithelial (SAE) cells. As shown in Fig. 3A, ESI-09 treatment led to significantly less infectious particles in infected SAE cells (MOI of 1 for 15 h). We also confirmed that ESI-09 could suppress RSV-induced cytokines/chemokines directly (Fig. 3B and C), similar to the results demonstrated in Fig. 2C and D for A549 and RPMI 2650 cells. Overall, these results suggest that ESI-09 regulates not only RSV replication but also RSV-induced host proinflammatory responses, and its effect on some cytokines/chemokines is cell type dependent.
FIG 3.
ESI-09 inhibits RSV replication and inflammatory responses in SAE cells. (A) SAE cells were infected with RSV and treated with ESI-09 as described for Fig. 2A and B, followed by virus titration. (B) The direct effect of ESI-09 on RSV-induced cytokines/chemokines was also assessed as described for Fig. 2C and D. (C) RSV genome copies were compared between ESI-09-treated and -untreated SAE samples from panel B. The titration data shown in panel A, net induction data shown in panel B, and genome copies shown in panel C are representative of three independent experiments and means ± SE. P < 0.05 (*) and P < 0.01 (**) relative to the DMSO-treated group.
Modulation of RSV-activated IRF and NF-κB by ESI-09.
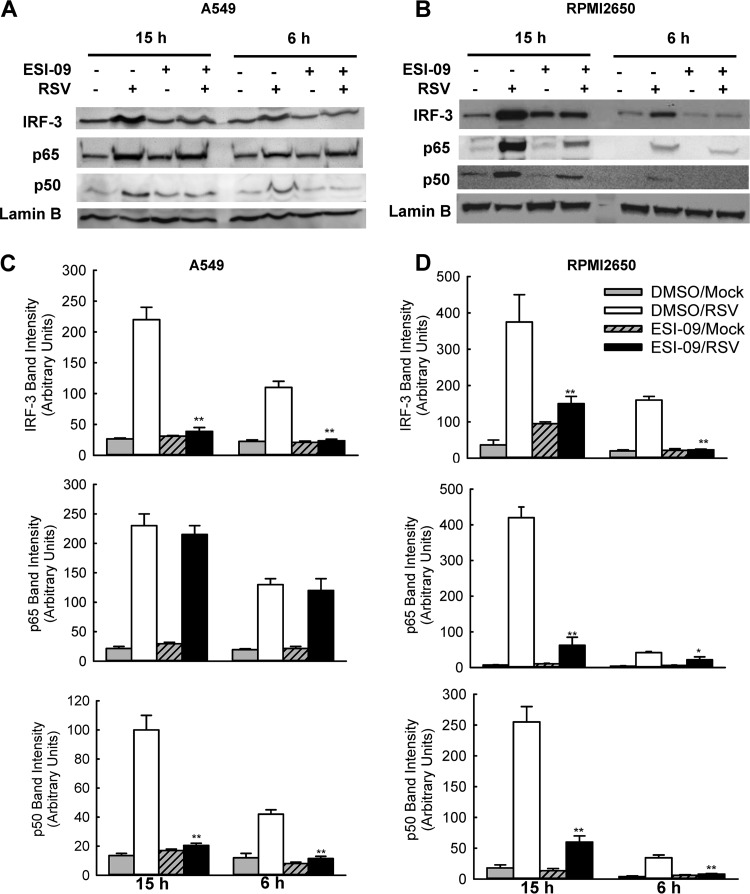
Given the significance of respiratory tract inflammation in RSV pathogenesis (32), it is important to study the molecular mechanism(s) by which ESI-09 regulates the induction of cytokines/chemokines by RSV. Transcription factors belonging to the interferon regulatory factor (IRF) and NF-κB families play an essential role in virus-induced expression of genes involved in the immune/inflammatory response, including chemokines, such as RANTES and IP-10, and cytokines, such as TNF-α and MCP-1 (reviewed in reference 33). Among the IRF family members, IRF-1, IRF-3, IRF-5, and IRF-7 are direct transducers of virus-induced signaling, with IRF-3 being necessary for RANTES gene expression in response to pneumovirus infections (34, 35). NF-κB is a superfamily of ubiquitous transcription factors composed of NF-κB1 or p50, NF-κB2 or p52, RelA or p65, and RelB and c-Rel proteins. A number of pneumovirus-inducible inflammatory genes require NF-κB for their transcription (36, 37). In response to viral infection, the nuclear translocation of IRFs and NF-κB proteins is a key step to the transcription of target genes, including those critical for inflammation.
To assess whether ESI-09 inhibits proinflammatory cytokines/chemokines via blocking IRF- and NF-κB-dependent pathways, A549 cells or nasal RPMI 2650 cells were infected with RSV at an MOI of 1, followed by treatment with ESI-09. Mock-infected and/or untreated cells were used as controls. As shown in Fig. 4, in ESI-09-free cells, RSV infection led to enhanced nuclear translocation of IRF-3, p65, and p50 at 6 and 15 h p.i. In both A549 and RPMI 2650 cells, RSV-induced nuclear translocation of IRF-3 and p50 was blocked by ESI-09. However, the inhibitory effect of ESI-09 on RSV-activated p65 in A549 was not as significant as its effect on p65 in RPMI 2650 cells, suggesting mechanisms underlying the distinct impact of ESI-09 on RSV-induced cytokines/chemokines profile of A549 and RPMI 2650 cells. It is also worth pointing out that the inhibitory effect of ESI-09 was observed as early as 6 h p.i., when RSV genome copies were comparable between untreated and ESI-09-treated cells (Fig. 1F). This observation, together with the selective inhibitory effect of ESI-09 on RSV-activated IRF-3 and p50 in A549 cells, further supported that the effects of ESI-09 on innate cytokine/chemokine responses can be independent from viral replication.
FIG 4.
ESI-09 inhibits RSV-induced NF-κB and IRF-3 activation. RSV-induced nuclear translocation of NF-κB and IRF-3 in response to ESI-09 treatment. A549 (A) or RPMI 2650 (B) cells were infected with RSV and treated with ESI-09 as described for Fig. 2A and B. At 6 or 15 h posttreatment, nuclear extracts of cells were prepared and subjected to Western blotting using an anti-IRF-3, anti-p65, or anti-p50 antibody. Lamin B was used as a control for equal loading of the samples. (C and D) The normalized band intensity from three experiments were summarized and shown for samples from panels A and B, respectively. In brief, the histogram function of Adobe Photoshop was used for intensity quantification. The mean NF-κB and IRF band intensity was normalized by the corresponding mean intensity of lamin B and expressed as mean ± SE. P < 0.05 (*) and P < 0.01 (**) relative to the DMSO-treated group.
Roles of EPAC2 in RSV production, syncytium formation, and virus-induced host responses.
