Abstract
Early life stress in the form of early institutional care has been shown to have wide-ranging impacts on the biological and behavioral development of young children. Studies of brain structure using magnetic resonance imaging have reported decreased prefrontal volumes, and a large literature has detailed decreased executive function (EF) in post-institutionalized (PI) youth. Little is known about how these findings relate to decision-making, particularly in PI youth entering adolescence—a period often characterized by social transition and increased reliance upon EF skills and the still-maturing prefrontal regions that support them. As decisionmaking in risky situations can be an especially important milestone in early adolescence, a clearer knowledge of the relationship between risky decision making and prefrontal structures in post-institutionalized youth is needed. The youth version of the Balloon Analogue Risk Task and a two-deck variant of the Iowa Gambling Task were used to assess risky decision-making in post-institutionalized youth and a community control group (N = 74, PI = 44, Non-adopted = 30; mean age = 12.93). Participants also completed a structural MRI scan for the assessment of group differences in brain structure. We hypothesized that participants adopted from institutions would display poorer performance on risky-decision making tasks and smaller brain volumes compared to non-adopted youth. Results indicated that later-adopted participants made fewer risky decisions than those experiencing shorter periods of deprivation or no institutional rearing. Further, decreased prefrontal volumes were observed in later-adopted youth and were significantly associated with task performance. Our results suggest that changes in risky-decision making behavior and brain structure are associated with the duration of early institutional care.
Keywords: Risk-taking, structural brain imaging, international adoption, early life stress, adolescence
1. Introduction
A growing body of evidence has demonstrated that early life stress (ELS) has wide ranging negative impacts on the developing child, including changes in brain structure and function, decreased cognitive performance, and altered recruitment of neurochemical systems (Nelson, Bos, Gunnar, & Sonuga-Barke, 2011). Studies of individuals who have experienced maltreatment including physical, emotional, and sexual abuse have shown increased behavioral problems, decreased cortical brain volumes, and deficits in decision-making ability and cognitive performance (Cowell, Cicchetti, Rogosch, & Toth, 2015; Hanson et al., 2015; Trickett, Noll, & Putnam, 2011; Weller & Fisher, 2013). Further, studies of children experiencing poverty have described deficits in working memory, differential neural connectivity during emotion regulation, increased homeostatic stress, and poorer physical health (Blair, Raver, Granger, Mills-Koonce, & Hibel, 2011; Chen, Matthews, & Boyce, 2002; Evans & Schamberg, 2009; Kim et al., 2013). Experiences of ELS have long lasting impacts and may contribute to additional vulnerability during periods of increased environmental susceptibility (e.g. adolescence; Casey, 2015). In cases of maltreatment and poverty, the experience of stress often extends over long periods of time, making it difficult to understand the effects of developmental timing on the relationship between the stressor and later outcomes. The study of youth adopted internationally from institutional care into well-resourced families, however, may provide insights into the impacts of ELS limited to the first years of life.
1.2. Decision-making in Adolescence Following Early Life Stress
The adolescent period has been characterized as an especially turbulent period in development. Models of adolescent decision-making, such as the dual systems model, suggest that an imbalance in maturity between brain regions involved in reward processing and regions implicated in behavior regulation results in increased sensitivity to the environment and limited ability to generate appropriate responses (Casey, 2015; Crone & Dahl, 2012). It may be that ELS affects decision-making processes specifically, which could be especially evident during the adolescent period. For example, maltreated children and adolescents have been shown to choose safe options in a risky task more frequently than non-maltreated youth (Guyer et al., 2006). In a slightly younger sample, Weller and Fisher (2013) reported that maltreated children tended to make risky choices to avoid losses instead of taking risks to gain rewards. These studies suggest a propensity to avoid losses in youth who have experienced ELS, a bias that may be an adaptive response to the experience of adversity in early life even if it may sacrifice the opportunity for gain (Boyce & Ellis, 2005; Humphreys et al., 2015).
Similar effects have been shown in children with a history of institutional rearing. Post-institutionalized (PI) and post-foster care adolescents failed to gain as many points as nonadopted (NA) youth on a risk taking task, a result that demonstrated lower sensation-seeking behavior in PI compared to NA children (Loman, Johnson, Quevedo, Lafavor, & Gunnar, 2014). Results from a similar task, in which participants could learn when they were more likely to lose points, demonstrated that PI youth took fewer risks and chose to “cash in” rewards more often than NA control youth. However, the tendency to save rewards more often in the PI group was mediated by separation anxiety scores, such that the relationship between PI status and choosing to “cash in” rewards was diminished (Humphreys et al., 2015). These findings suggest that PI adolescents make risky decisions differently than their NA counterparts, differences that could be related to deficits in other cognitive processes, such as executive function.
1.3. Early Institutional Care and Executive Function
Executive function (EF) deficits are important in understanding the long term impacts of early psychosocial deprivation in part due to research indicating that EF skills predict social cognition, academic competency, and emotion regulation in children and adolescents (Blair & Razza, 2007; Carlson & Wang, 2007; Hughes, 2011). In a study of international adoptees tested one year following adoption, Hostinar et al. (2012) reported global EF deficits, with EF being negatively related to both institutional quality and time spent in the biological family prior to entry into the institution.
Deficits in a wide range of EF sub-domains are evident in PI youth. Children who experienced early institutional care made significantly more errors in a spatial working memory task (Bos, Fox, Zeanah, & Nelson, 2009) and showed a deficit in performance on the backward digit span task compared to non-adopted groups (Beckett, Castle, Rutter, & Sonuga-Barke, 2010). Sub-domain EF differences also may depend on the duration of ELS. For example, PI children adopted after 12 months of age performed significantly worse on the memory, attention, and learning tasks of the Cambridge Neuropsychological Test Automated Battery (CANTAB) and NEPSY Developmental Neuropsychological Assessment when tested between 8 and 10 years of age (Pollak et al., 2010). Further, children adopted after 6 months of age made more errors on the Tower of London task than children adopted prior to 6 months or NA control children (Beckett et al., 2010), though not all researchers have observed group differences on the executive control aspects of the CANTAB (see Bos et al., 2009; Pollak et al., 2010). Additional findings demonstrate negative relations between the duration of institutional care and Stroop performance, inhibitory control, and working memory, further suggesting that the duration of institutionalization may have important implications for later cognitive development (Colvert et al., 2008; Merz, McCall, Wright, & Luna, 2013; Pollak et al., 2010). Differences in EF following ELS may be especially important when studying brain volume and decision-making. Deficits in EF are behaviors thought to be supported by the prefrontal cortex, which has been shown to be structurally different in PI children (Hodel et al., 2015; Merz, Harlé, Noble, & McCall, 2016). Further, risky decision-making tasks, which necessitate choice under circumstances that present the risk of gaining or losing rewards, require many of the same EF skills on which PI youth have been found to perform poorly for optimal performance (Buelow & Blaine, 2015).
