Abstract
Background/Objectives
A negative association between cigarette smoking and celiac disease has been observed but results were inconsistent across the published studies. A meta-analysis was conducted with the aim to identify all studies that investigated this association and to summarize the results of those studies.
Methods
A comprehensive literature review was conducted utilizing MEDLINE and Embase databases through March 2018 to identify all cohort studies and case-control studies that compared the risk of celiac disease among current and/or former smokers versus never-smokers. Effect estimates from each study were extracted and combined together using the random-effect, generic inverse variance method of DerSimonian and Laird.
Results
A total of seven studies with 307,924 participants fulfilled the eligibility criteria and were included in the meta-analysis. The pooled analysis found a significantly decreased risk of celiac disease among current smokers compared with never-smokers with the pooled odds ratio (OR) of 0.52 (95% confidence interval (CI), 0.32–0.84; I2 86%). However, the risk of celiac disease among former smokers was not significantly different from never-smokers with the pooled OR of 1.10 (95% CI, 0.76–1.60; I2 of 73%).
Conclusions
A significantly decreased risk of celiac disease among current smokers compared with never-smokers was demonstrated in this meta-analysis.
Keywords: Gluten enteropathy, celiac disease, tobacco, smoking, meta-analysis
Key points
A negative association between cigarette smoking and celiac disease has been observed although the results were inconsistent across the studies.
This meta-analysis summarized all available data and demonstrated a significantly decreased risk of celiac disease among current smokers compared with never-smokers.
The risk of celiac disease among former smokers was not significantly different from never-smokers.
Effects of cigarette smoking on immune system and gut permeability are the likely biological explanations.
Introduction
Celiac disease or gluten-sensitive enteropathy is a common disease of the gastrointestinal tract with the estimated prevalence in the United States (US) and Europe of approximately 0.7–1%.1 Classic clinical manifestations of celiac disease include diarrhea, steatorrhea, flatulence and complications of malabsorption although milder form of the disease, including subclinical disease, is probably even more common and is often not recognized.2–4
Cigarette smoking is still a major public health concern in the US that directly and indirectly led to 448,865 deaths in 2014.5 It is one of the strongest risk factors for several diseases such lung cancer, chronic obstructive pulmonary disease, coronary artery disease and cerebrovascular disease.6 However, interestingly, studies have suggested that cigarette smoking is associated with a lower risk of some gastrointestinal diseases, including ulcerative colitis,7,8 and primary sclerosing cholangitis.9 A similar negative association between cigarette smoking and celiac disease has been observed as well although the results were inconsistent across the published studies.10–16 The current systematic review and meta-analysis was conducted with the aim of identifying all studies that investigated this association and to summarize the results of those studies.
Methods
Information sources and search strategy
A systematic literature search of the MEDLINE and Embase databases was carried out from inception to March 2018 to identify all original studies that investigated the association between cigarette smoking and celiac disease. The systematic literature review was independently conducted by three investigators (K.W., P.P., and P.U.) using a search strategy that included the terms for “celiac disease”, “gluten enteropathy”, “smoking”, “tobacco”, and “cigarette” as described in online supplementary data 1. No language limitation was applied. A hand-search was also performed on references of selected retrieved articles. The meta-analysis was conducted according to the PRISMA (preferred reporting items for systematic reviews and meta-analysis) statement, which is provided as online supplementary data 2.
Selection criteria
Studies that were eligible for this meta-analysis must be either case-control studies or cohort studies that investigated the relationship between smoking status and risk of celiac disease. For case-control studies, cases must be patients with celiac disease, and controls must be individuals without celiac disease. The exposure of interest must be history of cigarette smoking. Since we planned to use never-smokers as the reference group, eligible studies must report the number of current and/or former smokers as well as never smokers for both cases and controls. Alternatively, studies may report odds ratios (ORs) and 95% confidence interval (CIs) of having celiac disease among current and/or former smokers versus never-smokers. For cohort studies, cases must be current and/or former smokers whereas comparators must be never-smokers. One of the outcomes of the study must be the occurrence of celiac disease after the entrance of the cohort for both cases and comparators, which could be reported as relative risk (RR) or hazard ratio (HR) with 95% CI. Inclusion was not limited by study size. When more than one article utilizing the same database/cohort was available, only one study with the most comprehensive data/analyses was included.