EPAC has two isoforms, EPAC1 and EPAC2, both of which are present in the lung. While EPAC1 is restrictedly located in the perinuclear region, EPAC2 is distributed cytoplasmically (38). EPAC2 has three subfamily members, EPAC2A, EPAC2B, and EPAC2C, with EPAC2B and EPAC2C being expressed specifically in adrenal gland and liver, respectively (39, 40). Therefore, the EPAC2 isoform in the lung is EPAC2A. EPAC1 and EPAC2A share extensive sequence and domain homology, except that EPAC2A contains an extra cyclic nucleotide-binding domain (CNBD) (41). The functions of EPAC1 and EPAC2 are also distinguished from each other. For example, EPAC2 has not been implicated in cancer, while EPAC1's role in cancer and the potential of EPAC1 as a target for cancer therapeutics has been extensively demonstrated (42). Therefore, it is possible that EPAC1 and EPAC2 also have distinct roles in controlling virus infection and/or inflammation. For the purpose of precise drug design and therapy development against RSV pathogenesis and virus replication, it is important to determine which EPAC isoform(s) contribute to antiviral responses and inflammation.
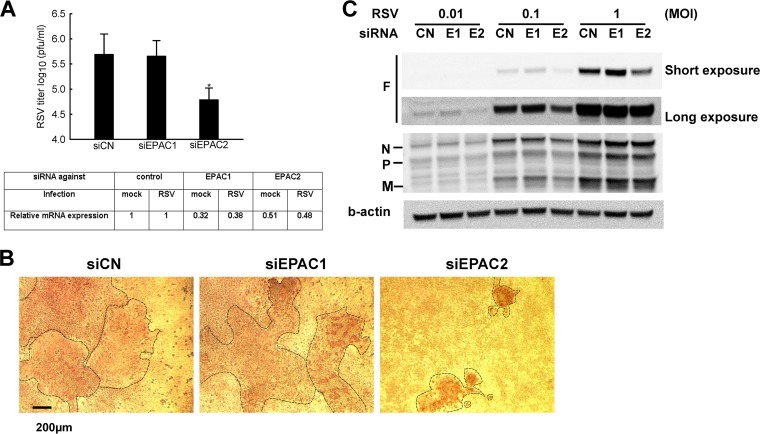
To investigate which EPAC isoform(s) are responsible for the regulation, we used knockdown (KD) experiments using EPAC1- and EPAC2-specific siRNAs in A549 cells, followed by RSV infection at an MOI of 1 for 15 h. Scrambled siRNA was used as a negative control. As shown in Fig. 5A, scrambled or EPAC1-specific siRNA-treated cells had comparable viral production, while the EPAC2 siRNA-treated cells produced significantly fewer infectious particles, close to 0.8 log less than those from control cells. In addition, progeny viruses had impaired function in CPE formation (Fig. 5B). Viruses harvested from control or EPAC1 KD cells, once inoculated to the HEp-2 cells, developed syncytia of similar sizes, while the syncytia developed by progeny viruses from EPAC2 KD were significantly smaller, suggesting that EPAC2 is responsible for the impaired syncytium formation. Since RSV F proteins are responsible for syncytium formation, we compared F protein expression in cells with different siRNA treatments. As shown in Fig. 5C, F protein expression was much decreased in EPAC2 KD cells, while there were no significant changes in N, P, and M proteins by EPAC2 KD.
FIG 5.
EPAC2 affects CPE formation. (A) The role of EPAC isoforms in RSV replication. A549 cells were transfected with 100 nM siRNA against EPAC1 (siEPAC1) or EPAC2 (siEPAC2). The scrambled siRNA was used as a control (siCN). At 48 h posttransfection, cells were mock infected or infected with RSV at an MOI of 1. At 2 h postinfection, the supernatant was washed with PBS and replaced with fresh medium. At 15 h p.i., cells from a 6-well plate were harvested for RSV titration or total RNA was extracted for qRT-PCR to measure EPAC1 or EPAC2 mRNA expression. Data shown are representative of three independent experiments and means ± SE. *, P < 0.05 relative to the siCN. (B) The effect of EPAC isoforms on syncytium formation. HEp-2 cells were used to titrate total viruses harvested and shown in panel A. At day 2 postinfection, HEp-2 cells were fixed, followed by immunostaining using polyclonal biotin-conjugated goat anti-RSV antibody and then streptavidin peroxidase polymer. The plaques were observed using a phase-contrast microscope. (C) A549 cells were transfected with siRNAs as described for panel A and infected with RSV at the indicated MOI. At 15 h p.i., cells were harvested to prepare total cell lysates, followed by Western blotting to check the expression of viral proteins using the indicated antibody. β-Actin was used as a control for equal loading of the samples. Data shown are representative of three independent experiments.
Regarding smaller plaque formation by RSV progeny from EPAC2 KD cells (Fig. 5B), it is possible that selective pressure on EPAC2 KD induced mutations in F protein and, subsequently, plaque malformation. However, this is not the case, as our sequencing data did not support this possibility. In addition, whole-genome sequencing suggested there were no mutations in other regions of RSV. Since F protein, not other viral proteins, was suppressed by EPAC2 KD, it is possible that the viral progeny had less assembled F proteins on their surfaces, resulting in impaired viral entry and subsequently smaller plaques. It is also possible that EPAC2 KD actually impaired fusion function of F protein in addition to its expression. Overall, why the viral progeny from EPAC2 KD cells produces smaller plaques is still unclear.
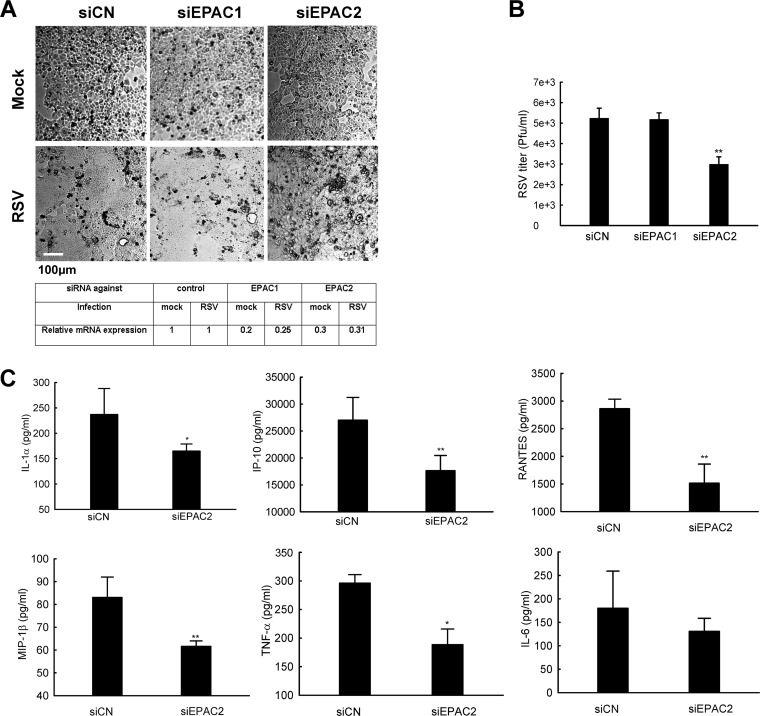
siRNAs were also used to knock down EPAC1/2 in nasal cells to investigate which isoform(s) is important for RSV replication and the host response. As shown in Fig. 6A, most control and EPAC1 KD RPMI 2650 cells fused together after infection. However, the configuration of EPAC2 KD cells was kept intact after infection. In addition, significantly fewer infectious particles were found in EPAC2 KD RPMI 2650 cells (Fig. 6B), which is consistent with KD results for A549 cells. RSV-induced secretion of cytokines/chemokines was also suppressed in RPMI 2650 cells with EPAC2 KD (Fig. 6C). Such an inhibition was not observed in EPAC1 KD cells (data not shown).