1.4. Early Life Stress and Prefrontal Brain Structure
Stress, even in small, time-limited amounts has been shown to alter prefrontal cortex (PFC) function and connectivity (Liston, McEwen, & Casey, 2009). Alterations to the PFC following stress may be impacted directly by stress-mediating physiological systems. For example, hormones produced in response to stressors may have a negative impact on neuronal survival in the PFC (Lupien, McEwen, Gunnar, & Heim, 2009). Indeed, research has shown that children fostered from Romanian institutions exhibit decreased total gray matter volume compared to Romanian children who had never been institutionalized (Sheridan, Fox, Zeanah, McLaughlin, & Nelson, 2012). Similarly, youth adopted internationally from institutional care exhibited a bilateral decrease in prefrontal gray matter volume compared to NA comparison youth (Hodel et al., 2015). Further, Hodel et al. (2015) also reported differences in surface area and a small difference in cortical thickness when comparing PI to NA youth. Reduced cortical thickness in PI youth compared to never institutionalized youth has also been associated with increased inattention and impulsivity (McLaughlin et al., 2014). These changes in brain structure may be related to the specific stressors associated with time spent in institutional care. Such care is often characterized by daily regimentation, lack of individual caregiver attention, low levels of psychosocial investment from caregivers, and minimal social interaction (Zeanah et al., 2009). Given the extreme plasticity of the brain in the first years of life, the deprivation of social care common in institutions could have substantial impacts on the brains and behavior of PI children. Nelson et al. (2011) suggest that the formation of individual attachment relationships is a biologically expected environment for normative development of brain structure and function. Development of these systems may be hindered by the lack of individualized care provided in institutional settings for the care of children who have been abandoned or separated from their parents.
1.5. The Current Study
Given the links between early institutional care, executive function, and prefrontal volume, we anticipated effects of institutional care on risky decision-making during adolescence. As reviewed above (see section 1.2), adolescence is a particularly vulnerable period of development and one in which making advantageous decisions can have a large positive impact on long-term outcomes. To date, it is unclear whether the effects of ELS apply specifically to executive function, or if they extend to non-EF contexts, such as risky-decision making. In maltreated youth, decrements are specific to inhibitory control rather than global cognitive ability (Cowell et al., 2015), though it is unclear whether this kind of specific decrement continues into adolescence or exists in other populations. For this reason, the current study was designed to investigate the associations between early institutional care, risky decision-making, and brain volume in adolescence. We used the Balloon Analogue Risk Task (BART) and the Iowa Gambling Task (IGT) to assess sensation seeking and risky decision-making in PI and NA adolescents, a comparison that provides a useful model for understanding the long-term behavioral effects of stress confined to the first years of life. Participants also completed a structural MRI scan. While much of the prior literature in PI populations that has investigated behavior or brain structure have examined them individually, our study aims to shed light on the contributions of ELS to risky decision-making and its association with brain structure in adolescence.
The sample used in this analysis is a subset of those we reported on previously (Hodel et al., 2015). That analysis showed a reduction in prefrontal volume, surface area, and cortical thickness in PI youth relative to youth without ELS histories. Based on literature describing decision-making following early institutional care we predicted that PI youth would gain fewer points on the BART and exhibit lower accuracy scores on the IGT than NA comparison youth. In addition, we hypothesized that a decomposition of the IGT into high- and low-payout conditions would reveal differences in risk-taking behavior due to differences in PI adolescents’ sensitivity to reward. Finally, as previous literature has suggested that performance on the BART and IGT involve similar prefrontal regions including the ACC, OFC, and dorsolateral prefrontal cortex (dlPFC; Bechara, Damasio, Damasio, & Anderson, 1994; Hartstra, Oldenburg, Van Leijenhorst, Rombouts, & Crone, 2010; Li, Lu, D’Argembeau, Ng, & Bechara, 2010; Rao, Korczykowski, Pluta, Hoang, & Detre, 2008), we predicted that specified prefrontal volumes would correlate with behavioral performance. Brain analyses were limited to a priori regions of interest based on previous research investigating the developmental outcomes of stress and structural and functional brain imaging findings and included prefrontal cortex (aggregate), orbitofrontal cortex (OFC) and the anterior cingulate cortex (ACC; Hartstra et al., 2010; Hodel et al., 2015; Li et al., 2010; Liston et al., 2009). While differences in cortical surface area, and thickness suggest ontologically distinct mechanisms of change (Panizzon et al., 2009; Raznahan et al., 2011), we did not have a priori hypotheses specific to these mechanisms. As such, these exploratory analyses investigated cortical volume (used most commonly across studies), surface area, and thickness of the regions of interest measured separately for the left and right hemispheres.
2. Method
2.1. Participants
Our final sample included 74 adolescents (50 female, Mage = 12.93 years, SD = 0.58, range = 11.75 – 14.09 years): 44 PI youth adopted into Minnesota families and 30 NA controls raised in their biological families (PI Group: 28 female, Mage = 13.00 years, SD = 0.66; NA Group: 22 female, Mage = 12.83 years, SD = 0.43). The PI sample represents a subset of a larger study (e.g. Gunnar et al., 2012; Hodel et al., 2015) drawn from the International Adoption Project Registry maintained at the University of Minnesota. Participants were included in the subsample if they had high-quality imaging and behavioral data. All of the PI youth were adopted by 72 months of age and came from diverse regions of the world, including Colombia (5%), Ecuador (2%), Russia/Eastern Europe (45%), India/Nepal (16%), China (27%), and Vietnam (5%). Adolescents were excluded for serious illness (e.g. cancer), known genetic conditions (e.g. Down Syndrome), Fetal Alcohol Syndrome, neurological conditions (e.g. epilepsy), developmental disorders (e.g. Autism Spectrum Disorder, Pervasive Developmental Disorder), known IQ below 80, or the presence of MRI exclusions (e.g. orthodontic braces, metal in body, claustrophobia). PI adolescents were not excluded for current diagnosis of psychological/psychiatric disorders such as ADHD or anxiety disorders as excluding PI youth for psychiatric disorders may remove variance of interest. Prior research in internationally adopted populations has reported increases in behaviors that result in future diagnoses (e.g. impulsivity that may result in a diagnosis of ADHD; Zeanah et al., 2009). As a result, removing PI youth with psychiatric diagnoses may serve to remove variance in the behaviors of interest when comparing adopted to non-adopted youth in the context of risky decision-making. Previous research with internationally adopted youth has also used this method (McLaughlin et al., 2014; Tottenham et al., 2011).
Non-adopted (NA) comparison youth were recruited from a community participant pool maintained by the University of Minnesota. Adolescents in the NA group were excluded for psychological or psychiatric disorders and for birth complications, in addition to the exclusions listed for PI youth. Parents of all participants completed verbal and written consent procedures and participants completed both verbal and written assent procedures. Both the parents and adolescents were compensated for their efforts in the study. Study procedures were approved by the University of Minnesota Institutional Review Board.
2.2. IQ Measurement
Global cognitive function was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). Given expected differences in cognitive function between groups, full-scale IQ was used as a covariate in analyses of behavioral measures.
2.3. Questionnaire Measures
2.3.1. Impulsivity.
Ratings of impulsivity, which evaluate individual participants’ ability to inhibit automatic responses, were collated from the MacArthur Health Behavior Questionnaire (HBQ; Essex et al., 2002) which was collected previously when participants were 8 −13 years of age. The HBQ is a parent-report measure of multiple health behaviors including ADHD symptoms and signs of impulsivity. Among these questions were items like “[My child] does dangerous things without thinking” or “jumps from one activity to another.”
