Abstract
Background
As of now, no established model for the transition from childhood to adulthood in coeliac disease exists. We aim to describe the dietary compliance and the quality of life of a population of young coeliac disease patients around transition age and to develop a practical tool (TRANSIT-CeD disk) which can be used during the transition process effectively to transmit young adults to the adult healthcare giver.
Methods
We consecutively recruited all coeliac disease patients with a paediatric diagnosis (≤16 years) and aged between 9 and 20 years at the time of the study. The patients were asked to answer some questions concerning their adherence to a gluten-free diet, knowledge about coeliac disease, relationship with healthcare givers and quality of life.
Results
We included 58 subjects, mean age 14.5 ± 3.6 years, of which 62% were girls/young women. We observed that dietary compliance was independently and positively related to age at diagnosis and coeliac disease knowledge, while quality of life was only independently and positively related to coeliac disease knowledge.
Conclusion
A good coeliac disease knowledge is positively related to dietary compliance and quality of life. With the help of the TRANSIT-CeD disk we proposed, paediatricians and adult gastroenterologists can follow the patients during the transition and identify some points to work on.
Keywords: Compliance, quality of life, gluten-free diet, coeliac disease, AYA (adolescents young adults)
Key summary
Summarise the established knowledge on this subject
The transition from childhood to adulthood is a crucial phase in the management of all chronic diseases.
The management of the disease is often guided by the parents and caregivers of the adolescents.
At the moment no established model for transition in coeliac disease exists.
What are the significant and/or new findings of this study?
Compliance and quality of life improve with a better knowledge of the disease.
Coeliac disease patients diagnosed later in life are better able to follow the gluten-free diet independently of their knowledge of the disease.
With the help of a practical tool, paediatricians and adult gastroenterologists can follow the patients during the transition and identify some points to work on.
Introduction
The transition from childhood to adulthood is a crucial phase in the management of all chronic diseases,1 which should call for active collaboration between patients, parents and physicians, rather than being just a transfer of duties.2 The parents and caregivers of the adolescents, who may drop out of medical assistance once they come of age, often guide the management of the disease. The Child and Adolescent Health Measurement Initiative estimates that every year in the USA 1 million adolescents with special needs pass into adult healthcare. A vast majority of them is likely not to be ready for the process (http://www.cahmi.org/).
Coeliac disease (CeD) is currently one of the most common chronic diseases affecting adolescents/young adults (AYAs) and for which a transition to adult healthcare should be provided.
At the moment no established model for transition in CeD exists.3 Most of the reports in the literature on transition care are descriptive, with recommendations based on clinical experience. In the absence of evidence-based recommendations, each institution bases its model on local factors.
In Europe, children and adolescents’ compliance with the gluten-free diet (GFD) varies a lot and occasional lapses/dietary transgressions are quite common.4–8 AYAs report less compliance with the GFD compared to younger children, in particular at social gatherings.9 The reasons/conditions for the reported low compliance to the GFD are pressure from peers and the stigma of ‘being different from others’ experienced on school trips or at social gatherings, such as parties or going out with friends.10, 11 Moreover, in childhood and early adolescence, the GFD is managed by caregivers both at home and school, with very little involvement from the child with CeD. That is why during adolescence the GFD should be managed jointly by caregivers and AYAs, who will gradually become responsible for buying food and also preparing meals, taking over the caregivers’ job.3 In fact, according to Kurppa et al. only age at diagnosis and age at present were major determinants of adherence.12 Poor dietary adherence has been associated with a poor quality of life (QoL), but whether one causes the other remains unknown.13–16 In fact, a recent long-term longitudinal study suggested that subsequent deterioration of the QoL was associated with a lack of dietary adherence.17 Moreover, adolescents do not all share the same knowledge and self-management of the disease and therefore they might be in need of different transition processes.
The transition process should be based mainly on the collaboration between paediatrician/paediatric gastroenterologist and general practitioner/adult gastroenterologist who should take care of the adolescents through this challenging phase. For this to happen the previous literature recommends more than one outpatient visit, although the presence of both specialists cannot always be guaranteed during each visit at the clinical practice.18
Therefore, a rapid and practical tool to evaluate dietary compliance, QoL, CeD knowledge and self-management of CeD (TRANSIT-CeD disk) should be developed to track the patient’s progress and be used easily during the visit.
The present study aims at defining which factors influence dietary compliance and QoL, and providing a practical visual tool, a disk with scales and scores to assess the global status of the AYA at the time of transmission to the adult healthcare giver.