FIG 6.
EPAC2 affects RSV-induced fusion in RPMI 2650 cells. (A) The impact of EPAC isoforms on RSV syncytium formation. RPMI 2650 cells were treated with siRNAs and infected with viruses as described for Fig. 4. The impact of EPAC isoforms on syncytium formation was investigated through a phase-contrast microscope. Total RNA extracts were also prepared for qRT-PCR to confirm gene suppression by siRNAs as indicated. (B) The role of EPAC isoforms in RSV replication. Total viruses, harvested from RPMI 2650 cells (12-well plate), were titrated in HEp-2 cells using immunostaining. Data shown are representative of three independent experiments and means ± SE. *, P < 0.05 relative to the siCN. (C) The regulatory function of EPAC isoform in host innate responses to RSV. Supernatants were harvested from the cells shown in panel A, and the net induction of cytokines/chemokines by RSV infection was measured by Bio-Plex. Data are representative of three independent experiments and are expressed as mean ± SE normalized luciferase activity. P < 0.05 (*) and P < 0.01 (**) relative to the siCN-treated and RSV-infected group.
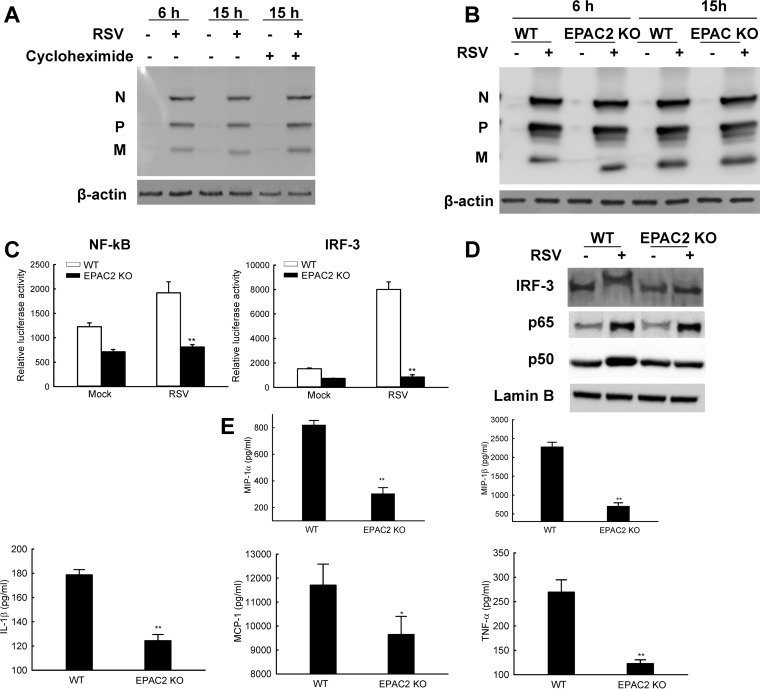
We also used mouse embryonic fibroblasts (MEFs) lacking the EPAC2 gene to confirm the role of EAPC2 in RSV infection. The EPAC2 mice were generously provided by Ju Chen from the University of California–San Diego. As shown in Fig. 7A, the MEFs are susceptible to RSV infection. However, the viral replication possibly was not significant in MEFs, as there were no virus proteins accumulated during the infection (lane 2 versus lane 4). Since the comparable expression does not completely exclude the possibility of de novo viral protein synthesis, we used cycloheximide to inhibit protein synthesis and investigated whether untreated cells have more viral proteins than treated ones. If they do, new viral proteins are synthesized. In brief, samples from a 15-h infection were left untreated or treated with 20 μM cycloheximide. Infected cells, regardless of the treatment, had similar viral protein expression (lane 4 versus lane 6), supporting that new viral proteins were barely synthesized in MEFs, at least in the course of a 15-h infection. We also found that the abundance of viral proteins in wild-type (WT) and EPAC2 knockout (KO) cells were comparable at both 6 and 15 h p.i. (Fig. 7B), suggesting that regulated cellular responses by EPAC2, if there are any in MEFs, are unlikely to be associated with virus replication. To investigate whether EPAC2 is critical for RSV-induced host responses, luciferase reporter plasmids harboring the binding sites of transcriptional factors NF-κB or IRF-3 were transfected into the MEFs. As shown in Fig. 7C, RSV infection enhanced luciferase activities controlled by NF-κB and IRF-3 binding sites in WT MEFs compared to that of mock infection. However, the induced activities were not that significant in EPAC2 KO MEFs. We also compared the nuclear translocation of NF-κB and IRF-3 in WT and EPAC2 KO cells to investigate the impact of EPAC2 on the innate response. Usually, IRF-3 is constitutively expressed in an unstimulated form without infection (43). Following viral infections, IRF-3 undergoes a shift in molecular weight for its transcription activities due to the virus-induced C-terminal phosphorylation (44). As shown in Fig. 7D, the activation of IRF-3 was significantly induced by RSV in WT cells. The nuclear abundance of P65 and P50 was also significantly enhanced by RSV in WT MEFs (second lane versus first lane). However, IRF-3 and p50 activation was impaired in EPAC2 KO cells, while p65 nuclear translocation was not affected (lane 4 versus lane 2). Given the fact that the replication is not so permissive in MEFs, at least before 15 h p.i., the effect of EPAC2 on the activation of transcription factors confirmed a direct role of EPAC2 in innate response to RSV infection. We next examined whether reduced activity of these transcription factors affected RSV-induced cytokines/chemokines. The result showed that, upon RSV infection, the EPAC2 KO MEFs produced less IL-1β, MIP-1α, MIP-1β, MCP-1, and TNF-α than WT MEFs (Fig. 7E). Collectively, these data demonstrate that EPAC2 can regulate RSV-induced host innate responses directly and is a key host component in controlling virus replication and inflammatory pathogenesis.
