Abstract
Background
Endoscopic ultrasound (EUS)-guided drainage is the procedure of choice for pancreatic fluid collection (PFC) management. Recently developed lumen-apposing fully covered self-expandable metal stents (LAMSs) may facilitate drainage, especially of necrotic and complex PFCs.
Objective
To evaluate the feasibility and efficacy of a newly developed LAMS (Nagi, Taiwong Medical Co. Ltd, South Korea) in the drainage of PFCs.
Methods
Retrospective analysis of LAMS drainage of PFCs from seven centres. Patient demographic, EUS and radiological findings, PFCs aetiology, procedural technical and clinical success, and adverse events were evaluated.
Results
Sixty-seven patients with mean age 58.8 ± 14 years (68.7% males) were included in the analysis. Of these, 44 patients had pseudocyst (PP) and 23 patients had walled-off pancreatic necrosis (WOPN). Technical success was achieved in 98.5% of cases and clinical success in 94%. The adverse event rate was 24.2%, higher and mostly due to stent migration and occlusion in the WOPN group as compared to the PP group, despite the time to stent removal being significantly lower in the WOPN group.
Conclusions
PFC drainage using the Nagi stent is highly feasible and effective, with a relatively safe profile. Future studies enrolling more patients with complex PFCs are needed to clearly establish the role of this stent in PFC management.
Keywords: Pancreatic fluid collection, pancreatitis, pseudocyst, walled-off necrosis, EUS-guided drainage
Key summary
Summarize the established knowledge on this subject:
- Endoscopic ultrasound-guided drainage is the technique of choice for pancreatic fluid collection (PFC) management.
- Recently, new dedicated anchoring lumen-apposing fully covered self-expandable metal stents (LAMSs) have been developed, allowing better drainage and easy direct access into the PFC cavity for direct endoscopic necrosectomy.
- The Nagi stent is a new dedicated anchoring LAMS, with few published studies showing its potential and heterogeneous results in small populations.
What are the significant and/or new findings of this study?
- Nagi stent positioning is feasible and effective for the drainage of PFCs.
- Adverse events are relatively low, and are mainly due to spontaneous stent migration and bleeding.
- No differences between the pseudocyst (PP) and walled-off pancreatic necrosis (WOPN) groups in terms of demographic characteristics and PFC aetiology, symptoms leading to drainage, dimension of collection and recurrence were observed.
- Compared to the PP group, the WOPN group experienced a significantly higher rate of stent migration, with shorter time to stent removal.
Introduction
According to the revised Atlanta classification, pancreatic fluid collections (PFCs) include acute peri-pancreatic fluid collections and acute necrotic collections, which, over time, turn into pancreatic pseudocysts (PPs) and walled-off pancreatic necrosis (WOPN), respectively.1 Currently, available treatment options for symptomatic PFCs include surgical, percutaneous and endoscopic drainage. Surgery is associated with high morbidity and mortality, whereas percutaneous treatment increases infection risk and fistula formation.2–4 Therefore, PFC endoscopic drainage has been increasingly used as a minimally invasive alternative. In particular, endoscopic ultrasound (EUS)-guided transluminal drainage, for its safety and effectiveness, is now becoming widely accepted as the first-line therapy for PFC drainage in many tertiary centres.2,3,5–9
EUS allows an accurate assessment of surrounding vessels and PFC wall thickness, showing the best transmural approach in the shortest distance. Under EUS guidance, the clinical success rate for PFC drainage is ∼90%, with a complication rate of ∼11%.2,3,10
Traditional endoscopic drainage of PFCs has included the use of a variety of accessories. Multiple plastic stents (7–10 Fr) were conventionally used because of their safety and effectiveness. However, a major criticism is related to their narrow lumen causing premature occlusion, leading to multiple revisions in 17.7–27% of cases.2,3,11 This is especially true for WOPN, which are characterized by solid debris, and therefore the insertion of multiple stents or nasocystic drainage is often necessary. Fully covered self-expandable metal stents (FCSEMSs), initially used for biliary strictures, have become available for PFC drainage, replacing the use of multiple plastic stents. FCSEMSs offer the advantage of a larger diameter lumen, allowing more efficient drainage when an excessive amount of debris is present and offering access to the cyst cavity for eventual direct endoscopic necrosectomy (DEN). However, these stents were initially tubular without anchoring flanges and could therefore migrate, resulting in inefficient drainage and adverse events.2,3
Recently, new dedicated anchoring lumen-apposing fully covered self-expandable metal stents (LAMSs) have been developed for PFC drainage, with wide-flared ends, minimizing the risk of migration, and providing a large lumen allowing better drainage of the PFC and easy direct access into the PFC cavity for DEN. Therefore, this new type of stent might allow faster patient recovery compared to the use of plastic stents and FCSEMSs.