Material and methods
Study population
The study was designed as a prospective observational study. We consecutively recruited all coeliac patients with a paediatric diagnosis (≤16 years) and aged between 9 and 20 years at the time of the study, who visited the adult and paediatric coeliac centres of the AOU San Giovanni di Dio e Ruggi di Aragona (Salerno) from November 2016 to July 2017. All patients had been on a GFD at the time of the study for at least two years, but had had positive CeD-specific serology (anti-transglutaminase IgA and anti-endomysial IgA in the absence of IgA deficiency) and, when requested by the ESPGHAN criteria, positive intestinal biopsy, grade 3 according to Marsh–Orberuber or a B-Co grade histology according to Corazza–Villanacci classification before starting the GFD.19–21 None of the patients had undergone a transition protocol.
Data collection
Demographics and clinical information were collected during the visit with data recorded in a dedicated database. We performed a clinical interview, assessing symptoms reported by the AYA, and administered the questionnaires to quantify the AYA’s responses on specific domains. Data on antibody antitransglutaminase IgA at the time of the study and the years of GFD were collected.
Questionnaires
The patients were asked to answer some questionnaires exploring the following four domains: knowledge about CeD; relationship with healthcare givers; adherence to GFD; and QoL.
CeD knowledge
Based on the previous literature22–24 we designed an ad hoc five-item questionnaire testing the patient’s knowledge of CeD that included questions concerning:
the definition of CeD (able to define–unable to define);
the definition of gluten (able to define–unable to define);
the duration of disease (the answer is known–unknown answer);
the ability to read a food label identifying sources of gluten (able to recognise–unable to recognise); and
the ability to identify food containing gluten (able to recognise–unable to recognise pictures of gluten-containing food in a list of 10 that are commonly consumed by AYAs).
For the first four answers the score was 1 for the correct answers and 0 for the wrong ones. For the safe food identification, the score 1 was attributed to the correct identification of seven out of the 10 listed foods.
The sum of the scores makes the knowledge score as follows: 0, not at all aware; 1, slightly aware; 2, somewhat aware; 3, moderately aware; 4, extremely aware.
Self-management of CeD
On the basis of already published standardised questionnaires, such as Am I on Trac25 and STARx,26 we designed the CeD Transit (scoring 0 to 16) scale with four questions exploring: ability to book an appointment with the doctor; simple medical language comprehension (two questions); and active participation to the medical visit.
I can make an appointment with my doctor if I need one;
I am able to understand what my doctor is saying;
I personally ask questions of my doctor; and
I go to my doctor by myself.
Scoring as follows: 0, never; 1, rarely; 2, at times; 3, often; 4, always.
We then classified five levels of patient’s self-management based on the score as follows: 0, not confident; 1–4, somewhat not confident; 5–8, neither confident nor not confident; 9–12, somewhat confident; 13–16, very confident.
The GFD compliance score
The GFD compliance (scoring from 0 to 4) was assessed by asking the patient to answer the question: ‘Do you ever voluntarily eat gluten-containing food?’.
Patients were invited to answer one of the following five possibilities: often, many lapses (0); at times (1); on special occasions (2); rarely (3); never (4).
Quality of life
Quality of life (scoring from 0 to 4) was assessed by asking the patient to summarise his/her QoL perception in the previous week through the question: ‘How have you felt in the last week?’
Patients were invited to answer one of the following five possibilities: very poor (0); poor (1); moderately well (2); well (3); very well (4).
The TRANSIT-CeD disk
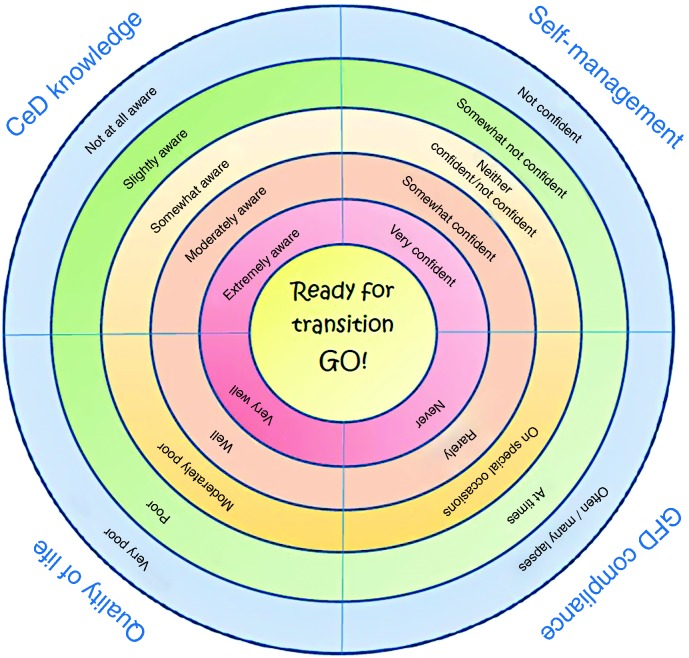
The TRANSIT-CeD disk summarises the scores of the principal questionnaires we used (Figure 1). The disk can be filled in during the visit that should start the transition process and can be used any time, until the first/second visit by the adult healthcare giver ends the process.