2.3.2. Sensation seeking.
Child-report of sensation seeking was assessed using the Sensation Seeking Scale for Children (SSSC; Russo et al., 1993). Sensation seeking is defined as a need for varied, novel, and complex experiences or sensations and a willingness to take physical and social risks to have these experiences (Zuckerman, 1979). The SSSC is comprised of 26 forced choice questions on thrill and adventure seeking, drug and alcohol seeking, and social disinhibition which are combined to create a total sensation seeking score. Forced choice questions required participants to choose which of two opposite options is most like themselves (e.g. “I enjoy the feeling of riding my bike fast down a big hill” or “Riding my bike fast down a big hill is too scary for me”). Analyses in the current study utilized the total sensation seeking score. Both the SSSC and HBQ impulsivity measures were analyzed in order to examine group differences in risky decisionmaking behavior outside of the laboratory context.
2.4. Behavioral Tasks
2.4.1. BART-Y.
Participants completed the youth version of the Balloon Analogue Risk Task (BART-Y; Lejuez et al. 2007), a task designed to measure sensation seeking and impulsivity. A recent metaanalysis reported small but significant associations between the BART and sensation seeking and impulsivity (r = 0.14 and r = .10, respectively; (Lauriola, Panno, Levin, & Lejuez, 2014). During the task, participants were directed to inflate an on-screen balloon by clicking a button that read “Pump.” With each pump, the balloon inflated slightly and the participant was given a small amount of money. The balloon on the screen was programmed to pop sometime between the first and 128th pump, at which point any money accrued for that balloon was lost. The probability of the balloon popping increased with each pump. Participants could choose to keep the money they had accrued at any point within a trial by clicking a button that read “Get $$$.” Choosing to save the money ended the trial. Participants were told that the bigger they made the balloon before clicking “Get $$$” the more money would accrue in the prize meter. After the 30th trial, the participant received on-screen feedback about the total amount accrued across the task. Performance on the BART-Y was measured using an adjusted average number of pumps per trial, calculated by averaging the number of pumps only for trials during which the balloon did not explode. This measure has been suggested as the most representative metric of performance on the BART as the number of pumps is constrained during trials in which the balloon pops, which serves to limit the between-subject variability when an overall average is calculated (Lejuez, Aklin, Zvolensky, & Pedulla, 2003). Data from the BART-Y were checked to ensure that all values were in the expected range and the adjusted average pumps metric was assessed for accuracy following its computation.
2.4.2. Iowa Gambling Task.
A two-deck variant of the Iowa Gambling Task (IGT) was used to evaluate risky decision-making and was completed during MRI scanning (Hartstra et al., 2010), though only the behavioral data is reported here. Participants were instructed to attempt to accrue as many points as possible by choosing cards from two decks, one of which would provide a net gain and one that would not. To this end, participants used a button box to draw from one of two decks of cards on each trial. Each card presented both the number of points gained (from 50 to 700 points) and the number of points lost (from 0 to −2,100 points). As a result, each trial resulted in a net gain or loss of points (i.e. the total number of points gained and lost on a given card). Participants were provided with both gain and loss feedback on every trial, as well as a running total of points earned in the game. The probability of the loss value being 0 on any given card was 50% (i.e. 7 out of every 14 cards contained some loss). Participants completed 12 blocks of 14 trials each, with the goal of maximizing the number of points earned. Each block presented two new decks of cards with unique levels of gain and loss.
2.4.3. IGT task conditions.
Each pair of decks differed in number of points gained or lost per card. Every pair of decks was manipulated so that choosing one of the decks exclusively would result in a net gain by the end of the block, while choosing the other deck exclusively would result in a net loss. To earn the most points, participants needed to learn which decks were advantageous (provided a net gain) based upon the feedback they received following the selection of each card.
Each pair of decks was categorized by one of two conditions, a high-payout condition and a low-payout condition. In the high-payout condition, the deck that provided larger rewards on individual trials would also result in a net gain across the block, while the low-paying deck would result in a net loss. Conversely, in the low-payout condition, the deck that provided smaller rewards on each trial resulted in a net gain across the block, while the deck paying large rewards resulted in a net loss (i.e. was associated with large losses). Two decks were used (as opposed to four decks in the original IGT) to ensure that sufficient learning occurred over a relatively short testing period (Hartstra et al., 2010), and to allow for repeated learning experiences across new pairs of decks. Further, the inclusion of two payout conditions ensured that participants had to be flexible in learning the optimal strategy instead of choosing to only play low-payout decks across all trials. Further, differences in group performance between the high- and low-pay conditions allows for an investigation of reward-sensitivity in the context of risky-decision making. Participant-level data from the IGT was inspected to ensure that participants sampled from both decks during the task.
2.5. MRI Acquisition and Processing
A Siemens 3T Trio MRI scanner equipped with a 32-channel head coil was used for all MRI sessions. Initial participant position was verified using a sagittal scout series. A T1weighted, three-dimensional magnetization prepared rapid gradient echo (MPRAGE) series was used to acquire 240 contiguous 1 mm slices in the sagittal plane (TR = 2530 ms, TE = 3.65 ms, FOV = 256 mm, flip angle = 7°, voxel size = 1.0mm3). Cortical segmentation was performed with the Freesurfer image analysis suite which uses the atlas defined by Desikan and colleagues (Desikan et al., 2006). This segmentation was used to assess our regions of interest—aggregate prefrontal, OFC, and ACC (version 5.1.0; http://surfer.nmr.mgh.harvard.edu). Aggregate prefrontal cortex was a combination of medial orbital frontal, lateral orbital frontal, pars orbitalis, pars triangularis, pars opercularis, caudal middle frontal, anterior cingulate, middle frontal, superior frontal, and frontal pole cortices. The OFC region of interest included both lateral orbitofrontal and parsorbitalis cortex and the ACC region of interest included both anterior and rostral anterior cingulate. Data processing included motion correction, removal of non-brain tissue with hybrid watershed and surface deformation procedures (Ségonne et al., 2004), automated Tailarach transformation, segmentation of subcortical structures, intensity normalization, automated topology correction and surface deformation procedures to identify transitions to new tissue types (Dale, Fischl, & Sereno, 1999; Fischl et al., 2004; Ségonne, Pacheco, & Fischl, 2007; Sled, Zijdenbos, & Evans, 1998). Further procedures included surface inflation, registration to atlases using cortical folding patterns, and parcellation of the cerebral cortex by gyral and sulcal structure (Desikan et al., 2006; Fischl, Sereno, & Dale, 1999; Fischl, Sereno, Tootell, & Dale, 1999). Good test-retest reliability has been shown across scanners and field-strengths (Han et al., 2006).
In addition to the default processing steps completed in Freesurfer, all individual structural images were visually inspected by trained staff. Images were inspected for large structural abnormalities (e.g. enlarged or asymmetrical ventricles) and excess motion artifact. Following segmentation, image outputs were visually inspected for gross segmentation errors in the areas of interest. A total of 13 participants were excluded following these quality assurance steps including 9 for excess motion, 3 due to poor segmentation, and 1 for a venous anomaly in the PFC.