Retrieved articles were reviewed for their eligibility independently by the same three investigators (K.W., P.P., and P.U.) with disagreements resolved by consensus. The Newcastle-Ottawa quality assessment scale was used to appraise the quality of the studies in three domains, which included the recruitment of participants, the comparability between the groups, as well as the ascertainment of the outcome of interest for cohort study, and the ascertainment of the exposure of interest for case-control study.17
Data abstraction
The investigators used a structured information collection form to extract the following data from each study: title of the study, name of the first author, publication year, year of the study, country where the study was conducted, number of subjects, demographics of subjects, methods used to identify and verify smoking status and celiac disease, adjusted effect estimates with 95% CI, and covariates that were adjusted in the multivariable analysis.
To ensure the accuracy, this data extraction process was independently performed by two investigators (K.W. and P.P.) and was reviewed by the senior investigator (P.U.).
Statistical analysis
Data analysis was performed using the Cochrane Collaboration’s Review Manager 5.3 software (London, UK). Adjusted point estimates from each study were consolidated by the generic inverse variance method of DerSimonian and Laird, which assigned the weight of each study for the pooled analysis based on its variance.18 As the outcome of interest was relatively uncommon, we planned to use the RR and HR of cohort studies as an estimate for the OR to calculate the pooled effect estimates with OR of case-control studies. In light of the high likelihood of high between-study variance because of different study designs, populations, and methodologies, a random-effect model was used. Cochran’s Q test and I2 statistic were used to quantify the between-study heterogeneity. A value of I2 of 0–25% represents insignificant heterogeneity, 26–50% represents low heterogeneity, 51–75% represents moderate heterogeneity, and more than 75% represents high heterogeneity.19 Funnel plot was used to assess for the presence of publication bias. A comprehensive Meta-analysis 3.0 software (Englewood, NJ) was used to perform Egger’s regression test for evaluation of publication bias.20 For all analyses, a P-value less than 0.05 was considered statistically significant.
Results
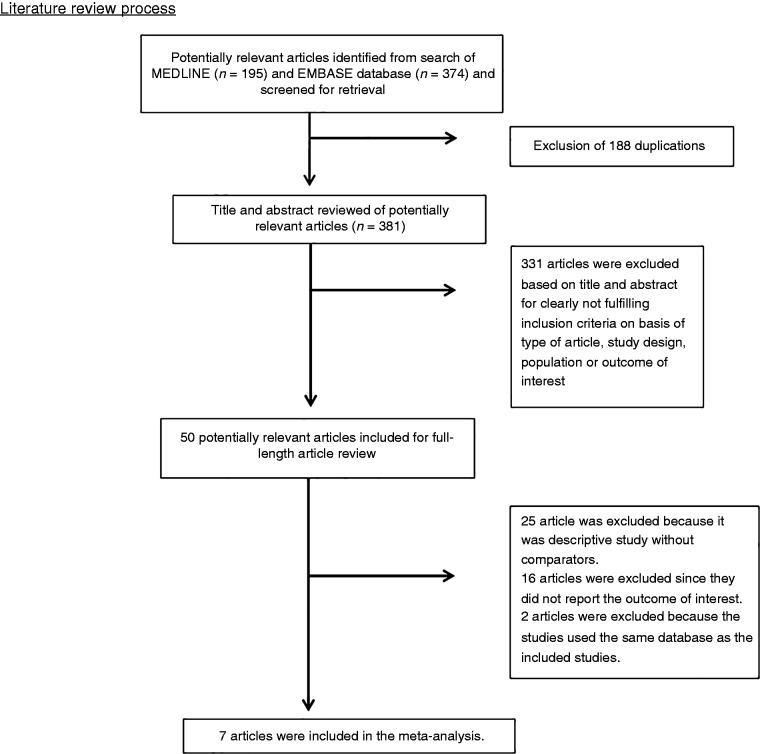
A total of 569 potentially eligible articles were identified using the described search strategy (195 from Medline and 374 from EMBASE). After the exclusion of 188 duplicated articles, titles and abstracts of 381 unique articles were reviewed. Another 331 articles were excluded at this stage since they were case reports, case series, correspondence, review articles, in vitro studies, animal studies or interventional studies, leaving 50 articles for full-text review. Of these, 16 were excluded after full-length review as they did not report the outcome of interest, while 25 articles were excluded since they were descriptive studies without comparative analysis. A total of nine studies fulfilled the eligibility criteria but four studies utilized the same databases,14/21;10/22 and only the studies with the most comprehensive data were included.10,14 Finally, seven studies with 307,924 participants were included in the meta-analysis.10–16 The literature retrieval, review and selection process are shown in Figure 1. The characteristics and quality appraisal of the included studies are presented in Table 1. Inter-rater agreement for the quality assessment using the Newcastle-Ottawa scale was high with a kappa statistic of 0.69.