However, data on the feasibility and clinical usefulness of these devices are limited. The Nagi stent (Taewoong Medical Co. Ltd, South Korea) is a new dedicated anchoring LAMS, with very few published studies showing its potential and heterogeneous results in small populations.
Therefore, the primary aims of this study were to evaluate the technical and clinical success rate and the adverse events of the use of this novel lumen-apposing stent for symptomatic PFC management in a large cohort of patients. As a secondary aim, outcomes of patients with either PPs or WOPNs were compared.
Methods
This is a multicentric, retrospective study conducted across seven tertiary care centres in Italy, after ethical approval by the Institutional Review Board of each centre (Comitato Etico Ospedale San Raffaele, 9 June 2016).
Endoscopy Databases of practicing endosonographers were queried for all patients who had undergone PFC EUS-guided drainage using Nagi stents between 2013 and 2016. A standardized datasheet recording patient demographics, PFC aetiology and type, technique used for insertion, adverse events, and technical and clinical success was created from outpatient and hospital records. Only patients who underwent PFC EUS-guided drainage with attempt of Nagi stent insertion and with a ≥ 3-month follow-up were included.
Data collection was performed according to the principles of the Declaration of Helsinki.
Definitions
PFCs were defined as PPs and WOPNs according to the revised Atlanta classification,1 and subsequently as sterile or infected based on the presence of signs of infection (fever, increased WBC or CRP, and/or evidence of gas bubbles at imaging examinations). PFCs were evaluated through computed tomography (CT) and EUS. The indications for PFC drainage were: refractory abdominal pain, impaired gastric outlet, anorexia and infected PFCs, according to current guidelines.5,6 Presence of regional varices, suspected cystic neoplasms, coagulopathy (International Normalized Ratio. INR > 1.5), thrombocytopenia (platelets < 50.000/mm3) or absence of close proximity of PFC walls to EUS probe (>1 cm) were exclusion criteria. Technical success was defined as the ability to position and deploy the stent. Clinical success was defined as at least a 50% decrease in the PFC size based on radiological evaluation (either CT scan and/or magnetic resonance imaging) analysis at 30 days, associated with clinical resolution of the symptoms considered the primary indication to the procedure.
Adverse events were defined as: immediate peri-procedural (<24 hours), post-procedural early (<7 days) and delayed (>7 days).
Device and procedure description
The Nagi stent is specifically designed to create a temporary cystogastrostomy or cystoduodenostomy, and consists of a 20-mm long nitinol structure with a silicone coating. It is doted of bilateral anchoring wide flares (24 mm) at both extremities of the stent, designed to hold the stomach or duodenal wall in apposition to the PFC wall. A variety of lengths (10, 20 and 30 mm) and diameters (10, 12, 14 and 16 mm) are currently available. The stent is mounted on a coaxial delivery system, 10 Fr in diameter, and can be passed through the 3.8-mm channel of a therapeutic echoendoscope. The stent is equipped with three fluoroscopic markers (distal, central and proximal) and one endoscopic marker on the enteral side in order to facilitate placement. The delivery system allows the insertion of a standard 0.035-inch guidewire.
All procedures were performed under deep sedation, using a therapeutic linear echoendoscope, by endosonographers with > 5 years of practice. Written informed consent was obtained from each included patient. An access tract was created using either a 19G needle or cystotome as per endoscopist preference, followed by the placement of a 0.035-inch guidewire advanced within the cavity to form several loops under radiological guidance. After removing the needle, the fistula tract was dilated using a dilating balloon or the cystotome, and the stent delivery catheter advanced over the guidewire into the cavity. Distal flange deployment was done under EUS and/or radiological guidance, while proximal flange deployment was performed under endoscopic visualization Fig. 1. Stent diameter selection was at endoscopist discretion. In cases of WOPN, stents with larger diameter were preferred to allow access to the cavity for the eventual necessity of DEN. DEN sessions were performed using an upper endoscope advanced through the stent at the scheduling preference of the endoscopist until complete resolution of the necrosis was achieved.