Figure 1.
TRANSIT-CeD disk summarising the 1–5 scores of the main questionnaires and visual analogue scale.
Statistical analysis
Categorical and continuous variables were expressed as frequency and mean and standard deviation (SD), respectively. Differences in frequencies between groups were calculated using the χ2 test. First, univariate linear regression models were used to assess whether demographic and clinical variables (age at test, time on GFD, age at diagnosis, self-management of the disease, CeD knowledge) were related to the QoL or compliance. Second, full multivariate models were fitted that included all covariates, significantly related to the QoL or compliance identified in univariate analysis using a statistical significance cut-off level of P < 0.05. All tests were two-tailed with the significance level set at P < 0.05. STATA 11 software was used to analyse the data.
Results
During the study period we recruited 65 eligible patients. Three parents did not sign the informed consent, two AYAs were not sufficiently autonomous to answer the questionnaires, and for another two of them we lacked full information on the diagnosis. We therefore included 58 patients, mean age 14.5 ± 3.6 years (range 9–20.9), of which 62% were girls/young women. All AYAs had been on GFD from 6.2 ± 4.8 years. However, 12 (20.6%) patients had positive serum tissue transglutaminase at the time of the evaluation. Fifty per cent of them had classic symptoms at the time of diagnosis (Table 1). We observed that none of the patients were not confident with the management of the disease, while 48% of the patients were neither confident nor not confident (Table 2). None reported ‘often’ or ‘at times’ lapses of the GFD, 21 of them declared ‘never’ having lapsed although five of them had a positive transglutaminase IgA. Most of them were somewhat/moderately aware of the disease, and half of them declared to have been moderately well in the previous week (Table 2). Compliance was positively related to age at test, age at diagnosis and CeD knowledge (Table 3). However, when we designed a multivariate linear regression model, only age at diagnosis (coefficient 0.045, 95% confidence interval (CI) 0.006–0.08) and CeD knowledge (0.19, 95% CI 0.032–0.36) were independently and positively related to compliance with GFD. Therefore, the better the knowledge and the older the patient at the time of diagnosis the better the compliance.
Table 1.
Characteristics of the study population.
| Number of patients | 58 |
|---|---|
| Age (years) at time of questionnaires, mean ± SD | 14.5 ± 3.6 (range 9–20.9) |
| Girls/young women (%) | 36 (62.1) |
| Age (years) at diagnosis, mean ± SD | 8.3 ± 5.1 |
| Years of GFD, mean ± SD | 6.2 ± 4.8 |
| a-Ttg IgA positive at time of questionnaires (%) | 12 (20.7) |
| Symptoms at diagnosis | |
| • Asymptomatic (%) | 12 (20.7) |
| • Classic presentation (%) | 31 (53.4) |
| • Non-classic presentation (%) | 15 (25.9) |
| Mother’s education | |
| • Secondary school (%) | 13 (22.4) |
| • High school degree/college (%) | 45 (77.6) |
| Father’s education | |
| • Secondary school (%) | 24 (41.4) |
| • High school degree/college (%) | 34 (58.6) |
GFD: gluten-free diet; a-Ttg: antibody antitransglutaminase.
Table 2.