Figure 1.
Literature review process.
Table 1.
Main characteristics of the studies included in this meta-analysis.
| Study |
Williams et al.16 |
Todi et al.11 |
Patel et al.13 |
Vazquez et al.15 |
| Country | UK | UK | US | Argentina |
| Study design | Case-control study | Case-control study | Case-control study | Case-control study |
| Year | 1988 | 1997 | 2001 | 2001 |
| Total number of participants | 157 (76 cases and 81 controls) | 444 (330 cases and 114 controls) | 328 (82 cases and 246 controls) | 261 (87 cases and 174 controls) |
| Participants | Cases: Cases were adult patients with celiac disease who were prospectively recruited from the outpatient clinic of the East Birmingham Hospital, UK. Controls: Controls were individuals without celiac disease who were prospectively recruited from the same outpatient clinic of the same hospital. | Cases: Cases were adult patients with celiac disease who were prospectively recruited from the outpatient clinic of the Castle Hill Hospital, UK. Controls: Controls were individuals without celiac disease who were prospectively recruited from the accident and emergency department of the same hospital. Controls were sex and age-matched to cases. | Cases: Cases were adult patients with celiac disease identified form the database of the Mayo Clinic, Rochester, MN, from January 1993 to June 1998. Controls: Controls were individuals without celiac disease identified from the database of the same hospital at the same period. Controls were sex, age calendar year and geography-matched to cases. | Cases: Cases were consecutive adult patients with celiac disease prospectively recruited from Gastroenterology Hospital of Buenos Aires, Argentina, from July 1997 to December 1997. Controls: Controls were individuals without celiac disease who were prospectively recruited from outpatient clinic of the same hospital at the same period. Controls were sex and age-matched to cases. |
| Determination of smoking status | Smoking status were obtained from direct interview | Smoking status were obtained from direct interview | Smoking status was obtained from health questionnaires | Smoking status was obtained from health questionnaires |
| Diagnosis of celiac disease | Celiac disease was diagnosed based on small bowel biopsies showing villous atrophy. | N/A | Celiac disease was diagnosed based on small bowel biopsies showing villous atrophy along with clinical or histological response to gluten-free diet or positive serologies | Celiac disease was diagnosed based on small bowel biopsies showing severe mucosal atrophy, serological test and clinical or histological response to a gluten-free diet |
| Confounder adjusted in multivariate analysis | None | None | Age and date of visit | None |
| Quality assessment (Newcastle-Ottawa scale) | Selection: 3 Comparability: 1 Exposure: 2 | Selection: 3 Comparability: 2 Exposure: 2 | Selection: 3 Comparability: 2 Exposure: 2 | Selection: 3 Comparability: 2 Exposure: 2 |
| Study |
Suman et al.14 |
Austin et al.10 |
Ludvigsson et al.12 |
|
| Country | UK | UK | Sweden | |
| Study design | Case-control study | Case-control study | Retrospective cohort study | |
| Year | 2003 | 2009 | 2014 | |
| Total number of participants | 414 (138 cases and 276 controls) | 598 (370 cases and 228 controls) | 305,722 | |