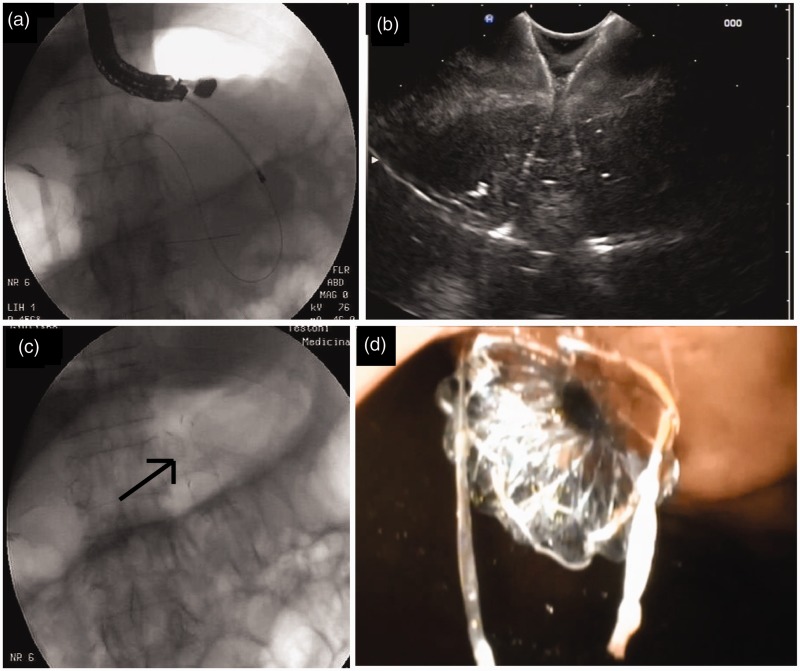
Figure 1.
Fluoroscopic view of a 10 Fr cystotome onto the guidewire used to perform fistula (a). Endosonographic (b), fluoroscopic (c) black arrow) and endoscopic (d) views of the stent after its deployment.
Outcome measures
Primary endpoints included technical success, clinical success and immediate peri-procedural, post-procedural early and delayed adverse events.
The secondary endpoint was to compare characteristics and outcomes of patients with PPs versus WOPNs.
Evaluations were performed at baseline, 30 or 60 days after stent removal with a visit, and with a radiological evaluation performed after stent removal based on medical judgement.
Statistical analysis
Continuous variables are presented as mean ( ± SD) and categorical variables are presented as number (percentage). Categorical data were compared by Fisher's test and continuous variables by Student's t-test. Logistic regression was used to calculate odds ratios (ORs) and their 95% confidence intervals (CIs).
Statistical analyses were performed using MedCalc version 13 (MedCalc Software, Belgium). A p-value < 0.05 was considered statistically significant.
Results
Patient demographics and PFC characteristics
Sixty-seven patients (mean age 58.8 ± 14; 68.7% males) were enrolled. Patient demographics, PFC characteristics and aetiologies, and indications to drainage are detailed in Table 1.
Table 1.
Patient demographic and pancreatic fluid collection characteristics.
| Patients | 67 |
| Sex | |
| Males | 46 (68.7%) |
| Females | 21 (31.3%) |
| Age, years (mean ± SD) | 58.8 ± 14 |
| PFC subtype | |
| Sterile PP | 27 (40.3%) |
| Infected PP | 17 (25.4%) |
| Sterile WOPN | 12 (17.9%) |
| Infected WOPN | 11 (16.4%) |
| PFC aetiology | |
| Gallstone acute pancreatitis | 42 (63%) |
| Alcoholic acute pancreatitis | 8 (12%) |
| Post-ERCP acute pancreatitis | 2 (3%) |
| Chronic pancreatitis | 9 (13%) |
| Post-surgical pancreatitis | 6 (9%) |
| PFC Symptoms | |
| Abdominal pain | 40 (59.7%) |
| Nausea and vomiting | 25 (37.3%) |
| Fever | 27 (40.3%) |
| Other | 5 (7.5%) |
| Size at CT scan: baseline, cm (mean ± SD) | 12.4 ± 7.9 |
| Size at EUS: baseline, cm (mean ± SD) | 10.7 ± 3.7 |
CT: computed tomography; ERCP: endoscopic retrograde cholangiopancreatography; EUS: endoscopic ultrasound sonography; PFC: pancreatic fluid collection; PP: pseudocyst; WOPN: walled-off pancreatic necrosis.