The results of the questionnaires.
| N patients (%) | |
|---|---|
| Self-management | |
| Not confident | 0 |
| Somewhat not confident | 17 (29.3) |
| Neither confident/not confident | 28 (48.3) |
| Somewhat confident | 10 (17.2) |
| Very Confident | 3 (5.2) |
| Compliancea | |
| Often, many lapses | 0 |
| At times | 0 |
| On special occasions | 16 (27.6) |
| Rarely | 21 (36.2) |
| Never | 21 (36.2) |
| Knowledge | |
| Not at all aware | 5 (8.6) |
| Slightly aware | 8 (13.8) |
| Somewhat aware | 14 (24.1) |
| Moderately aware | 20 (34.5) |
| Extremely aware | 11 (19) |
| Health-related quality of lifeb | |
| Very poor | 0 |
| Poor | 8 (13.8) |
| Moderately well | 29 (50) |
| Well | 13 (22.4) |
| Very well | 8 (13.8) |
Do you ever voluntarily eat gluten-containing food?
How have you felt in the last week?
Table 3.
The linear regression analysis of compliance and quality of life versus several parameters.
| Unadjusted coefficient | Unadjusted coefficient | |
|---|---|---|
| Compliance | Quality of life | |
| Age at test | 0.062 (0.004–0.119)a | 0.05 (–0.1–0.11) |
| Time on GFD | –0.02 (–0.07–0.02) | 0.01 (–0.04–0.06) |
| Age at diagnosis | 0.05 (0.01–0.09)a | 0.01 (–0.3–0.6) |
| Self-management | 0.13 (–0.12–0.38) | 0.31 (0.04–0.59)a |
| CeD knowledge | 0.23 (0.06–0.39)a | 0.24 (0.05–0.4)a |
| Health-related quality of life | 0.2 (–0.03–0.43) | – |
| Compliance | – | 0.25 (–0.39–0.54) |
Linear regression P < 0.05
GFD: gluten-free diet; CeD: coeliac disease.
QoL was positively related to CeD knowledge and self-management (Table 3). However, when we performed a multivariate model, only knowledge was statistically significantly related to QoL (coefficient 0.2, 95% CI 0.007–0.39). Therefore, the better the disease knowledge the better the QoL. Finally, we found that both CeD knowledge and disease self-management were independently and positively correlated with the age at the time of the test (data not shown). Therefore, in our population, which did not perform any transition process, self-management and knowledge improved as age increased.
For each patient, the final scores of all the scales were reported in the TRANSIT-CeD disk and then were used on subsequent visits.
Discussion
This study is a snapshot of Italian AYAs with CeD during their transition to adult healthcare, a transition that at the moment does not follow any standardised protocol.
In fact, only in very recent times has a consensus outlined the critical steps and pitfalls of the transition process in CeD healthcare.3
The data from our study indicate that compliance and QoL improve with a better knowledge of the disease, independently of the patient’s age at the time of the test and from his/her relationship with the doctor. However, patients diagnosed later in life are better able to follow the GFD, independently of their knowledge of the facts and figures of CeD. Moreover, our results report that self-management and knowledge improved as age increased.
Our data show that 20.6% of AYAs have positive gluten-related serology, meaning that lapses are frequent. Five out of 21 (23.8 %) AYAs who declared no dietary lapses showed positive serology, indicating that they are underestimating or not aware of gluten contamination in food. Taking into account that serology becomes positive only when a significant amount of gluten is eaten for a long time, it is possible that a more significant percentage of AYAs do not comply with the GFD.
The transition process deals with the need to make the AYAs feel responsible, giving him/her the means for autonomous and safe behaviour.
How to initiate the transition? In some conditions, paediatricians and gastroenterologists see the AYA on the same visit, and this is likely to be the best way to start. In some others, when co-presence is not possible, the paediatrician and the gastroenterologist of the referral centres should meet regularly to revise the cases that should be transferred. In some other cases, when the AYA has to visit a specific referral centre that is located at a great distance, the paediatrician prepares the AYA for the actual transfer to another doctor, in the time made available by his/her personal resources. He/she also supplies a good report of the initial diagnosis details, including anthropometry, biopsy or only serology sustaining the diagnosis, symptoms, human leukocyte antigen status and problems, a transition document as recently suggested by an expert team.3 We suggest including in the transition process the TRANSIT disk, a visual summary of the crucial issues to deal with the AYA during the transition process (Figure 1). A team of experts3 has recently suggested that the moment of the transition to adult care is also the time to revise the diagnosis. Experts believe that some diagnoses in childhood may be imprecise, or even wrong. Therefore, the report of data such as histology and serology, but also the overall response to GFD, may be of importance. Adult gastroenterologists in the case of discordant or doubtful tests at diagnosis may also consider the possibility of reintroducing gluten for a short period of time to re-evaluate the diagnosis (gluten challenge), which is better done with the informed consent of the AYA. Table 4 shows an example of the CeD pass, a document that might be used with the TRANSIT-CeD disk.