| Participants | Cases: Cases were patients aged between 18 and 70 years who were diagnosed with celiac disease at the Poole Hospital NHS Trust, UK from 1990 to 2000. Controls: Controls were individuals without celiac disease recruited from ear-nose-throat or orthopedic outpatient clinics of the same center from 2000 to 2001. Controls were sex and age-matched to cases. | Cases: Cases were patients aged 16 years and over with celiac disease identified from the registries of the University Hospital, Nottingham, and Derbyshire Royal Infirmary, Derby, UK. Controls: Controls were individuals without celiac disease randomly identified from the general practitioner registry of the Nottingham Family Health Services. Controls were sex and age-matched to cases. | Participants were construction workers who underwent annual health examination provided by the Swedish national occupational health service from 1971 to 1993. Participants were categorized into current, former and never smokers at the entrance of cohort. Individuals with history of celiac disease prior to the first examination were excluded. | |
| Determination of smoking status | Smoking status were obtained from direct interview | Smoking status was obtained from postal health questionnaires. National smoking data were also used as a second comparator to allow for possible bias and sampling error as a consequence of a low response rate. | Smoking status was obtained from health questionnaires and a face-to-face interview by dedicated nurses. | |
| Diagnosis of celiac disease | Celiac disease was diagnosed from clinical presentation, histology of small bowel biopsies consistent with celiac disease and clinical or histological response to gluten-free diet | Celiac disease was diagnosed based on small bowel histology showing villous atrophy and clinical or histological response to gluten withdrawal | Celiac disease was diagnosed based on duodenal and jejunal biopsies showing villous atrophy. Data of the biopsies were obtained from 28 pathology department across the Sweden from October 2006 to February 2008 | |
| Confounder adjusted in multivariate analysis | None | None | Age, sex and decade | |
| Quality assessment (Newcastle-Ottawa scale) | Selection: 3 Comparability: 2 Exposure: 2 | Selection: 3 Comparability: 2 Exposure: 2 | Selection: 3 Comparability: 1 Outcome: 3 | |
ICD: International classification of disease; MONICA: multinational monitoring of trends and determinants in cardiovascular disease; IgA EMA: immunoglobulin A class antiendomysial antibody; IgA AGA: immunoglobulin A class antigliadin antibody; anti-EMA: antiendomysial antibody; anti-tTG: antitransglutaminase antibody.
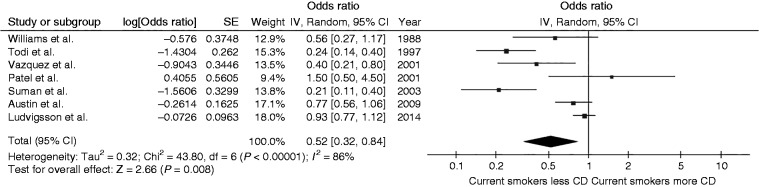
Risk of celiac disease among current smokers
The pooled analysis found a significantly decreased risk of celiac disease among current smokers compared with never-smokers with the pooled OR of 0.52 (95% CI, 0.32–0.84) as demonstrated in Figure 2. The between-study heterogeneity was high with an I2 of 86%.
Figure 2.
Forest plot of the risk of celiac disease among current smokers versus never-smokers.
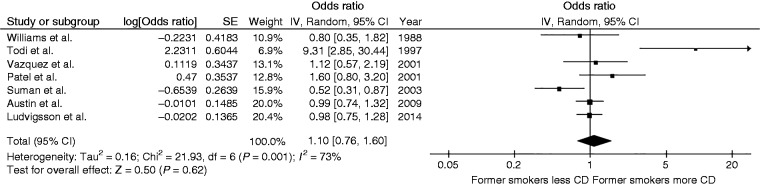
Risk of celiac disease among former smokers
The risk of celiac disease among former smokers was not significantly different from never-smokers with the pooled OR of 1.10 (95% CI, 0.76–1.60) as demonstrated in Figure 3. The between-study heterogeneity was moderate, with an I2 of 73%.