Technical success rate
Successful LAMS placement was achieved in 66/67 patients (98.5%), as assessed by radiological and endoscopic imaging. In all cases, the stent was inserted through the stomach wall. All technical details are reported in Table 2. Unsuccessful LAMS deployment in one subject was caused by stent malpositioning. This patient underwent plastic double pig-tail stent positioning during the same session after Nagi removal.
Table 2.
Technical procedure details, adverse events and clinical success.
| Stent placement | |
| Transgastric | 67 (100%) |
| Transduodenal | 0 |
| Access for fistula track: | |
| 19-gauge EchoTip needle | 58 (86.6%) |
| Cystotome | 8 (11.9%) |
| Dilation of fistula track | |
| Graduated balloon | 29 (43.3%) |
| Cystotome | 38 (56.7%) |
| Nagi stent diameter | |
| 10 mm | 5 (7.5%) |
| 12 mm | 13 (19.4%) |
| 16 mm | 49 (73.1%) |
| Technical success | 66 (98.5%) |
| Direct endoscopic necrosectomy | 8 (12%) |
| Adverse events (overall) | 16 (24.2%) |
| Peri-procedural adverse events | 1 (1.5%) |
| Bleeding | 0 |
| Perforation | 1 (1.5%) |
| Suprainfection | 0 |
| Post-procedural early-onset adverse events | 4 (13.4%) |
| Bleeding | 3 (4.6%) |
| Perforation | 0 |
| Suprainfection | 1 (1.5%) |
| Post-procedural delayed-onset adverse events | 11 (16.7%) |
| Bleeding | 2 (3%) |
| Perforation | 0 |
| Suprainfection | 1 (1.5%) |
| Stent migration | 5 (7.5%) |
| During DEN session | 2 (3%) |
| Spontaneous | 3 (4.5%) |
| Stent occlusion | 2 (3.0%) |
| Other | 1 (1.5%) |
| Clinical success | 63 (94%) |
| Size at EUS: 30 days, cm (mean ± SD) | 3.8 ± 2.9 |
| Days to stent removal | 40.7 ± 30.6 |
| Recurrence | 2 (3%) |
DEN: direct endoscopic necrosectomy; EUS: endoscopic ultrasound sonography.
Clinical success rate
Clinical success was achieved in 63/66 (94%) subjects who underwent successful stent placement, as shown in Table 2. PFC size decreased significantly from baseline (10.7 ± 3.7 cm) to 30 days after stent placement (3.8 ± 2.9 cm) (p < 0.0001). Among the three patients in whom clinical success was not obtained, two were due to stent occlusion (PFC resolution was obtained in one with surgical necrosectomy and in the other one with the positioning of double pig-tail stents through the Nagi) and one had stent migration during DEN (with PFC resolution obtained with the positioning of double pig-tail stents replacing the Nagi stent). DEN was performed in 8/66 (12.1%) patients with an infected WOPN.
A logistic regression analysis was performed to evaluate whether patient characteristics, the aetiology of the pancreatitis or type of PFC was associated with the PFC resolution. Neither patient characteristics (age OR 1.00 95% CI 0.95–1.04, p = 0.76; sex OR 0.58 95% CI 0.14–2.38; p = 0.45), aetiology of pancreatitis (biliary OR 2.26 95% CI 0.66–7.75, p = 0.19; alcoholic OR 0.34 95% CI 0.07–1.69; p = 0.19; chronic pancreatitis OR 0.42 95% CI 0.09–1.99, p = 0.27) nor type of PFC (WOPN versus PP OR 0.55 95% CI 0.16–1.89, p = 0.34) were associated with a reduced or increased rate of PFC resolution.
Procedure-related adverse events, stent migration and occlusion
Only one peri-procedural perforation was reported as an immediate adverse event, happening in the patient where the stent could not be adequately positioned. Fifteen post-procedural adverse events were reported, 4 (13.4%) with early onset and 11 (16.7%) with delayed onset. Most of them were bleedings (all requiring radiological intervention, except for one requiring surgical intervention) and stent migration (7.5%), while stent occlusion occurred in two (3%) patients. There was no mortality directly related to the procedure. Details are reported in Table 2.
Among patients with clinical resolution of PFC, LAMSs remained implanted for a mean of 40.7 ± 30.6 days. Stent removal was successful in all patients.