FIG 7.
EPAC2 directly regulates RSV-induced host immunity. (A) Limited RSV protein accumulation along the course of infection. WT MEF cells were mock infected or infected with RSV at MOI of 1. At 2 h p.i., 20 μM cycloheximide was added as indicated. Untreated cells were used as controls. At 6 or 15 h p.i., total cell lysates were prepared and subjected to Western blotting using an anti-RSV antibody. β-Actin was used as a control for the efficacy of cycloheximide treatment. (B) RSV replication in wild-type (WT) or EPAC2 knockout (KO) MEFs. MEFs, with or without EPAC2, were mock infected or infected with RSV at an MOI of 1. At 6 or 15 h p.i., total cell lysates were harvested and subjected to Western blotting using an anti-RSV antibody. β-Actin was used as a control for equal loading of the samples. Data shown are representative of three independent experiments. (C) The impact of EPAC2 on the activation of NF-κB and IRF-3. WT or EPAC2 KO cells in triplicate were transfected with a luciferase reporter plasmid containing the human NF-κB (left) or IRF-3 (right) promoter. At 30 h posttransfection, cells were mock infected or infected with RSV for 15 h and harvested to measure luciferase activities. **, P < 0.01 relative to the RSV-infected WT MEFs. (D) EPAC2-controlled nuclear translation of NF-κB and IRF-3. WT or EPAC2 KO cells were mock infected or infected with RSV. At 15 h p.i., nuclear extracts of cells were prepared and subjected to Western blotting using an anti-IRF-3, anti-p65, or anti-p50 antibody. Lamin B was used as a control for equal loading of the samples. Data shown are representative of two independent experiments. (E) EPAC2-regulated cytokine/chemokine induction. WT and EPAC2 KO cells were mock infected or infected with RSV for 15 h, and supernatant was collected to measure cytokines/chemokines. Net induction data shown are representative of three independent experiments and means ± SE. **, P < 0.01 relative to the WT.
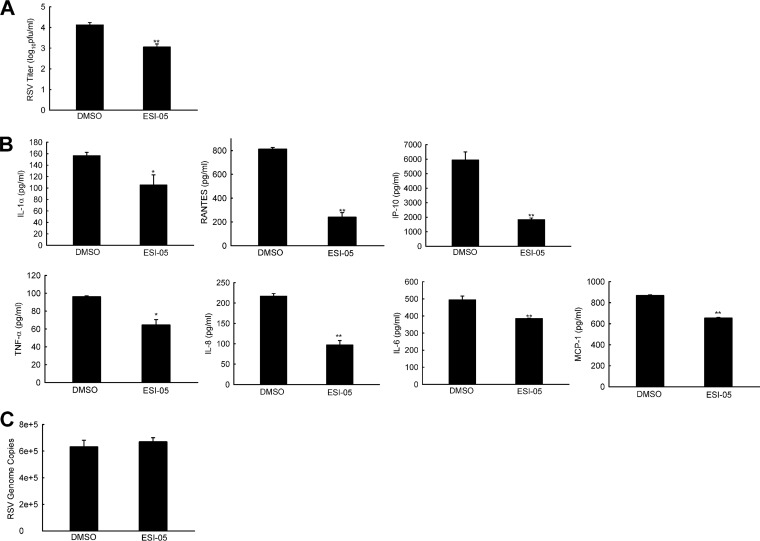
Recently, a small-molecule ESI-05 has been characterized as a specific and potent EPAC2 antagonist (45). It inhibits cAMP-mediated EPAC2 guanine exchange factor (GEF) activity with an IC50 of 0.5 μM but shows no inhibition of EPAC1 activity at 25 μM. When 10 μM ESI-05 was added to RSV-infected SAE cells (MOI of 1), there was a significant reduction in infectious particles at 15 h p.i., demonstrating an inhibitory role of ESI-05 in RSV replication (Fig. 8A). Such inhibition was also observed in A549 cells and nasal RPMI 2650 cells (data not shown). We also used a higher MOI of RSV (MOI of 5) to investigate the role of ESI-05 in RSV-induced inflammation at early time points p.i., similar to what was demonstrated for ESI-09 in Fig. 3. As shown in Fig. 8B, the induction of inflammatory mediators was significant after SAE cells were infected with RSV for 4 h. ESI-05 treatment led to less induction of chemokines/cytokines. Since RSV genome copies were comparable in cells treated with/without ESI-05 (Fig. 8C), the data supported the importance of EPAC2 in controlling RSV-induced inflammatory responses. In summary, our data demonstrated that EPAC2 is of importance to regulate both RSV replication and associated inflammation. Therefore, it could be a promising therapeutic target to control pulmonary RSV load and RSV-induced pathogenesis. The EPAC2-specific siRNA and inhibitors also have a potential therapeutic application for RSV treatment.
FIG 8.
Role of ESI-05 in RSV replication and RSV-induced innate immunity. (A) Viral replication regulation by ESI-05. SAE cells were mock infected or infected with RSV at an MOI of 1. At 2 h p.i., the cells were washed with PBS, followed by ESI-05 treatment. At 15 h p.i., the total viruses were harvested and titrated. DMSO was used as a vehicle control for ESI-05. (B) The effect of ESI-05 on RSV-induced host innate immunity. SAE cells were infected with RSV at an MOI of 5, followed by ESI-5 treatment at 2 h p.i. At 4 h p.i., the supernatants were harvested, followed by Bio-Plex analysis to investigate the impact of ESI-05 on the induction of cytokines/chemokines by RSV. (C) The samples, after the removal of supernatant, were harvested for total RNA preparation, followed by RT-PCR to quantify the RSV genome. The net induction of proinflammatory mediators and viral genome data are representative of three independent experiments. P < 0.05 (*) and P < 0.01 (**) relative to the DMSO-treated RSV-infected group.
DISCUSSION
RSV is a leading cause of severe lower respiratory tract infections in children as well as in other populations. Overall, there is no vaccine or effective treatment besides supportive measures. Although palivizumab is available for preventing RSV-associated hospitalizations, it is not very cost-effective and is mainly limited to high-risk infants for the first RSV season (7). Here, we identified a new potential role of EPAC molecules in airway epithelial cells in response to RSV infection. Our results suggested EPAC2, but not EPAC1, is critical for controlling RSV replication and virus-induced host responses. Therefore, EPAC2 can serve as an important potential target in the development of new therapies.