Figure 3.
Forest plot of the risk of celiac disease among former smokers versus never-smokers.
Sensitivity analysis
A sensitivity analysis was conducted by including the studies by Thomason et al.22 and Snook et al.21 that were excluded because of the concern over potential patient duplication with the studies by Suman et al.14 and Austin et al.10, respectively, instead of those two studies to investigate if the decision to include one of the two studies with potential overlap had a significant effect on the outcomes of this meta-analysis. We found that the outcomes of this sensitivity analysis were similar to the original analysis that a significantly decreased risk of celiac disease among current smokers compared with never-smokers was observed (pooled OR 0.46, 95% CI 0.26–0.81, I2 87%) whereas the risk of celiac disease among former smokers was not significantly different from never-smokers (pooled OR 1.12, 95% CI 0.76–1.66, I2 68%).
Evaluation for publication bias
A funnel plot was constructed based on effect estimate and accuracy of each study to assess for the presence of publication bias. The funnel plots for current smokers versus never-smokers analysis and former smokers versus never-smokers analysis are shown as Figures 4 and 5, respectively. Both funnel plots were relatively symmetric and, therefore, were not suggestive of the presence of publication bias. In addition, there was no evidence of publication bias by Egger’s regression test with the p-value of 0.13 and 0.35 for current smokers versus never-smokers analysis and former smokers versus never-smokers analysis, respectively.
Figure 4.
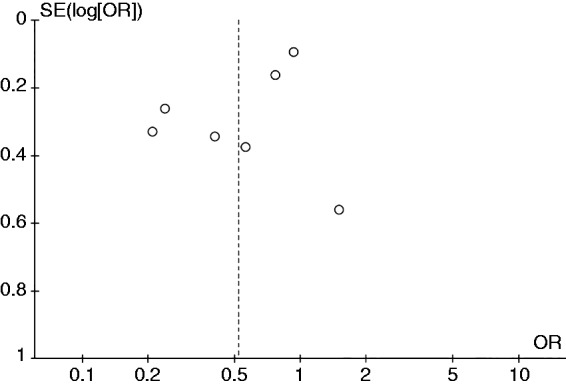
Funnel plot of the risk of celiac disease among current smokers versus never-smokers.
Figure 5.
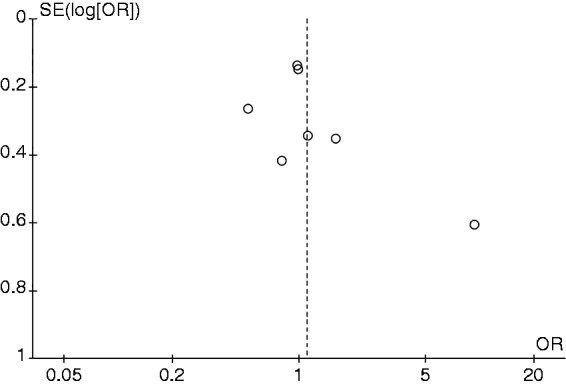
Funnel plot of the risk of celiac disease among former smokers versus never-smokers.