2.6. Data Analysis Strategy
Based on previous work, we divided the PI group into earlier- and later-adopted subgroups using a median split of age at adoption. The use of a categorical predictor is supported by previous analyses that have shown significant differences between children adopted prior to specific age benchmarks (Colvert et al., 2008). Our analyses include an earlier-adopted PI group (adopted prior to 12 months of age; PI-EA), a later-adopted PI group (adopted between 12 – 72 months; PI-LA), and a non-adopted control group (NA).
Group differences in impulsivity, sensation seeking, performance on the BART-Y and IGT, and brain structure were assessed using analysis of covariance (ANCOVA). Bonferroni correction for multiple comparison was included when interpreting pairwise comparisons unless otherwise noted. Sex and age at assessment were included as covariates of non-interest in all analyses. IQ was included as a covariate in analyses of sensation seeking and impulsivity. A number of research groups have assessed the value of including IQ in analyses of the IGT, with results suggesting primary roles for both cognitive and affective processes (e.g. Toplak, Sorge, Benoit, West, & Stanovich, 2010; Webb, DelDonno, & Killgore, 2014). In other words, performance on the IGT may be best predicted by a combination of IQ and decision-making ability in an emotional context. For this reason, analyses of risky decision-making are reported both with and without IQ included in models of task performance. All covariates were meancentered. The relationships between questionnaire measures and task performance were investigated with Pearson correlation.
Structural MRI measures investigated cortical gray matter volume, cortical thickness, and surface area differences between groups. Statistical analyses of group differences in brain structure were meant to confirm the expected direction of effect found in a previous investigation of brain structure in the sample that this behavioral subset is a part of (Hodel et al., 2015), and are thus not corrected for multiple comparisons. Tests of the association between brain structure and task performance on the BART-Y and IGT included only the a priori regions of interest described, aggregate prefrontal, OFC, and ACC. These brain-behavior associations were exploratory and are presented with a Bonferroni adjusted alpha of 0.0167 to account for the number of regions of interest tested. All imaging analyses included estimated total intracranial volume (eTIV), age at assessment, and sex as mean-centered covariates to account for potentially confounding influences on brain anatomy. Data analysis was completed in SPSS (version 24).
3. Results
3.1. Group Demographics
Non-adopted and adopted youth did not differ on most demographic variables including household income, parent education level, sex, or age at assessment (p’s > .2). There was, however, a significant difference in IQ (t(64) = 2.16, p = .034). PI youth scored seven points lower on the WASI than their non-adopted peers (PI: M = 110.61, SD = 12.87; NA: M = 117.04, SD = 10.57).
3.2. Group Differences in Sensation Seeking and Impulsivity
Differences were found between the groups on sensation seeking scores (F(2, 55) = 3.924, p = .026). Post-hoc pairwise comparisons revealed that PI-LA youth scored significantly lower than NA youth but not PI-EA youth (Bonferroni corrected p = .021 and p = .279, respectively). PI-EA scores did not differ from those of NA youth.
A significant effect of group on impulsivity scores was also found (F(2, 53) = 4.654, p = .014). Both PI-LA and PI-EA groups were reported to be more impulsive than the NA group (Bonferroni corrected p = .048 and p = .036, respectively), but did not differ from each other.
3.3. Group Differences in Risky Decision-making
3.3.1. BART-Y
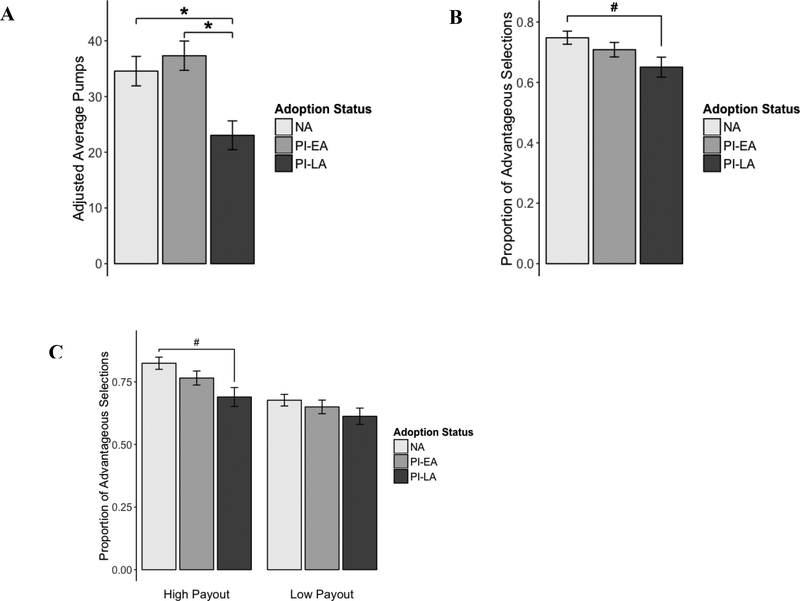
A significant main effect of group was observed on the BART-Y (F(2, 56) = 6.406, p <.01). PI-LA adolescents pressed significantly fewer times than the NA and PI-EA groups (Bonferroni corrected p = .006 and p = .007, respectively; PI-EA: M = 36.53, SD = 11.26; PI-LA: M = 23.04, SD = 9.7; NA: M = 34.56, SD = 14.25). The NA and PI-EA groups did not differ in the adjusted number of pumps per balloon (see Figure 1A).
Figure 1.
Mean adjusted average number of pumps on the BART-Y (A), mean accuracy on the IGT (B), and mean accuracy on high- and low-payout blocks of the IGT (C) for early-, late- and non-adopted adolescents. Error bars show ±1 SE from the mean. PI-EA = early adopted (<12 months), PI-LA = later adopted ≥12 months), NA = non-adopted, raised in biological family. * p < 0.05, # p < 0.10
When IQ was added to the model the group effect remained significant (F(2, 50) = 6.323, p < .01). PI-LA youth pressed significantly less, on average, than did the NA or PI-EA groups (Bonferroni corrected p = .005 and p = .011, respectively; PI-EA: M = 35.37, SD = 10.44; PI-LA: M = 22.19, SD = 8.09; NA: M = 34.80, SD = 14.74). No significant difference was evident between the PI-EA or NA groups.
3.3.2. IGT
A mean accuracy score was calculated to evaluate group differences in performance on the IGT. The proportion of advantageous choices across all trials of the task was used in the group-level analyses. There was only a trend-level main effect of group (F(2, 68) = 2.932, p = .06). The PI-LA group made marginally fewer advantageous choices over the course of the task compared to NA adolescents but did not differ from PI-EA adolescents (Bonferroni corrected p = .055 and p = .447, respectively; PI-EA: M = .71, SD = .12; PI-LA: M = .65, SD = .15; NA: M = .74, SD = .12). Once again, no difference was found between PI-EA and NA groups (see Figure 1B). No PI vs. NA group differences in mean IGT performance approached significance when IQ was entered into the model.