Table 4.
The CeD pass, an example of the transition document that should accompany the AYA to adult healthcare.
| Name _________________________________ date of birth________________ |
|---|
| Diagnosis of coeliac disease, year and site |
| Serology at diagnosis (please indicate the value with range of normality) |
| Histology at diagnosis (please indicate grade of lesions) |
| HLA status if available |
| Associated diseases (thyroid diabetes, other) |
| Clinical response to gluten-free diet |
| Notes |
AYA: adolescent/young adult; CeD: coeliac disease; HLA: human leukocyte antigen.
The most recent consensus on the transition in CeD points out that the patient and his/her family are the fulcrum of the transitional process, with the physician providing both with the right information, balancing the parents’ authority and the autonomy needs of the AYA.3 The transition occurs at a particular time in the life of AYAs when the latter work hard to overcome the dependence boundaries with their parents. Our data show the importance of AYAs’ CeD knowledge. Therefore, the transition process should offer the opportunity to improve the education of the patients on CeD management, as well as their health from childhood, continuing into adolescence. By doing so, the patients will become adults with an adequate perception and knowledge of their disease. Our study reported that, independently of the CeD knowledge and without any transition process, dietary compliance (but not the QoL), improves as age at diagnosis increases. A direct correlation between age at diagnosis and dietary adherence is in line with a previous study.12 Moreover, in our study, CeD knowledge and disease self-management were independently and positively correlated with age at the evaluation, therefore these data suggest not to start the transition process too early, in particular in patients who have been diagnosed before.
This study has some limitations. It captures the knowledge, QoL, disease management and compliance of a limited number of subjects who had not undergone a transition process at the time of research. This evaluation has been carried out on subjects with a large spectrum of ages, 9–20 years, and with different levels of education and maturity. Moreover, as with all studies which make use of questionnaires, reporting bias cannot be avoided. We did not use standardised questionnaires to evaluate QoL and compliance, because we aimed to find a practical and fast way to assess these aspects during a common visit and not for research use. However, our study also has some important strengths. It furthers the existing information on factors that influence dietary compliance and QoL, it provides an opportunity to understand the point of view of adolescents at the age of transition, and helps to develop a practical tool to assess the global status of the AYA to be passed on to the adult healthcare giver, helping to identify some points to work on.
Future prospective studies with a large sample size will be able to evaluate the benefits and the advantages of using our TRANSIT-CeD disk in clinical practice during the transition process.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a previous approved document by the institution’s human research committee. The study was approved by the local ethics committee institutional board, Campania Sud (prot/SCCE n 95281 – 12/10/2015).
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Informed consent
Written informed consent was obtained from each patient.
References
- 1.McManus MA, Pollack LR, Cooley WC, et al. Current status of transition preparation among youth with special needs in the United States. Pediatrics 2013; 131: 1090–1097. [DOI] [PubMed] [Google Scholar]
- 2.Disabato JA, Cook PF, Hutton L, et al. Transition from pediatric to adult specialty care for adolescents and young adults with refractory epilepsy: a quality improvement approach. J Pediatr Nurs 2015; 30: e37–e45. [DOI] [PubMed] [Google Scholar]
- 3.Ludvigsson JF, Agreus L, Ciacci C, et al. Transition from childhood to adulthood in coeliac disease: the Prague consensus report. Gut 2016; 65: 1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciacci C, Ciclitira P, Hadjivassiliou M, et al. The gluten-free diet and its current application in coeliac disease and dermatitis herpetiformis. Unit Eur Gastroenterol J 2015; 3: 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charalampopoulos D, Panayiotou J, Chouliaras G, et al. Determinants of adherence to gluten-free diet in Greek children with coeliac disease: a cross-sectional study. Eur J Clin Nutr 2013; 67: 615–619. [DOI] [PubMed] [Google Scholar]