Discussion
The current study is the first systematic review and meta-analysis to summarize all available studies on the risk of celiac disease among smokers. The pooled analysis found an almost 50% decreased risk of celiac disease among current smokers compared with never-smokers. However, a similar risk reduction was not observed among former smokers. This observation may suggest that cigarette smoking could be a protective environmental factor against the development of celiac disease although the effect appears to attenuate after smoking cessation. The reasons as to why cigarette smoking may decrease the risk of celiac disease is not well understood but there are few possible explanations.23
The first explanation is based on the immunomodulatory effects of cigarette smoking on both cellular and humoral immune function.24,25 Since activation of T cells in the gut mucosa by gliadin is the pivotal step in the pathogenesis of the mucosal inflammation of celiac disease,2 26,27 it is possible that smoking may alter the capacity of T cells to respond to gliadin and, thus, lower the chance of development of celiac disease. Some studies also suggested that cigarette smoking may decrease the expression of tissue transglutaminase enzyme,28,29 an enzyme that plays an important role in the amplification of response of T cells to gliadin through conversion of glutamine residues into glutamic acid, which creates deamidated gluten peptides that can bind efficiently to human leukocyte antigen (HLA)-DQ2/-DQ8 presented on antigen-presenting cells.30
The second explanation is related to the fact that cigarette smoking has been shown to reduce gut permeability.31 As epithelial barrier impairment resulting in increased gut permeability of immunogenic gluten peptides is considered one of the early events in the pathogenesis of celiac disease,30 smoking may help lowering the risk of celiac disease by decreasing the permeability of the gut.
Although the literature review was comprehensive, and the included studies were of high quality as reflected by the quality assessment scores, we acknowledge that the current study has some limitations.
First, between-study statistical heterogeneity was not low in both analyses. We suspect that the differences in the study populations, designs, and methodologies were responsible for the variation. Second, confounders may play a role in this meta-analysis as it is a meta-analysis of observational studies and most of the included studies did not adjust their effect estimates for potential confounders (except for age and sex). Third, almost all of the included studies were conducted in the US and Europe. Therefore, generalizability of the results to other regions around the globe could be limited.
In summary, this systematic review and meta-analysis found a significantly decreased risk of celiac disease among current smokers compared with never-smokers. However, a similar risk reduction was not observed among former smokers.
Supplemental Material
Supplemental material for Cigarette smoking and risk of celiac disease: A systematic review and meta-analysis by Karn Wijarnpreecha, Susan Lou, Panadeekarn Panjawatanan, Wisit Cheungpasitporn, Surakit Pungpapong, Frank J. Lukens and Patompong Ungprasert in United European Gastroenterology Journal
Authors' contributions
All authors had access to the data and a role in writing the manuscript.
Funding
None
Conflict of interest statement for all authors
We do not have any financial or non-financial potential conflicts of interest.
Authors’ contributions
All authors had access to the data and a role in writing the manuscript.
Disclosure
The authors have no commercial associations that might be a conflict of interest about this article. No funding support for this article.
Informed consent
Informed consent is not applicable for this study as it did not directly involve human subjects.
Ethics approval
The need for ethic approval for this study was exempted as it did not directly involve human subjects or animals.
References
- 1.Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol 2012; 107: 1538–1544. quiz 1537, 1545. [DOI] [PubMed] [Google Scholar]
- 2.Barker JM, Liu E. Celiac disease: pathophysiology, clinical manifestations, and associated autoimmune conditions. Adv Pediatr 2008; 55: 349–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rostami Nejad M, Hogg-Kollars S, Ishaq S, et al. Subclinical celiac disease and gluten sensitivity. Gastroenterol Hepatol Bed Bench 2011; 4: 102–108. [PMC free article] [PubMed] [Google Scholar]