A follow-up decomposition of the task, in which high-payout and low-payout trials were analyzed separately, revealed similar effects of group on accuracy during high-payout trials. During high-payout trials, PI-LA youth chose from the advantageous deck less often than PI-EA and NA youth (F(2, 68) = 4.677, p < .05; Bonferroni corrected p = .216 and p = .010, respectively; PI-EA: M = .77, SD = .14; PI-LA: M = .69, SD = .17; NA: M = .82, SD = .13). There was no significant difference in accuracy between PI-EA and NA adolescents. No differences were found between groups in the low-payout condition (F(2, 68) = .993, p > .376; see Figure 1C).
Contrary to the results reported above, the inclusion of IQ in models evaluating performance on high- and low-payout trials of the IGT resulted in no main effect of group, but did reveal a significant relationship between IQ and accuracy on high-payout trials (F(1, 59) = 5.570, p < .05). The main effect of IQ during high-payout trials suggested that youth with higher IQs made significantly more advantageous choices during high-payout trials. No differences were found between groups in the low-payout condition, nor did IQ predict performance (F(2, 59) = .223, p = .800 and F(1, 59) = .328, p = .569, respectively).
3.4. Associations Between Sensation Seeking, Impulsivity, and Decision-making
No significant association was found between sensation seeking scores and BART-Y performance in the full sample, nor was there a correlation between HBQ impulsivity and BART-Y scores. In contrast, although the mean number of advantageous choices on the IGT was not significantly correlated with sensation seeking scores, IGT performance was negatively correlated with HBQ impulsivity (r = −.26, p = .03). There were no significant effects of impulsivity or sensation seeking on either of the decision-making tasks when PI status was included in the analyses.
3.5. Group Differences in Brain Structure
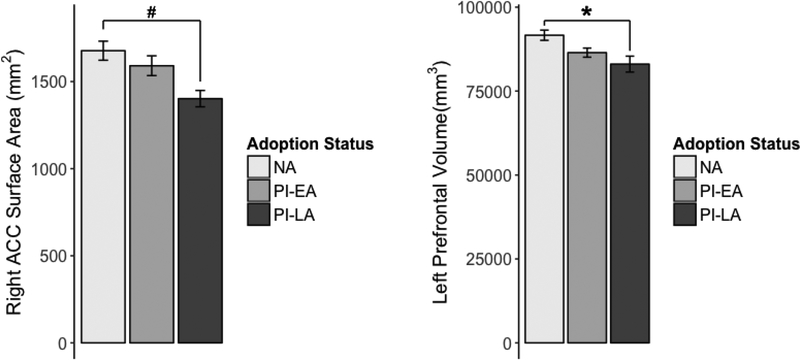
Group differences in brain structure in this subset of participants largely confirmed the previous results of Hodel et al. (2015) in the larger sample of PI youth. While there was a trendlevel difference between the aggregate PI group and the NA group in left prefrontal volume (F(1, 56) = 3.111, p = .083), this effect was driven by the late adopted youth in the sample. PI-LA children exhibited significantly smaller left prefrontal volume compared to control youth (uncorrected p = .043). Left prefrontal volume did not differ between PI-EA youth and PI-LA or NA youth (uncorrected p = .274 and p = .289, respectively; see Figure 2). Smaller right anterior cingulate surface area was also found in PI-LA vs. NA youth, though it only reached a marginal level of significance (uncorrected p = .055). These models included sex, age at assessment, and total intracranial volume as covariates.
Figure 2.
Mean right ACC surface area (A) and left prefrontal cortex volume (B) for early-, late- and non-adopted adolescents. Error bars show ±1 SE from the mean. PI-EA = early adopted (< 12 months), PI-LA = later adopted ≥ 12 months), NA = non-adopted, raised in biological family. * p < 0.05, # p < 0.1
3.6. Brain Structure and Task Performance
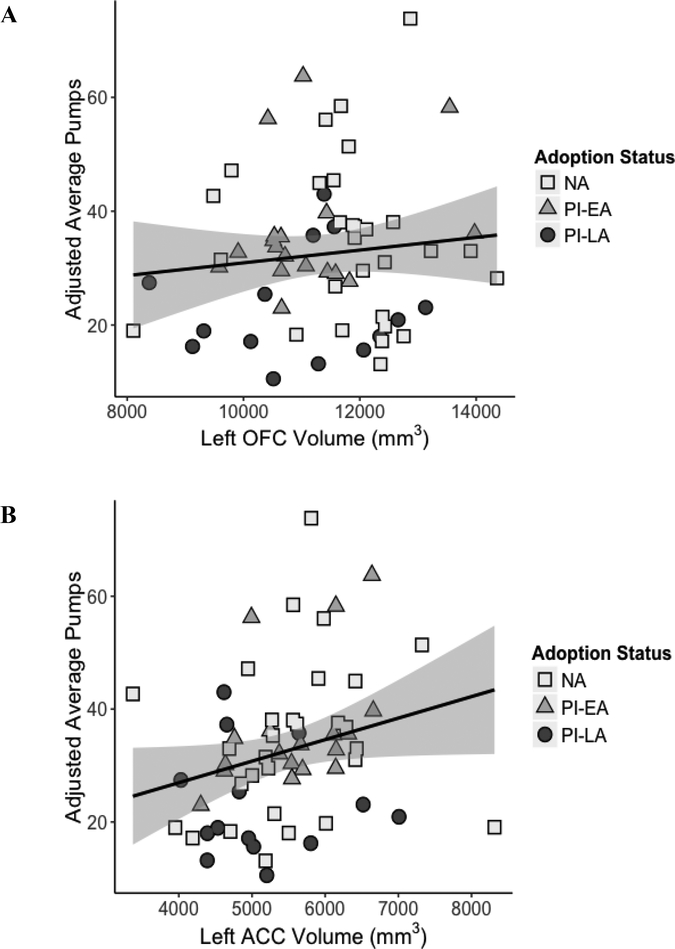
No association between brain structure and task performance was found when all participants were included. However, consistent with an effect of duration of institutional care, left OFC volume was significantly positively associated with adjusted average pumps on the BART-Y, while left ACC volume trended in the same direction but failed to reach significance at the corrected significance criteria (Bonferroni adjusted alpha = 0.0167; F(1,22) = 8.195, p = .009 and F(1,22) = 4.450, p < .047, respectively). These analyses controlled for age at adoption, age at assessment, sex, total intracranial volume, and IQ. Figure 3 depicts this relationship. Aggregate prefrontal, right OFC, and right ACC volumes did not significantly predict BART performance, nor were regional brain volumes significant predictors of IGT performance.
Figure 3.
Associations between BART-Y performance and the left OFC volume (p = .009) (A) and left ACC volume (p = .047) (B) of early-, late-, and non-adopted adolescents. Gray region represents ±1 SE from the mean. PI-EA = early adopted (<12 months), PI-LA = later adopted (≥12 months), NA = non-adopted, raised in biological family.