The primary infected site for RSV is airway epithelial cells, which produce many antiviral and inflammatory mediators in response to infection (31). It is well known that RSV infection enhances the production of several cytokines/chemokines, including IP-10, RANTES, and MIP-1β, in airway epithelial cells (16, 46, 47). The expression of these cytokines/chemokines promotes activation and recruitment of immune cells, resulting in increased secretion of inflammatory cytokines/chemokines (7, 10). Several reports have shown that excessive host responses induced by inflammatory mediators may be linked to hyperresponsiveness and airway damage during virus clearance (20, 44). In addition, chemokine inhibition by RANTES antibodies showed a profound reduction in airway hyperreactivity in mice, resulting in a decrease in lung pathology and damage (12). Thus, properly regulating RSV-induced airway epithelial host responses can be a key step for intervention strategies. In this study, we found that EPAC2-deficient cells produced fewer inflammatory mediators in response to RSV infection, supporting that EPAC2 can be a target to regulate the inflammation response. Although the induction of many proinflammatory mediators is virus replication dependent, we found that EPAC2 can also control cytokine/chemokine induction independently from its control of viral replication. As shown in Fig. 7 and 8, the induction of proinflammatory mediators was significantly suppressed in EPAC2-deficient cells or cells treated with ESI-05 when genome copies were comparable between control and EPAC2-deficient/impaired cells at a specific assay window. On the other hand, since the activation of NF-κB and IRF-3 is a necessary step for the induction of many antiviral or proinflammatory mediators (36, 48), the suppressed nuclear translocation/activation of NF-κB and IRF-3 could be a cause for reduced induction of proinflammatory mediators (Fig. 7C and D). In summary, this is the first report demonstrating the signaling from EPAC to nuclear transcriptional factors NF-κB and IRF-3 in the context of viral infection, revealing a new pathway contributing to RSV-induced inflammation.
Pulmonary RSV load also plays a significant role in controlling virus-induced pathogenesis (49). Therefore, we studied the effect of EPAC on viral replication in this study as well. Our results showed the suppression of RSV replication by EPAC2 gene silencing/deletion. We believe that EPAC2-regulated F protein expression and viral genome replication contribute to the RSV replication suppression by EPAC2 KD or EPAC2-specific inhibitor (Fig. 1F and 5). Overall, given that viral replication and inflammatory cytokines/chemokines are key risk factors for RSV disease severity (50–54), the inhibitory effect of EPAC2-specific inhibitor ESI-05, EPAC2-specific siRNA, or EPAC2 deletion on the production of infectious progeny viruses and cytokine/chemokine induction supported EPAC2 as a promising therapeutic target to control RSV replication and pathogenesis.
cAMP is a second messenger which plays an essential role in many biological processes. Recently, the development of inhibitors specific to cAMP and its effector molecules has enabled researchers to study the importance of this signaling under various cellular conditions, including viral infection. Recent research shows that the effect of cAMP/PKA and cAMP/EPAC signaling on viral replication is cell type dependent. For example, the inhibition of human immunodeficiency virus type 1 replication in macrophage and T cells was mediated by cAMP/PKA and EPAC/Rap1 signaling, respectively (5, 39). The contribution of EPAC isoform to viral replication seems virus dependent. For example, replication of Middle East respiratory syndrome coronavirus (MERS-CoV) is controlled by EPAC1 (48), while our data demonstrate that EPAC2 plays a significant role in RSV infection. Taken together, these results indicate that cAMP, PKA, and EPAC are important molecules in medicating viral infection. The reason why cells or viruses use a distinct but related molecule(s) for the regulation needs to be explored in a future study.
The EPAC protein family is composed of EPAC1 and EPAC2, which are encoded by two independent genes in mammals. They share some similar domains but also have distinct ones. All EPACs have a C-terminal catalytic region containing a CDC25 homology domain (CDC25-HD), a Ras association (RA) domain, a Ras exchange motif (REM), and at least one cyclic nucleotide-binding domain (cNBD-B). EPAC2 has three subtypes: EPAC2A, EPAC2B, and EPAC2C. As discussed, EPAC2B and EPAC2C express specifically in adrenal gland and liver, respectively. Compared to other EPACs, EPAC2C is the only isoform which lacks a Disheveled/Egl-10/pleckstrin (DEP) domain, and EPAC2A is the only isoform with two additional domains, cNBD-B and RA. Several investigators have proposed that these two additional domains of EPAC2 are responsible for its subcellular localization and biological functions (15, 32). Whether EPAC2-specific domains are responsible for control of RSV replication and virus-induced host responses will be investigated in our future research.
In conclusion, we have shown that modulation of cellular EPAC2 protein significantly impacts cellular responses and viral replication in two airway epithelial models of RSV infection, suggesting EPAC2 is a promising therapeutic target for RSV infection. Given the importance of EPAC in cancer, diabetes, heart failure, inflammation, and neurological disorders, EPAC-specific modulators are being urgently investigated to explore their physiological functions, mechanisms of regulation, and therapeutic applications (55, 56). We are currently investigating the antiviral spectrum of other EPAC inhibitors.
MATERIALS AND METHODS
Cell lines, virus, and antibodies.
HEp-2 (human epithelial type 2), A549 (human alveolar type II-like epithelial), and RPMI 2650 (human nasal septum epithelial) cells all were from the ATCC, Manassas, VA, and maintained as previously described (24, 46, 57). SAE (small airway epithelial) cells, isolated from the normal human lung distal portion, were purchased from Lonza (distributed via Fisher Scientific, Pittsburgh, PA). RSV long strain was propagated in HEp-2 cells at 37°C and purified by sucrose gradient as described previously (24, 25, 46). Viral titer was determined by immunostaining in HEp-2 cells using polyclonal biotin-conjugated goat anti-RSV antibody (7950-0104; Bio-Rad, Hercules, CA) and streptavidin peroxidase polymer (S2438; Sigma-Aldrich, St. Louis, MO) sequentially, as previously described (24, 25). Monoclonal antibody against β-actin was from Sigma (A1978). Primary antibodies against lamin B (sc-374015), p50 (sc-8414), IRF-3 (sc-9082), and goat anti-mouse IgG-HRP (sc-2031) were purchased from Santa Cruz Biotechnology (Santa Cruz Biotechnology, Santa Cruz, CA). Primary antibody for p65 detection was from Cell Signaling Technology (4764; Cell Signaling Technology, Denvers, MA). Horseradish-coupled secondary antibodies were purchased from Santa Cruz as well (sc-2030).
Cytokine and chemokine quantification.
To investigate the role of EPAC in regulating host innate immune responses, RSV-induced chemokines and cytokines were quantified by using a multianalytic human (M50-0KCAF0Y) or mouse (M60-009RDPD) cytokine/chemokine profiling kit from Bio-Rad (Hercules, CA) according to the manufacturer's instructions. Data were analyzed using the multiplex analysis software from Bio-Rad.
qRT-PCR.