Subgroup analysis of patients with PP versus WOPN
There were no differences between the PP and WOPN groups in terms of demographic characteristics and PFC aetiology, symptoms leading to drainage, collection dimension and recurrence, as showed in Table 3. Patients with WOPN experienced a significantly higher rate of stent migration (17.4 versus 2.3%; p = 0.044), and shorter time to stent removal as compared to patients with PP (48.1 ± 35.2 versus 29.3 ± 16.9; p = 0.018). No significant difference was seen in terms of peri-procedural bleeding or suprainfection. Other characteristics and outcomes are reported in Table 3.
Table 3.
Subgroup analysis comparing pseudocyst versus walled-off pancreatic necrosis.
| PP (n = 44) N (%) | WOPN (n = 23) N (%) | p-value | |
|---|---|---|---|
| Sex (male) | 30 (68.2%) | 16 (69.6%) | 1 |
| Age (mean ± SD) | 58.9 ± 15.5 | 58.8 ± 12.8 | 0.98 |
| AP Biliary aetiology | 29 (65.9%) | 14 (60.9%) | 0.79 |
| AP Other aetiologies | 15 (34.1%) | 9 (39.1%) | 0.23 |
| Size at CT scan (mean ± SD) | 12.8 ± 9.5 | 11.5 ± 3.6 | 0.53 |
| Size at EUS (mean ± SD) | 10.6 ± 3.9 | 10.7 ± 2.9 | 0.91 |
| Technical success | 43 (97.7%) | 23 (100%) | 1 |
| Clinical success | 42 (95.5%) | 21 (91.3%) | 0.6 |
| Adverse events (overall) | 7 (15.9%) | 9 (39.1%) | 0.067 |
| Peri-procedural AEs | 1 (2.3%) | 0 | 1 |
| Post-procedural early-onset AEs | 3 (6.8%) | 1 (4.3%) | 1 |
| Post-procedural delayed-onset AEs | 3 (6.8%) | 8 (34.8%) | 0.0057 |
| Stent migration | 1 (2.3%) | 4 (17.4%) | 0.044 |
| Stent occlusion | 0 | 2 (8.7%) | 0.11 |
| Days to stent removal | 48.1 ± 35.2 | 29.3 ± 16.9 | 0.0186 |
| Recurrence | 1 (2.3%) | 1 (4.4%) | 1 |
AEs: adverse events; AP: acute pancreatitis; CT: computed tomography; EUS: endoscopic ultrasound sonography; PP: pancreatic pseudocyst; WOPN: walled-off pancreatic necrosis.
Discussion
LAMSs are an innovative therapeutic approach for PFC drainage with good efficacy and safety, characterized by a wider lumen compared to plastic stents, which reduce the risk of occlusion and allow access to the cavity to perform DEN, and equipped with anchoring flanges to reduce the risk of dislocation.9–13 This multicentre study including patients who underwent Nagi stent positioning for PFCs showed, among 67 patients, a technical success rate of stent placement of 98.5%, a PFC resolution rate of 94% and an overall adverse events rate of 24.2%.
These data are in line with the available literature on EUS-guided drainage of PFCs with LAMSs (see Table 4).
Table 4.
Studies evaluating drainage of pancreatic fluid collections with lumen apposing metal stents.
| Study | Patients (n) | Type of stent | Technical success | Clinical success |
|---|---|---|---|---|
| Itoi et al., 201228 | 15 | Axios | 100% | 100% |
| Gornals et al., 201324 | 9 | Axios | 88.9% | 100% |
| Yamamoto et al., 201327 | 9 (4 WOPN, 5 PP) | Nagi | 100% | 77.8% |
| Chandran et al., 201425 | 47 (9 WOPN, 38 PP) | Nagi | 98% | 76% |
| Walter et al., 201529 | 61 (46 WOPN, 15 PP) | Axios | 98% | WOPN 81%, PP 93% |
| Rinninella et al., 201530 | 93 (52 WOPN, 18 PP) | Hot Axios | 98.9% | WOPN 90.4%, PP 100% |
| Bapaye et al., 201518 | 19 | Nagi | 100% | 100% |
| Shah et al., 201513 | 33 | Axios | 91% | 93% |
| Huggett et al., 201517 | 19 WOPN | Nagi | 100% | 100% |
| Sharaiha et al., 201631 | 124 WOPN | Axios | 100% | 86.3% |
| Siddiqui et al., 201626 | 82 (68 WOPN, 12 PP) | Axios | 97.5% | WOPN 88%, PP 100% |
| Mukai et al., 201615 | 21 (19 WOPN, 2 PP) | Nagi | 100% | 100% |
| Lakhtakia et al., 201614 | 205 WOPN | Nagi | 99% | 96.5% |
| Vazquez-Sequeiros et al., 201632 | 211 (99 WOPN, 112 PP) | Axios/FCSEMS | 97% | 94% |
| Siddiqui et al., 201733 | 86 WOPN | Axios | 97.7% | 90% |
| Venkatachalapathy et al., 201834 | 116 (70 WOPN, 46 PP) | Hot Axios | 99.1% | 94% |
FCSEMS: fully covered self-expandable metal stent; PP: pancreatic pseudocyst; WOPN: walled-off pancreatic necrosis.