4. Discussion
Our results replicate and extend previous findings in the literature that suggest altered risky decision-making in youth who have experienced early adversity. Similar to Loman et al. (2014), we found that being adopted from institutional care after 12 months of age was associated with pressing less frequently on the BART-Y, and, extending the previous literature to a new task, with making fewer advantageous choices on a variant of the Iowa Gambling Task compared to non-adopted youth. Importantly, these differences were only apparent when comparing PI-LA adolescents to control youth. PI-EA youth and NA youth did not differ significantly from each other in either task. Our results show high concordance with those of previous studies suggesting that the effects of early life institutional care might be best understood in the context of the duration of such care. Previous research has demonstrated that EF deficits seem to be especially apparent in children adopted after 6 months (Colvert et al., 2008). Similarly, our results suggest that adoption after 12 months of age is associated with differences in risky decision-making. This finding, that risky decision-making behavior may differ in adolescence as a function of duration in an institutional care setting (or the age of transition out of the institution), is a novel one and suggests a new area of research in populations that have experienced ELS.
As expected, PI youth differed significantly from non-adopted participants in impulsivity and sensation seeking. Both PI-EA and PI-LA groups were significantly more impulsive than non-adopted youth, as indicated on a parent report measure. PI-LA youth also exhibited significantly lower sensation seeking scores when compared to the PI-EA and NA groups. What may be surprising, however, was the lack of association between scores of impulsivity or sensation seeking and measures from the behavioral tasks. The BART-Y is moderately correlated with a sensation seeking questionnaire different from the one used here (Lejuez et al., 2007), but was not related to sensation seeking in the prior study of international adoption that used it (Loman et al., 2014). Further decomposition of these effects in future analyses may reveal intra-group relationships that clarify the lack of significance in the whole group. For example, difficulty inhibiting automatic responses in combination with low sensation seeking may result in increased withdrawal in risky situations (e.g. fewer pumps on the BART-Y).
Buelow and Blaine (2015) reported similarities between the BART and a hot-decision making task, which might suggest the BART is a test of risk-taking propensity. Considering the BART-Y to be a measure of risk-taking propensity suggests that the marginal relationship observed between the BART-Y and sensation seeking seen in the PI-LA participants could indicate reduced motivation to gain rewards, resulting in a decreased likelihood of risk taking during uncertain hot-decision making scenarios. This interpretation would be consistent with previous findings in the PI literature which reported a failure by PI children to show accuracy improvements when a reward was available, compared to trials without the possibility of reward, in an incentivized anti-saccade task, an improvement that is typical in control children (Mueller et al., 2012). In contrast, Buelow and Blaine (2015) suggest that the Iowa Gambling Task involves a cooler cognitive process, due to the high learning demands of the task. In our study, group differences in performance on the IGT were no longer significant when IQ was entered into the model. It may be that the two-deck version of the IGT used in this study reflects individual differences in learning task conditions rather than risk-taking in adolescents. While this variant was used to ensure that participants would be able to improve their performance across a short imaging paradigm (Hartstra et al., 2010), it may be that the inclusion of the high- and low-payout rules placed greater emphasis on the learning of different rules across trials than a four deck IGT, subsequently emphasizing cold cognition over and above reward motivation. This interpretation is consistent with prior PI literature that has reported mixed results in similarly cold EF tasks such as the CANTAB (e.g. Beckett et al., 2010, Bos et al., 2009; Pollak et al., 2010)
Consistent with prior literature, smaller brain volumes were associated with the experience of early institutional care. Smaller prefrontal volumes have been reported in adolescents who spent time in institutional care (Merz et al., 2016). Similarly, within our subset of the larger sample, our findings mirrored those reported by our group previously (Hodel et al., 2015). Thus, youth who experience institutional care early in life exhibit long term changes in prefrontal brain structure. Among these changes may be decreased surface area in the anterior cingulate, which reached a trend level of significance in this study and is a topic for future inquiry. Following from the significant differences found in the prefrontal cortex of PI youth, we report significant relationships between these regions and the BART-Y. This relationship lends additional importance to prior research investigating brain structure in PI youth, suggesting that decreased brain volume associated with institutional care may have significant effects on risk taking behavior in adolescence.
The current study is limited in several ways. While the design provides a natural sample of time-limited early life stress, it does not account for experiences in the years between adoption and testing, prenatal differences, or variation in quality of care prior to, and following, institutional entry. Data from an increased number of time points following adoption would provide a more complete picture of brain development in adolescence following early institutional care. Additionally, while our results suggest group differences as a function of being earlier or later adopted, we are unable to draw concrete conclusions regarding thresholds for the duration of institutionalization or time of transition into adoptive homes. These sample characteristics constrain analyses to simple associations between brain structure and behavioral performance without directional specificity or causal attributions. Further, the single time-point nature of the data results in a number of statistical tests being performed when attempting to identify brain-behavior relationships following ELS. As such, the exploratory analyses presented here, some of which were not corrected for multiple comparisons, should be further investigated in future research to confirm the direction of effect.
Behaviorally, the relationship between the BART-Y and the Iowa Gambling Task is difficult to disentangle. While both tasks tap decision-making processes under variably risky conditions, it is unclear what, if any, construct overlap is present. Though the direction of effects in both tasks is consistent with prior literature, there are several possible conclusions that could be drawn regarding the effects of ELS. For example, the observed effects could be due to group differences in contingency learning, differences in hot vs. cold cognition, or differential sensitivities to reward or risk. Alternatively, poorer performance exhibited by PI youth on these decision-making tasks might reflect increased risk aversion in adolescence following early institutional care. The popping of the balloon during the BART-Y or loss of points during the IGT may have made PI-LA youth in particular warier when making choices during these tasks. Future studies may consider using tasks that explicitly test reward sensitivity (i.e. an incentivized anti-saccade task), in addition to the BART-Y and assessment of EF, to improve the specificity of data interpretation.
A final limitation of our study is the inclusion of PI youth with psychiatric diagnoses while excluding NA adolescents for the presence of psychiatric disorder. As argued above (section 2.1 Participants), we believe that the removal of PI youth with psychiatric diagnoses such as ADHD or anxiety disorders may remove variance of interest following ELS (e.g. impulsivity that may result in an ADHD diagnosis). However, the converse may also be true. Excluding NA adolescents for psychiatric diagnoses, in a developmental period during which rates of such disorders increase, may serve to inflate group differences. Due to this characteristic of our sample, the reported results should be interpreted with care and balance the benefit of considering phenotypic differences known to be associated with ELS against their potential for resulting in psychiatric diagnosis.
In summary, the current study extends our knowledge of the effects of early institutional care on risky decision-making. Behavioral results on a pair of risk taking tasks indicate that there are specific differences in risky decision-making between PI and NA adolescents. Further, this research corroborates prior associations between structural brain development and early institutional care, extending them to include brain-behavior relationships. Our analyses of the brain regions linked with risky decision-making showed significant associations between brain structure and behavioral performance. This finding supports the widely-held notion that poor outcomes following early life stress may be the consequence of biological pathways leading to cognitive deficits that persist across development. Finally, the current study provides support for the expectation that the timing and duration of ELS plays an important role in determining biological and cognitive outcomes.