Total cellular RNA was extracted using TRIzol reagents (Thermo Fisher Scientific, Waltham, MA). qRT-PCR, used to examine gene expression and viral replication, was performed using SYBR as we previously described (24, 25, 46). The primers used to quantify the EPAC genes are available upon request.
Western blot analysis.
Total cellular lysates or cytosol and nuclear extracts were prepared for uninfected or infected cells as previously described (58). Proteins were then quantified with a protein quantification kit from Bio-Rad, followed by fractionation using SDS-PAGE denaturing gels and protein transfer to polyvinylidene difluoride membranes as previously described (47, 59). Membranes were blocked with 5% milk in TBS-Tween 20 and incubated with the proper primary antibodies according to the manufacturer's instructions.
Preparation of MEFs.
Mouse embryos from wild-type and EPAC2−/− mice (a generous gift from Ju Chen, University of California at San Diego) were harvested at 14 to 15 days of gestation. Embryos were placed in prechilled Dulbecco's phosphate-buffered saline (DPBS), and their heads, tails, legs, and red organs were discarded. The leftover was then washed with PBS, followed by tissue mincing into very small pieces using a sterile razor blade. The tissue pieces were then transferred to a 50-ml conical tube and digested in 1 ml 0.1% trypsin-EDTA containing 10 μl of DNase I (10 mg/ml)/embryo for 15 min at 37°C. The digested tissues were then dissociated into cells by rocking the tube for 5 min, followed by pipetting and filtering through a 70-μm nylon mesh. The cells were suspended in Dulbecco's modified Eagle's medium (DMEM) containing 10% (vol/vol) fetal bovine serum, 10 mM glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin, followed by centrifugation at 1,000 rpm for 10 min. The supernatants were discarded and cell pellets were washed again with DMEM. The isolated cells were then plated on a flask or dish. For all experiments, cells were used at passage numbers 3 to 4.
Reporter gene assays.
MEFs cells were transfected in triplicate with luciferase reporter gene plasmids containing multiple copies of NF-κB binding sites (NF-κB-Luc) or the IRF-3 binding site (PRDIII-I-Luc and IRF-3-Luc) using FuGene 6 (Roche, Indianapolis, IN), as previously described (60, 61). At 30 h posttransfection, cells were infected with RSV for 15 h and lysed to measure luciferase reporter activity.
Statistical analysis.
Statistical significance was determined using analysis of variance (ANOVA). A P value of less than 0.05 was considered significant. Means ± standard errors (SE) are shown.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute of Allergy and Infectious Diseases, NIH (1R01AI107033-01 and R21AI113771-01A1), and a Flight Attendant Medical Research Institute Clinical Innovator Award to X.B.
We thank Animesh Chandra for assistance with manuscript editing and Cynthia Tribble for graphics preparation. A. Chandra is supported by the UTMB CTSA, funded by NCATS grant UL1TR001439. We thank Kimberly Palkowetz for technical assistance.
We have no conflicts of interest to report.
REFERENCES
- 1.Falsey AR. 1998. Respiratory syncytial virus infection in older persons. Vaccine 16:1775–1778. doi: 10.1016/S0264-410X(98)00142-X. [DOI] [PubMed] [Google Scholar]
- 2.Jensen TO, Stelzer-Braid S, Willenborg C, Cheung C, Andresen D, Rawlinson W, Clezy K. 2016. Outbreak of respiratory syncytial virus (RSV) infection in immunocompromised adults on a hematology ward. J Med Virol 88:1827–1831. doi: 10.1002/jmv.24521. [DOI] [PubMed] [Google Scholar]
- 3.Mazur NI, Martinon-Torres F, Baraldi E, Fauroux B, Greenough A, Heikkinen T, Manzoni P, Mejias A, Nair H, Papadopoulos NG, Polack FP, Ramilo O, Sharland M, Stein R, Madhi SA, Bont L. 2015. Lower respiratory tract infection caused by respiratory syncytial virus: current management and new therapeutics. Lancet Respir Med 3:888–900. doi: 10.1016/S2213-2600(15)00255-6. [DOI] [PubMed] [Google Scholar]
- 4.McClure DL, Kieke BA, Sundaram ME, Simpson MD, Meece JK, Sifakis F, Gasser RA Jr, Belongia EA. 2014. Seasonal incidence of medically attended respiratory syncytial virus infection in a community cohort of adults ≥50 years old. PLoS One 9:e102586. doi: 10.1371/journal.pone.0102586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco JCG, Boukhvalova MS, Morrison TG, Vogel SN. 2018. A multifaceted approach to RSV vaccination. Hum Vaccin Immunother 14:1734–1745. doi: 10.1080/21645515.2018.1472183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Resch B. 2017. Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection. Hum Vaccin Immunother 13:2138–2149. doi: 10.1080/21645515.2017.1337614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beavo JA, Brunton LL. 2002. Cyclic nucleotide research–still expanding after half a century. Nat Rev Mol Cell Biol 3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- 9.Giordano D, Magaletti DM, Clark EA, Beavo JA. 2003. Cyclic nucleotides promote monocyte differentiation toward a DC-SIGN+ (CD209) intermediate cell and impair differentiation into dendritic cells. J Immunol 171:6421–6430. doi: 10.4049/jimmunol.171.12.6421. [DOI] [PubMed] [Google Scholar]
- 10.Rybalkin SD, Rybalkina I, Beavo JA, Bornfeldt KE. 2002. Cyclic nucleotide phosphodiesterase 1C promotes human arterial smooth muscle cell proliferation. Circ Res 90:151–157. doi: 10.1161/hh0202.104108. [DOI] [PubMed] [Google Scholar]
- 11.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. 1998. A family of cAMP-binding proteins that directly activate Rap1. Science 282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 12.Springett GM, Kawasaki H, Spriggs DR. 2004. Non-kinase second-messenger signaling: new pathways with new promise. Bioessays 26:730–738. doi: 10.1002/bies.20057. [DOI] [PubMed] [Google Scholar]