The largest study evaluating the use of Nagi stents to date is, to our knowledge, the one by Lakhtakia et al., which evaluated its use in 205 WOPN patients with a technical success of 99% and an initial clinical success of 74.6%, which is lower compared to our results but with a lower rate of adverse events (3.9%).14
Mukai et al. investigated 21 patients with PFCs (2 PPs and 19 WOPN) treated with Nagi stent positioning,15 reporting that technical success was 100% and that final clinical success was 100%. Nevertheless, compared to our study, Mukai et al. also positioned a nasocystic tube in 28.6% of their patients in order to perform lavages of the cavity, which could explain their higher clinical success rate. In addition, their adverse event rate was quite high (28.6%), characterized mostly by stent migration; in these patients, plastic stents were positioned to prevent PFC recurrence. Interestingly, nasocystic tube positioning has been recently demonstrated, as reported by a network meta-analysis by Gurusamy et al., to result in fewer adverse events and a shorter hospital stays compared to EUS-guided drainage alone.16
In the study by Huggett et al., the performance of the Nagi stent was evaluated in 19 WOPN patients. Clinical success was 100% while the stent migration rate was 21%, higher compared to our study in the WOPN group, probably due to the higher rate of DEN required in their cohort of patients (about 74%).17
Bapaye et al. performed another study from three centres, enrolling 19 patients, using Nagi stents;18 technical and clinical success was seen in all patients (100%), with complications occurring in 10% of patients (one with stent migration and one with bleeding).
Most of the current literature regarding the role of LAMSs in PFC management is restricted to small case series and case reports performed at single centres, often including all PFCs and not performing subgroup analysis according to the type of PFC.2,3,7,8,14–24 Two studies performed at a multicentre level, one using Nagi stents and the other using another similar novel saddle-shaped LAMS (AXIOS, Xlumena), reported that the initial resolution rate was higher and sustained for PPs although not statistically significant, whereas it was not uniformly successful in WOPN.25,26
In fact, Chandran et al. performed a national multicentre study on the use of Nagi stents including 48 PFCs with a technical success of rate 98.1% (similar to our findings) and clinical success of 76.6%, which is quite low compared to our study and probably due to the fact that participating centres were not all referral centres. Early adverse events occurred in 18.6% of patients while late adverse events reached 26%, quite a bit higher compared to our study.25
Another reported advantage of LAMSs compared to plastic stents is the possibility of performing DEN through the larger calibre of these stents, with associated minimal incidence of adverse events.2,3,11–14 In our cohort, we performed DEN in 8 patients (12.1%). The low rate of DEN required, compared to other studies using different LAMSs,3 was probably due to the efficient drainage through these wide-calibre metal stents. Also, the study by Chandran et al. reported a rate of DEN of 19.2% and of stent dislodgement during DEN of 44.4%,25 while in our study only two patients had stent dislodgement during DEN (25% of patients undergoing DEN). Other studies with smaller sample sizes did not investigate this technical aspect, and thus probably underestimated the rate of adverse events related to WOPN drainage.2,3,27
Our study is one of the few to have performed a logistic regression evaluating whether patient characteristics, aetiology of pancreatitis or type of collection were associated with an increased or reduced rate of PFC resolution, but no significant result was seen in our population.
Interestingly, in our study, stents positioned for WOPN were removed significantly earlier compared to stents positioned for PP. In fact, patients with WOPN were kept hospitalized until resolution of the PFC and the stents were removed as soon as resolution was observed, while PP patients were discharged and the stents were removed during second scheduled hospitalization periods.
Unlike the results reported by Chandran et al., in which the stent migration rate was 20.4%, in our experience, spontaneous stent migration was similar to that of previous studies reporting that this occurs uncommonly.2,3,25
The major limitation of this study is due to its retrospective nature and the lack of a control population. Other limitations include follow-up method variability and timing after stent positioning across the different centres, as well as the lack of long-term follow-up and variability in endoscopic drainage techniques.