Future research should continue to investigate the impact of ELS from multiple levels of analysis, including biological and cognitive outcomes. Investigations of brain structure should specifically test associations between ELS and cortical thickness and surface area in the hopes of increasing mechanistic specificity. Specifically, investigations of cortical thickness and surface area could elucidate the potential for genetic influences or sexual dimorphism in brain development following ELS (Panizzon et al., 2009; Raznahan et al., 2011). Additionally, a continuing emphasis on the timing, duration, and transition from stress has great potential for understanding models of ELS (Hodel, 2018). Another important consideration resulting from this work is whether or not the combination of increased impulsivity and decreased sensation seeking might be associated with negative behavioral outcomes later in adolescence. Research that explicitly addresses the behavioral outcomes that may result from altered risky decision-making in PI youth is an important next step toward understanding the impacts of ELS on subsequent development. Further study of risky decision-making should emphasize procedures that allow researchers to disentangle the effects of reward sensitivity, sensation seeking, risk aversion, and differences in probabilistic learning ability.
HIGHLIGHTS.
Youth adopted internationally exhibit more impulsivity than non-adopted youth
Lower levels of sensation seeking are found in adopted youth than non-adopted youth
Youth adopted after 12 months made fewer advantageous choices on a gambling task
Risky decision-making is positively associated with OFC volume in adopted youth
Acknowledgements
This research was supported by a NIMH Grant to MRG and KMT (P50-MH79513, Project II), a University of Minnesota Graduate School Fellowship Award (ASH), a Ruth L. Kirschstein National Research Service Award (T32-HD007151 to ASH), the University of Minnesota Center for Neurobehavioral Development (T32-MH73129), and the University of Minnesota Center for Magnetic Resonance Research (P41 RR008079, P41 EB015894, and P30 NS076408). The authors thank the Minnesota Supercomputing Institute (MSI) at the University of Minnesota for providing resources that contributed to the research results reported within this paper (http://www.msi.umn.edu), collaborators at the Center for Brain, Gene, and Behavioral Research Across Development located at the Sackler Institute for Developmental Psychobiology, as well as members of the Cognitive Development and Neuroimaging Lab (KMT) and the Human Developmental Psychobiology Lab (MRG) for assistance with participant recruitment, scheduling, and testing.
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bechara A, Damasio AR, Damasio H, & Anderson SW (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50(1–3), 7–15. 10.1016/0010-0277(94)90018-3 [DOI] [PubMed] [Google Scholar]
- Beckett C, Castle J, Rutter M, & Sonuga-Barke EJ (2010). Institutional deprivation, specific cognitive functions, and scholastic achievement: English and Romanian Adoptee (ERA) study findings. Monographs of the Society for Research in Child Development, 75(1), 125–142. 10.1111/j.1540-5834.2010.00553.x [DOI] [PubMed] [Google Scholar]
- Blair C, Raver CC, Granger D, Mills-Koonce R, & Hibel L (2011). Allostasis and allostatic load in the context of poverty in early childhood. Development and Psychopathology, 23(3), 845–857. 10.1017/S0954579411000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, & Razza RP (2007). Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development, 78(2), 647–663. 10.1111/j.1467-8624.2007.01019.x [DOI] [PubMed] [Google Scholar]
- Bos KJ, Fox N, Zeanah CH, & Nelson CA (2009). Effects of early psychosocial deprivation on the development of memory and executive function. Frontiers in Behavioral Neuroscience, 3(September), 1–7. 10.3389/neuro.08.016.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, & Ellis BJ (2005). Biological sensitivity to context: I. An evolutionarydevelopmental theory of the origins and functions of stress reactivity. Development and Psychopathology, 17(2), 271–301. 10.1017/S0954579405050145 [DOI] [PubMed] [Google Scholar]
- Buelow MT, & Blaine AL (2015). The assessment of risky decision making: A factor analysis of performance on the Iowa Gambling Task, Balloon Analogue Risk Task, and Columbia Card Task. Psychological Assessment, 27(3), 777–785. 10.1037/a0038622 [DOI] [PubMed] [Google Scholar]
- Carlson SM, & Wang TS (2007). Inhibitory control and emotion regulation in preschool children. Cognitive Development, 22(4), 489–510. 10.1016/j.cogdev.2007.08.002 [DOI] [Google Scholar]
- Casey BJ (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology, 66(1), 295–319. 10.1146/annurev-psych-010814-015156 [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews K, & Boyce WT (2002). Socioeconomic differences in children’s health: How and why do these relationships change With age? Psychol Bull, 128(2), 295–329. 10.1037//0033-2909.128.2.295 [DOI] [PubMed] [Google Scholar]
- Colvert E, Rutter M, Kreppner J, Beckett C, Castle J, Groothues C, … Sonuga-Barke EJS (2008). Do theory of mind and executive function deficits underlie the adverse outcomes associated with profound early deprivation?: Findings from the English and Romanian adoptees study. Journal of Abnormal Child Psychology, 36(7), 1057–1068. 10.1007/s10802-008-9232-x [DOI] [PubMed] [Google Scholar]
- Cowell RA, Cicchetti D, Rogosch FA, & Toth SL (2015). Childhood maltreatment and its effect on neurocognitive functioning: Timing and chronicity matter. Development and Psychopathology, 27(02), 521–533. 10.1017/S0954579415000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, & Dahl RE (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–650. 10.1038/nrn3313 [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Essex MJ, Boyce WT, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer DJ, & Group TMABW (2002). The confluence of mental, physical, social, and academic difficulties in middle childhood. II: Developing the MacArthur Health and Behavior Questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry, 41(5), 580–587. 10.1097/00004583-200205000-00016 [DOI] [PubMed] [Google Scholar]
- Evans GW, & Schamberg MA (2009). Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences, 106(16), 6545–6549. 10.1073/pnas.0811910106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, & Dale AM (1999). Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. NeuroImage, 9(2), 195–207. 10.1006/nimg.1998.0396 [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RBH, & Dale AM (1999). High-resolution inter-subject averaging and a surface-based coordinate system. Human Brain Mapping, 8(February 1999), 272–284. 10.1002/(SICI)1097-0193(1999)8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, … Dale AM (2004). Automatically Parcellating the Human Cerebral Cortex. Cerebral Cortex, 14(1), 11–22. 10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wenner JA, Thomas KM, Glatt CE, Mckenna MC, & Clark AG (2012). The brain-derived neurotrophic factor Val66Met polymorphism moderates early deprivation effects on attention problems. Development and Psychopathology, 24, 1215–1223. 10.1017/S095457941200065X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Kaufman J, Hodgdon HB, Masten CL, Jazbec S, Pine DS, & Ernst M (2006). Behavioral alterations in reward system function. Journal of the American Academy of Child & Adolescent Psychiatry, 45(9), 1059–1067. 10.1097/01.chi.0000227882.50404.11 [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, … Fischl B (2006). Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage, 32(1), 180–194. 10.1016/j.neuroimage.2006.02.051 [DOI] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, … Davidson RJ(2015). Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry, 77(4), 314–323. 10.1016/j.biopsych.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartstra E, Oldenburg JFE, Van Leijenhorst L, Rombouts SARB, & Crone EA (2010). Brain regions involved in the learning and application of reward rules in a two-deck gambling task. Neuropsychologia, 48(5), 1438–1446. 10.1016/j.neuropsychologia.2010.01.012 [DOI] [PubMed] [Google Scholar]