- 6.Hopman E, Koopman H, Wit J, et al. Dietary compliance and health-related quality of life in patients with coeliac disease. Eur J Gastroenterol Hepatol 2009; 21: 1056–1056. [DOI] [PubMed] [Google Scholar]
- 7.Roma E, Roubani A, Kolia E, et al. Dietary compliance and life style of children with coeliac disease. J Hum Nutr Dietet: the official journal of the British Dietetic Association 2010; 23: 176–182. [DOI] [PubMed] [Google Scholar]
- 8.Tapsas D, Falth-Magnusson K, Hogberg L, et al. Swedish children with celiac disease comply well with a gluten-free diet, and most include oats without reporting any adverse effects: a long-term follow-up study. Nutr Res (New York, NY) 2014; 34: 436–441. [DOI] [PubMed] [Google Scholar]
- 9.Errichiello S, Esposito O, Di Mase R, et al. Celiac disease: predictors of compliance with a gluten-free diet in adolescents and young adults. J Pediatr Gastroenterol Nutr 2010; 50: 54–60. [DOI] [PubMed] [Google Scholar]
- 10.MacCulloch K, Rashid M. Factors affecting adherence to a gluten-free diet in children with celiac disease. Paediatr Child Health 2014; 19: 305–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner G, Berger G, Sinnreich U, et al. Quality of life in adolescents with treated coeliac disease: influence of compliance and age at diagnosis. J Pediatr Gastroenterol Nutr 2008; 47: 555–561. [DOI] [PubMed] [Google Scholar]
- 12.Kurppa K, Lauronen O, Collin P, et al. Factors associated with dietary adherence in celiac disease: a nationwide study. Digestion 2012; 86: 309–314. [DOI] [PubMed] [Google Scholar]
- 13.Häuser W, Stallmach A, Caspary W, et al. Predictors of reduced health-related quality of life in adults with coeliac disease. Aliment Pharmacol Therapeut 2007; 25: 569–578. [DOI] [PubMed] [Google Scholar]
- 14.Zingone F, Swift GL, Card TR, et al. Psychological morbidity of celiac disease: a review of the literature. Unit Eur Gastroenterol J 2015; 3: 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciacci C, Zingone F. The perceived social burden in celiac disease. Diseases (Basel, Switzerland) 2015; 3: 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciacci C, D’Agate C, De Rosa A, et al. Self-rated quality of life in celiac disease. Dig Dis Sci 2003; 48: 2216–2220. [DOI] [PubMed] [Google Scholar]
- 17.Nachman F, del Campo MP, Gonzalez A, et al. Long-term deterioration of quality of life in adult patients with celiac disease is associated with treatment noncompliance. Dig Liver Dis: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 2010; 42: 685–691. [DOI] [PubMed] [Google Scholar]
- 18.Crowley R, Wolfe I, Lock K, et al. Improving the transition between paediatric and adult healthcare: a systematic review. Arch Dis Childhood 2011; 96: 548–553. [DOI] [PubMed] [Google Scholar]
- 19.Husby S, Koletzko S, Korponay-Szabo I, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012; 54: 136–160. [DOI] [PubMed] [Google Scholar]
- 20.Marsh MN, Haeney MR. Studies of intestinal lymphoid tissue. VI – Proliferative response of small intestinal epithelial lymphocytes distinguishes gluten- from non-gluten-induced enteropathy. J Clin Pathol 1983; 36: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corazza GR, Villanacci V. Coeliac disease. J Clin Pathol 2005; 58: 573–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wessels MMS, Te Lintelo M, Vriezinga SL, et al. Assessment of dietary compliance in celiac children using a standardized dietary interview. Clin Nutr (Edinburgh, Scotland) 2018; 37: 1000–1004. [DOI] [PubMed] [Google Scholar]
- 23.Ciacci C, Iavarone A, Mazzacca G, et al. Depressive symptoms in adult coeliac disease. Scand J Gastroenterol 1998; 33: 247–250. [DOI] [PubMed] [Google Scholar]
- 24.van Doorn RK, Winkler LM, Zwinderman KH, et al. CDDUX: a disease-specific health-related quality-of-life questionnaire for children with celiac disease. J Pediatr Gastroenterol Nutr 2008; 47: 147–152. [DOI] [PubMed] [Google Scholar]
- 25.Moynihan M, Saewyc E, Whitehouse S, et al. Assessing readiness for transition from paediatric to adult health care: revision and psychometric evaluation of the Am I ON TRAC for Adult Care questionnaire. J Adv Nurs 2015; 71: 1324–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen SE, Hooper SR, Javalkar K, et al. Self-management and transition readiness assessment: concurrent, predictive and discriminant validation of the STARx questionnaire. J Pediatr Nurs 2015; 30: 668–676. [DOI] [PubMed] [Google Scholar]