The strengths of this study are represented by its nationwide recruitment across seven Italian centres with experienced echoendoscopists and the sample size, which is one of the largest on the use of Nagi LAMSs for PFC drainage to date. Additionally, we evaluated the roles of Nagi stents for PPs and WOPN separately, while the majority of previously published studies have assessed the role of LAMS for PFC drainage in a pooled manner.
In conclusion, in our study, PFC EUS-guided drainage using LAMSs had a very high technical success rate and was a safe procedure, with a clinical success rate for PP that was very high (95.5%), while slightly lower for WOPN (91.3%). Randomized controlled trials are required to evaluate the benefits, safety and efficacy of different LAMS subtypes specifically designed for EUS-guided drainage in the management of PFCs.
Footnotes
*The authors contributed equally to this work.
Declaration of conflicting interests
The authors declare no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Ethics approval
This study was performed with approval of the Institutional Review Board of each center and was conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
Informed consent
Written, informed consent was obtained from each patient.
References
- 1.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62: 102–111. [DOI] [PubMed] [Google Scholar]
- 2.Tyberg A, Karia K, Gabr M, et al. Management of pancreatic fluid collections: A comprehensive review of the literature. World J Gastroenterol 2016; 22: 2256–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nabi Z, Basha J, Reddy DN. Endoscopic management of pancreatic fluid collections-revisited. World J Gastroenterol 2017; 23: 2660–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teoh AY, Dhir V, Jin ZD, et al. Systematic review comparing endoscopic, percutaneous and surgical pancreatic pseudocyst drainage. World J Gastrointest Endosc 2016; 8: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Italian Association for the Study of the Pancreas (AISP), Pezzilli R, Zerbi A, et al. Consensus guidelines on severe acute pancreatitis. Dig Liver Dis 2015; 47: 532–543. [DOI] [PubMed]
- 6.Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013; 13: e1–e15. [DOI] [PubMed] [Google Scholar]
- 7.Varadajulu S, Bang JY, Sutton BS, et al. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology 2013; 145: 583–590.e1. [DOI] [PubMed] [Google Scholar]
- 8.van Brunschot S, van Grinsven J, van Santvoort HC, et al. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: A multicentre randomised trial. Lancet 2018; 391: 51–58. [DOI] [PubMed] [Google Scholar]
- 9.Ang TL, Teoh AYB. Endoscopic ultrasonography-guided drainage of pancreatic fluid collections. Dig Endosc 2017; 29: 463–471. [DOI] [PubMed] [Google Scholar]
- 10.Singhal S, Rotman SR, Gaidhane M, et al. Pancreatic fluid collection drainage by endoscopic ultrasound: An update. Clin Endosc 2013; 46: 506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawakami H, Itoi T, Sakamoto N. Endoscopic ultrasound-guided transluminal drainage for peripancreatic fluid collections: Where are we now?. Gut Liver 2014; 8: 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe Y, Mikata R, Yasui S, et al. Short- and long-term results of endoscopic ultrasound-guided transmural drainage for pancreatic pseudocysts and walled-off necrosis. World J Gastroenterol 2017; 23: 7110–7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah A, Denicola R, Edirisuriya C, et al. Management of inflammatory fluid collections and walled-off pancreatic necrosis. Curr Treat Options Gastroenterol 2017; 15: 576–586. [DOI] [PubMed] [Google Scholar]
- 14.Lakhtakia S, Basha J, Talukdar R, et al. Endoscopic “step-up approach” using a dedicated biflanged metal stent reduces the need for direct necrosectomy in walled-off necrosis (with videos). Gastrointest Endosc 2017; 85: 1243–1252. [DOI] [PubMed] [Google Scholar]
- 15.Mukai S, Itoi T, Sofuni A, et al. Clinical evaluation of endoscopic ultrasonography-guided drainage using a novel flared-type biflanged metal stent for pancreatic fluid collection. Endosc Ultrasound 2015; 4: 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurusamy KS, Belgaumkar AP, Haswell A, et al. Interventions for necrotising pancreatitis. Cochrane Database Syst Rev 2016; 4: CD011383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huggett MT, Oppong KW, Pereira SP, et al. Endoscopic drainage of walled-off pancreatic necrosis using a novel self-expanding metal stent. Endoscopy 2015; 47: 929–932. [DOI] [PubMed] [Google Scholar]