- 13.Gong B, Shelite T, Mei FC, Ha T, Hu Y, Xu G, Chang Q, Wakamiya M, Ksiazek TG, Boor PJ, Bouyer DH, Popov VL, Chen J, Walker DH, Cheng X. 2013. Exchange protein directly activated by cAMP plays a critical role in bacterial invasion during fatal rickettsioses. Proc Natl Acad Sci U S A 110:19615–19620. doi: 10.1073/pnas.1314400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mestre MB, Colombo MI. 2012. cAMP and EPAC are key players in the regulation of the signal transduction pathway involved in the alpha-hemolysin autophagic response. PLoS Pathog 8:e1002664. doi: 10.1371/journal.ppat.1002664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao X, Mei F, Agrawal A, Peters CJ, Ksiazek TG, Cheng X, Tseng CT. 2014. Blocking of exchange proteins directly activated by cAMP leads to reduced replication of Middle East respiratory syndrome coronavirus. J Virol 88:3902–3910. doi: 10.1128/JVI.03001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira L, Rehmann H, Lao DH, Erickson JR, Bossuyt J, Chen J, Bers DM. 2015. Novel Epac fluorescent ligand reveals distinct Epac1 vs. Epac2 distribution and function in cardiomyocytes. Proc Natl Acad Sci U S A 112:3991–3996. doi: 10.1073/pnas.1416163112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Heijnen CJ, van Velthoven CT, Willemen HL, Ishikawa Y, Zhang X, Sood AK, Vroon A, Eijkelkamp N, Kavelaars A. 2013. Balancing GRK2 and EPAC1 levels prevents and relieves chronic pain. J Clin Investig 123:5023–5034. doi: 10.1172/JCI66241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan J, Mei FC, Cheng H, Lao DH, Hu Y, Wei J, Patrikeev I, Hao D, Stutz SJ, Dineley KT, Motamedi M, Hommel JD, Cunningham KA, Chen J, Cheng X. 2013. Enhanced leptin sensitivity, reduced adiposity, and improved glucose homeostasis in mice lacking exchange protein directly activated by cyclic AMP isoform 1. Mol Cell Biol 33:918–926. doi: 10.1128/MCB.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolakofsky D. 2016. Paramyxovirus RNA synthesis, mRNA editing, and genome hexamer phase: a review. Virology 498:94–98. doi: 10.1016/j.virol.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Sevajol M, Subissi L, Decroly E, Canard B, Imbert I. 2014. Insights into RNA synthesis, capping, and proofreading mechanisms of SARS-coronavirus. Virus Res 194:90–99. doi: 10.1016/j.virusres.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Luxon BA, Casola A, Garofalo RP, Jamaluddin M, Brasier AR. 2001. Expression of respiratory syncytial virus-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. J Virol 75:9044–9058. doi: 10.1128/JVI.75.19.9044-9058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Ding C, Wild C, Liu H, Wang T, White MA, Cheng X, Zhou J. 2013. Efficient synthesis of ESI-09, a novel non-cyclic nucleotide EPAC antagonist. Tetrahedron Lett 54:1546–1549. doi: 10.1016/j.tetlet.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Y, Chen H, Boulton S, Mei F, Ye N, Melacini G, Zhou J, Cheng X. 2015. Biochemical and pharmacological characterizations of ESI-09 based EPAC inhibitors: defining the ESI-09 “therapeutic window.” Sci Rep 5:9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng J, Ptashkin RN, Chen Y, Cheng Z, Liu G, Phan T, Deng X, Zhou J, Lee I, Lee YS, Bao X. 2015. Respiratory syncytial virus utilizes a tRNA fragment to suppress antiviral responses through a novel targeting mechanism. Mol Ther 23:1622–1629. doi: 10.1038/mt.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, Lee I, Ren J, Ajay SS, Lee YS, Bao X. 2013. Identification and functional characterization of tRNA-derived RNA fragments (tRFs) in respiratory syncytial virus infection. Mol Ther 21:368–379. doi: 10.1038/mt.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto K, Yamamoto S, Ogasawara N, Takano K, Shiraishi T, Sato T, Miyata R, Kakuki T, Kamekura R, Kojima T, Tsutsumi H, Himi T, Yokota SI. 2016. Clarithromycin prevents human respiratory syncytial virus-induced airway epithelial responses by modulating activation of interferon regulatory factor-3. Pharmacol Res 111:804–814. doi: 10.1016/j.phrs.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 27.Obata K, Kojima T, Masaki T, Okabayashi T, Yokota S, Hirakawa S, Nomura K, Takasawa A, Murata M, Tanaka S, Fuchimoto J, Fujii N, Tsutsumi H, Himi T, Sawada N. 2013. Curcumin prevents replication of respiratory syncytial virus and the epithelial responses to it in human nasal epithelial cells. PLoS One 8:e70225. doi: 10.1371/journal.pone.0070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsutsumi H, Kojima T, Hirakawa S, Masaki T, Okabayashi T, Yokota S, Fujii N, Himi T, Sawada N. 2011. Respiratory syncytial virus infection and the tight junctions of nasal epithelial cells. Adv Otorhinolaryngol 72:153–156. [DOI] [PubMed] [Google Scholar]
- 29.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. 1998. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 30.Guerrero-Plata A, Casola A, Suarez G, Yu X, Spetch L, Peeples ME, Garofalo RP. 2006. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am J Respir Cell Mol Biol 34:320–329. doi: 10.1165/rcmb.2005-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olszewska-Pazdrak B, Pazdrak K, Ogra PL, Garofalo RP. 1998. Respiratory syncytial virus-infected pulmonary epithelial cells induce eosinophil degranulation by a CD18-mediated mechanism. J Immunol 160:4889–4895. [PubMed] [Google Scholar]
- 32.Tripp RA. 2004. Pathogenesis of respiratory syncytial virus infection. Viral Immunol 17:165–181. doi: 10.1089/0882824041310513. [DOI] [PubMed] [Google Scholar]
- 33.Man SM, Karki R, Kanneganti TD. 2017. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casola A, Burger N, Liu T, Jamaluddin M, Brasier AR, Garofal RP. 2001. Oxidant tone regulates RANTES gene transcription in airway epithelial cells infected with respiratory syncytial virus: role in viral-induced interferon regulatory factor activation. J Biol Chem 276:19715–19722. doi: 10.1074/jbc.M101526200. [DOI] [PubMed] [Google Scholar]
- 35.Genin P, Algarte M, Roof P, Lin R, Hiscott J. 2000. Regulation of RANTES chemokine gene expression requires cooperativity between NF-kappa B and IFN-regulatory factor transcription factors. J Immunol 164:5352–5361. doi: 10.4049/jimmunol.164.10.5352. [DOI] [PubMed] [Google Scholar]
- 36.Bao X, Liu T, Spetch L, Kolli D, Garofalo RP, Casola A. 2007. Airway epithelial cell response to human metapneumovirus infection. Virology 368:91–101. doi: 10.1016/j.virol.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoboua F, Martel A, Duval A, Mukawera E, Grandvaux N. 2010. Respiratory-syncytial virus-mediated NF-kappaB p65 phosphorylation at serine 536 is dependent on RIG-I, TRAF6 and IKKbeta. J Virol 84:7267–7277. doi: 10.1128/JVI.00142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almahariq M, Tsalkova T, Mei FC, Chen H, Zhou J, Sastry SK, Schwede F, Cheng X. 2013. A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol Pharmacol 83:122–128. doi: 10.1124/mol.112.080689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niimura M, Miki T, Shibasaki T, Fujimoto W, Iwanaga T, Seino S. 2009. Critical role of the N-terminal cyclic AMP-binding domain of Epac2 in its subcellular localization and function. J Cell Physiol 219:652–658. doi: 10.1002/jcp.21709. [DOI] [PubMed] [Google Scholar]