- 4.Tursi A, Giorgetti G, Brandimarte G, et al. Prevalence and clinical presentation of subclinical/silent celiac disease in adults: an analysis on a 12-year observation. Hepatogastroenterology 2001; 48: 462–464. [PubMed] [Google Scholar]
- 5.Ma J, Siegel RL, Jacobs EJ, et al. Smoking-attributable mortality by State in 2014, U.S. Am J Prev Med 2018; 54: 661–670. [DOI] [PubMed] [Google Scholar]
- 6.Saha SP, Bhalla DK, Whayne TF Jr, et al. Cigarette smoke and adverse health effects: An overview of research trends and future needs. Int J Angiol 2007; 16: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas GA, Rhodes J, Green JT. Inflammatory bowel disease and smoking—a review. Am J Gastroenterol 1998; 93: 144–149. [DOI] [PubMed] [Google Scholar]
- 8.Zhai H, Huang W, Liu A, et al. Current smoking improves ulcerative colitis patients' disease behaviour in the northwest of China. Prz Gastroenterol 2017; 12: 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wijarnpreecha K, Panjawatanan P, Mousa OY, et al. Association between smoking and risk of primary sclerosing cholangitis: A systematic review and meta-analysis. United Eur Gastroenterol J 2018; 6: 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Austin AS, Logan RF, Thomason K, et al. Cigarette smoking and adult coeliac disease. Scand J Gastroenterol 2002; 37: 978–982. [DOI] [PubMed] [Google Scholar]
- 11.Todi D, Tsai H. Coeliac disease is associated with non-smoking and cessation of smoking. Gut 1997; 40(Suppl1): A11–A11. [Google Scholar]
- 12.Ludvigsson JF, Nordenvall C, Jarvholm B. Smoking, use of moist snuff and risk of celiac disease: a prospective study. BMC Gastroenterol 2014; 14: 120–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel AH, Loftus EV Jr, Murray JA, et al. Cigarette smoking and celiac sprue: a case-control study. Am J Gastroenterol 2001; 96: 2388–2391. [DOI] [PubMed] [Google Scholar]
- 14.Suman S, Williams EJ, Thomas PW, et al. Is the risk of adult coeliac disease causally related to cigarette exposure? Eur J Gastroenterol Hepatol 2003; 15: 995–1000. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez H, Smecuol E, Flores D, et al. Relation between cigarette smoking and celiac disease: evidence from a case-control study. Am J Gastroenterol 2001; 96: 798–802. [DOI] [PubMed] [Google Scholar]
- 16.Williams AJ, Asquith P, Stableforth DE. Susceptibility to tuberculosis in patients with coeliac disease. Tubercle 1988; 69: 267–274. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snook JA, Dwyer L, Lee-Elliott C, et al. Adult coeliac disease and cigarette smoking. Gut 1996; 39: 60–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomason K, West J, Logan RF, et al. Fracture experience of patients with coeliac disease: a population based survey. Gut 2003; 52: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasad S, Thomas P, Nicholas DS, et al. Adult endomysial antibody-negative coeliac disease and cigarette smoking. Eur J Gastroenterol Hepatol 2001; 13: 667–671. [DOI] [PubMed] [Google Scholar]
- 24.Holt PG. Immune and inflammatory function in cigarette smokers. Thorax 1987; 42: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol 2002; 2: 372–377. [DOI] [PubMed] [Google Scholar]
- 26.Biagi F, Parnell ND, Thomas PD, et al. A new model for the pathogenesis of celiac disease. Gastroenterology 1999; 116: 1277–1278. [DOI] [PubMed] [Google Scholar]
- 27.Godkin A, Jewell D. The pathogenesis of celiac disease. Gastroenterology 1998; 115: 206–210. [DOI] [PubMed] [Google Scholar]
- 28.Berntorp K, Ekman M, Berntorp E. Cigarette smoke impairment of human lymphocyte function by inhibition of transglutaminase. J Int Med 1989; 226: 73–79. [DOI] [PubMed] [Google Scholar]
- 29.Roth WJ, Chung SI, Raju L, et al. Macrophage transglutaminases: characterization of molecular species and measurement of enzymatic modification by cigarette smoke components. Adv Exp Med Biol 1988; 231: 161–173. [DOI] [PubMed] [Google Scholar]
- 30.Cukrowska B, Sowinska A, Bierla JB, et al. Intestinal epithelium, intraepithelial lymphocytes and the gut microbiota—Key players in the pathogenesis of celiac disease. World J Gastroenterol 2017; 23: 7505–7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prytz H, Benoni C, Tagesson C. Does smoking tighten the gut? Scand J Gastroenterol 1989; 24: 1084–1088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Cigarette smoking and risk of celiac disease: A systematic review and meta-analysis by Karn Wijarnpreecha, Susan Lou, Panadeekarn Panjawatanan, Wisit Cheungpasitporn, Surakit Pungpapong, Frank J. Lukens and Patompong Ungprasert in United European Gastroenterology Journal