- Hodel AS (2018). Rapid infant prefrontal cortex development and sensitivity to early environmental experience. Developmental Review, 48(December 2017), 113–144. 10.1016/j.dr.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel AS, Hunt RH, Cowell R. a., Van Den Heuvel SE, Gunnar MR, & Thomas KM (2015). Duration of early adversity and structural brain development in ostinstitutionalized adolescents. NeuroImage, 105, 112–119. 10.1016/j.neuroimage.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Stellern SA, Schaefer C, Carlson SM, & Gunnar MR (2012). Associations between early life adversity and executive function in children adopted internationally from orphanages. Proceedings of the National Academy of Sciences, 109(Supplement_2), 17208–17212. 10.1073/pnas.1121246109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C (2011). Changes and challenges in 20 years of research into the development of executive functions. Infant and Child Development, 20, 251–271. 10.1002/icd [DOI] [Google Scholar]
- Humphreys KL, Lee SS, Telzer EH, Gabard-Durnam LJ, Goff B, Flannery J, & Tottenham N (2015). Exploration-exploitation strategy is dependent on early experience. Developmental Psychobiology, 57(3), 313–321. 10.1002/dev.21293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, … Phan KL (2013). Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences of the United States of America, 110(46), 18442–7. 10.1073/pnas.1308240110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauriola M, Panno A, Levin IP, & Lejuez CW (2014). Individual differences in risky decision making: A meta-analysis of sensation seeking and impulsivity with the Balloon Analogue Risk Task. Journal of Behavioral Decision Making, 27, 20–36. [Google Scholar]
- Lejuez CW, Aklin W, Daughters S, Zvolensky M, Kahler C, & Gwadz M (2007). Reliability and validity of the youth version of the Balloon Analogue Risk Task (BART-Y) in the assessment of risk-taking behavior among inner-city adolescents. Journal of Clinical Child and Adolescent Psychology, 36(1), 106–111. 10.1080/15374410709336573 [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, & Pedulla CM (2003). Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. Journal of Adolescence, 26(4), 475–479. 10.1016/S0140-1971(03)00036-8 [DOI] [PubMed] [Google Scholar]
- Li X, Lu ZL, D’Argembeau A, Ng M, & Bechara A (2010). The Iowa Gambling Task in fMRI images. Human Brain Mapping, 31(3), 410–423. 10.1002/hbm.20875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, & Casey BJ (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences of the United States of America, 106(3), 912–7. 10.1073/pnas.0807041106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loman MM, Johnson AE, Quevedo K, Lafavor TL, & Gunnar MR (2014). Risktaking and sensation-seeking propensity in postinstitutionalized early adolescents. Journal of Child Psychology and Psychiatry and Allied Disciplines, 55(10), 1145–1152. 10.1111/jcpp.12208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434–445. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, & Nelson CA (2014). Widespread reductions in cortical thickness following severe early-life deprivation: A neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biological Psychiatry, 76(8), 629–638. 10.1016/j.biopsych.2013.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, Harlé KM, Noble KG, & McCall RB (2016). Executive function in previously institutionalized children. Child Development Perspectives, 0(0), 1–6. 10.1111/cdep.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, McCall RB, Wright AJ, & Luna B (2013). Inhibitory control and working memory in post-institutionalized children. Journal of Abnormal Child Psychology, 41(6), 879–890. 10.1007/s10802-013-9737-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Hardin MG, Korelitz K, Daniele T, Bemis J, Dozier M, … Ernst M(2012). Incentive effect on inhibitory control in adolescents with early-life stress: An antisaccade study. Child Abuse and Neglect, 36(3), 217–225. 10.1016/j.chiabu.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Bos K, Gunnar MR, & Sonuga-Barke EJS (2011). The neurobiological toll of early human deprivation. Monogaphs of the Society for Research in Child Development, 76(4), 127–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, … Kremen WS (2009). Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex, 19(11), 2728–2735. 10.1093/cercor/bhp026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, … Gunnar MR (2010). Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Development, 81(1), 224–236. 10.1111/j.1467-8624.2009.01391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Korczykowski M, Pluta J, Hoang A, & Detre JA (2008). Neural correlates of voluntary and involuntary risk taking in the human brain: An fMRI Study of the Balloon Analog Risk Task (BART). NeuroImage, 42(2), 902–910. 10.1016/j.neuroimage.2008.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, … Giedd JN (2011). How Does Your Cortex Grow? Journal of Neuroscience, 31(19), 7174–7177. 10.1523/JNEUROSCI.0054-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo MF, Stokes GS, Lahey BB, Christ MAG, McBurnett K, Loeber R, … Green SM. (1993). A sensation seeking scale for children: Further refinement and psychometric development. Journal of Psychopathology and Behavioral Assessment, 15(2), 69–86. 10.1007/BF00960609 [DOI] [Google Scholar]
- Ségonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, & Fischl B (2004). A hybrid approach to the skull stripping problem in MRI. NeuroImage, 22(3), 1060–1075. 10.1016/j.neuroimage.2004.03.032 [DOI] [PubMed] [Google Scholar]
- Ségonne F, Pacheco J, & Fischl B (2007). Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Transactions on Medical Imaging, 26(4), 518–529. 10.1109/TMI.2006.887364 [DOI] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, & Nelson CA (2012). Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences of the United States of America, 109(32), 12927–32. 10.1073/pnas.1200041109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos a P., & Evans a C.(1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging, 17(1), 87–97. 10.1109/42.668698 [DOI] [PubMed] [Google Scholar]
- Toplak ME, Sorge GB, Benoit A, West RF, & Stanovich KE (2010). Decisionmaking and cognitive abilities: A review of associations between Iowa Gambling Task performance, executive functions, and intelligence. Clinical Psychology Review, 30(5), 562–581. 10.1016/j.cpr.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, & Casey BJ (2011). Elevated amygdala response to faces following early deprivation. Developmental Science, 14(2), 190–204. 10.1111/j.1467-7687.2010.00971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickett PK, Noll JG, & Putnam FW (2011). The impact of sexual abuse on female development: lessons from a multigenerational, longitudinal research study. Development and Psychopathology, 23(2), 453–76. 10.1017/S0954579411000174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CA, DelDonno S, & Killgore WDS (2014). The role of cognitive versus emotional intelligence in Iowa Gambling Task performance: What’s emotion got to do with it? Intelligence, 44(1), 112–119. 10.1016/j.intell.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JA, & Fisher PA (2013). Decision-making deficits among maltreated children. Child Maltreatment, 18(3), 184–194. 10.1177/1077559512467846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, & Guthrie D (2009). Institutional rearing and psychiatric disorders in Romanian preschool children. American Journal of Psychiatry, 166(7), 777–785. 10.1176/appi.ajp.2009.08091438 [DOI] [PubMed] [Google Scholar]
- Zuckerman M (1979). Sensation seeking: Beyond the optimal level of arousal. 1979. Erlbaum Associates Hillsdale, NJ. [Google Scholar]