- 40.Ueno H, Shibasaki T, Iwanaga T, Takahashi K, Yokoyama Y, Liu LM, Yokoi N, Ozaki N, Matsukura S, Yano H, Seino S. 2001. Characterization of the gene EPAC2: structure, chromosomal localization, tissue expression, and identification of the liver-specific isoform. Genomics 78:91–98. doi: 10.1006/geno.2001.6641. [DOI] [PubMed] [Google Scholar]
- 41.Chen H, Wild C, Zhou X, Ye N, Cheng X, Zhou J. 2014. Recent advances in the discovery of small molecules targeting exchange proteins directly activated by cAMP (EPAC). J Med Chem 57:3651–3665. doi: 10.1021/jm401425e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almahariq M, Mei FC, Cheng X. 2016. The pleiotropic role of exchange protein directly activated by cAMP 1 (EPAC1) in cancer: implications for therapeutic intervention. Acta Biochim Biophys Sin (Shanghai) 48:75–81. doi: 10.1093/abbs/gmv115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin R, Heylbroeck C, Pitha PM, Hiscott J. 1998. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol Cell Biol 18:2986–2996. doi: 10.1128/MCB.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Servant MJ, ten Oever B, LePage C, Conti L, Gessani S, Julkunen I, Lin R, Hiscott J. 2001. Identification of distinct signaling pathways leading to the phosphorylation of interferon regulatory factor 3. J Biol Chem 276:355–363. doi: 10.1074/jbc.M007790200. [DOI] [PubMed] [Google Scholar]
- 45.Chen H, Tsalkova T, Chepurny OG, Mei FC, Holz GG, Cheng X, Zhou J. 2013. Identification and characterization of small molecules as potent and specific EPAC2 antagonists. J Med Chem 56:952–962. doi: 10.1021/jm3014162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren J, Liu T, Pang L, Li K, Garofalo RP, Casola A, Bao X. 2011. A novel mechanism for the inhibition of interferon regulatory factor-3-dependent gene expression by human respiratory syncytial virus NS1 protein. J Gen Virol 92:2153–2159. doi: 10.1099/vir.0.032987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren J, Wang Q, Kolli D, Prusak DJ, Tseng CT, Chen ZJ, Li K, Wood TG, Bao X. 2012. Human metapneumovirus M2-2 protein inhibits innate cellular signaling by targeting MAVS. J Virol 86:13049–13061. doi: 10.1128/JVI.01248-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casola A, Garofalo RP, Jamaluddin M, Vlahopoulos S, Brasier AR. 2000. Requirement of a novel upstream response element in RSV induction of interleukin-8 gene expression: stimulus-specific differences with cytokine activation. J Immunol 164:5944–5951. doi: 10.4049/jimmunol.164.11.5944. [DOI] [PubMed] [Google Scholar]
- 49.Hasegawa K, Jartti T, Mansbach JM, Laham FR, Jewell AM, Espinola JA, Piedra PA, Camargo CA Jr. 2015. Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: multicenter cohort studies in the United States and Finland. J Infect Dis 211:1550–1559. doi: 10.1093/infdis/jiu658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buckinham SC, Bush AJ, Devincenzo JP. 2000. Nasal quantity of respiratory syncytial virus correlates with disease severity in hospitalized infants. Pediatr Infect Dis 19:113–117. doi: 10.1097/00006454-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 51.DeVincenzo JP, El Saleeby CM, Bush AJ. 2005. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis 191:1861–1868. doi: 10.1086/430008. [DOI] [PubMed] [Google Scholar]
- 52.Hornsleth A, Loland L, Larsen LB. 2001. Cytokines and chemokines in respiratory secretion and severity of disease in infants with respiratory syncytial virus (RSV) infection. J Clin Virol 21:163–170. doi: 10.1016/S1386-6532(01)00159-7. [DOI] [PubMed] [Google Scholar]
- 53.Kakimoto Y, Seto Y, Ochiai E, Satoh F, Osawa M. 2016. Cytokine elevation in sudden death with respiratory syncytial virus: a case report of 2 children. Pediatrics 138:e20161293. doi: 10.1542/peds.2016-1293. [DOI] [PubMed] [Google Scholar]
- 54.Mejias A, Dimo B, Suarez NM, Garcia C, Suarez-Arrabal MC, Jartti T, Blankenship D, Jordan-Villegas A, Ardura MI, Xu Z, Banchereau J, Chaussabel D, Ramilo O. 2013. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med 10:e1001549. doi: 10.1371/journal.pmed.1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Z, Zhu Y, Chen H, Wang P, Mei FC, Ye N, Cheng X, Zhou J. 2017. Structure-activity relationships of 2-substituted phenyl-N-phenyl-2-oxoacetohydrazonoyl cyanides as novel antagonists of exchange proteins directly activated by cAMP (EPACs). Bioorg Med Chem Lett 27:5163–5166. doi: 10.1016/j.bmcl.2017.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang P, Liu Z, Chen H, Ye N, Cheng X, Zhou J. 2017. Exchange proteins directly activated by cAMP (EPACs): emerging therapeutic targets. Bioorg Med Chem Lett 27:1633–1639. doi: 10.1016/j.bmcl.2017.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bao X, Indukuri H, Liu T, Liao SL, Tian B, Brasier AR, Garofalo RP, Casola A. 2010. IKKepsilon modulates RSV-induced NF-kappaB-dependent gene transcription. Virology 408:224–231. doi: 10.1016/j.virol.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bao X, Kolli D, Liu T, Shan Y, Garofalo RP, Casola A. 2008. Human metapneumovirus small hydrophobic protein inhibits NF-kappaB transcriptional activity. J Virol 82:8224–8229. doi: 10.1128/JVI.02584-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bannister R, Rodrigues D, Murray EJ, Laxton C, Westby M, Bright H. 2010. Use of a highly sensitive strand-specific quantitative PCR to identify abortive replication in the mouse model of respiratory syncytial virus disease. Virol J 7:250. doi: 10.1186/1743-422X-7-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bao X, Liu T, Shan Y, Li K, Garofalo RP, Casola A. 2008. Human metapneumovirus glycoprotein G inhibits innate immune responses. PLoS Pathog 4:e1000077. doi: 10.1371/journal.ppat.1000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Y, Deng X, Deng J, Zhou J, Ren Y, Liu S, Prusak DJ, Wood TG, Bao X. 2016. Functional motifs responsible for human metapneumovirus M2-2-mediated innate immune evasion. Virology 499:361–368. doi: 10.1016/j.virol.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]