- 18.Bapaye A, Itoi T, Kongkam P, et al. New fully covered large-bore wide-flare removable metal stents for drainage of pancreatic fluid collections: Results of a multicenter study. Dig Endosc 2015; 27: 499–504. [DOI] [PubMed] [Google Scholar]
- 19.Tarantino I, Barresi L, Fazio V, et al. EUS-guided self-expandable stent placement in 1 step: a new method to treat pancreatic abscess. Gastrointest Endosc 2009; 69: 1401–1403. [DOI] [PubMed] [Google Scholar]
- 20.Fabbri C, Luigiano C, Cennamo V, et al. Endoscopic ultrasound-guided transmural drainage of infected pancreatic fluid collections with placement of covered self-expanding metal stents: A case series. Endoscopy 2012; 44: 429–433. [DOI] [PubMed] [Google Scholar]
- 21.Bang JY, Hawes R, Bartolucci A, et al. Efficacy of metal and plastic stents for transmural drainage of pancreatic fluid collections: a systematic review. Dig Endosc 2015; 27: 486–498. [DOI] [PubMed] [Google Scholar]
- 22.Penn DE, Draganov PV, Wagh MS, et al. Prospective evaluation of the use of fully covered self-expanding metal stents for EUS-guided transmural drainage of pancreatic pseudocysts. Gastrointest Endosc 2012; 76: 679–684. [DOI] [PubMed] [Google Scholar]
- 23.Weilert F, Binmoeller KF, Shah JN, et al. Endoscopic ultrasound-guided drainage of pancreatic fluid collections with indeterminate adherence using temporary covered metal stents. Endoscopy 2012; 44: 780–783. [DOI] [PubMed] [Google Scholar]
- 24.Gornals JB, De la Serna-Higuera C, Sanchez-Yague A, et al. Endosonography-guided drainage of pancreatic fluid collections with a novel lumen-apposing stent. Surg Endosc 2013; 27: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 25.Chandran S, Efthymiou M, Kaffes A, et al. Management of pancreatic collections with a novel endsocopically placed fully covered self-expandable metal stent: A national experience. Gastrointest Endosc 2015; 81: 127–135. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqui AA, Adler DG, Nieto J, et al. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: A large, retrospective, multicenter U.S. experience. Gastrointest Endosc 2016; 83: 699–707. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto N, Isayama H, Kawakami H, et al. Preliminary report on a new, fully covered metal stent designed for the treatment of pancreatic fluid collections. Gastrointest Endosc 2013; 77: 809–814. [DOI] [PubMed] [Google Scholar]
- 28.Itoi T, Nageshwar Reddy D, Yasuda I. New fully-covered self-expandable metal stent for endoscopic ultrasonography-guided intervention in infectious walled-off pancreatic necrosis (with video). J Hepatobiliary Pancreat Sci 2013; 20: 403–406. [DOI] [PubMed] [Google Scholar]
- 29.Walter D, Will U, Sanchez-Yague A, et al. A novel lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: A prospective cohort study. Endoscopy 2015; 47: 63–67. [DOI] [PubMed] [Google Scholar]
- 30.Rinninella E, Kunda R, Dollhopf M, et al. EUS-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery-enhanced delivery system: A large retrospective study (with video). Gastrointest Endosc 2015; 82: 1039–1046. [DOI] [PubMed] [Google Scholar]
- 31.Sharaiha RZ, Tyberg A, Khashab MA, et al. Endoscopic therapy with lumen-apposing metal stents is safe and effective for patients with pancreatic walled-off necrosis. Clin Gastroenterol Hepatol 2016; 14: 1797–1803. [DOI] [PubMed] [Google Scholar]
- 32.Vazquez-Sequeiros E, Baron TH, Pérez-Miranda M, et al. Evaluation of the short- and long-term effectiveness and safety of fully covered self-expandable metal stents for drainage of pancreatic fluid collections: Results of a Spanish nationwide registry. Gastrointest Endosc 2016; 84: 450–457.e2. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui AA, Kowalski TE, Loren DE, et al. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: Clinical outcomes and success. Gastrointest Endosc 2017; 85: 758–765. [DOI] [PubMed] [Google Scholar]
- 34.Venkatachalapathy SV, Bekkali N, Pereira S, et al. Multicenter experience from the UK and Ireland of use of lumen-apposing metal stent for transluminal drainage of pancreatic fluid collections. Endosc Int Open 2018; 6: E259–E265. [DOI] [PMC free article] [PubMed] [Google Scholar]