Abstract
Background
Functional dyspepsia is a common functional gastrointestinal disorder in which a variety of pathophysiological mechanisms such as increased intestinal permeability and low-grade inflammation are involved. The factor causing these alterations, however, has not been identified.
Objective
We aimed to evaluate the luminal bile salt content and receptor expression in patients with functional dyspepsia and healthy volunteers.
Methods
Gastroduodenoscopy was performed to obtain duodenal biopsies from 25 healthy volunteers and 25 patients with functional dyspepsia (Rome III) to measure duodenal bile salt receptor expression with Western blot. Duodenal fluid aspirates were collected at fixed time points during fasted and fed state conditions and bile salt composition analysis was performed by liquid chromatography-mass spectrometry/mass spectrometry.
Results
Patients (N = 17) displayed decreased fasted bile salt concentrations compared to healthy volunteers (N = 20) over time (1.8 ± 0.3 mM vs 3.6 ± 0.5 mM; p = 0.03). In addition, an increased expression of duodenal bile salt sensor vitamin D receptor was found in patients (3.7 ± 1.0-fold; p < 0.0005; N = 24 for both groups).
Conclusion
Patients with functional dyspepsia are characterized by a decreased duodenal bile salt concentration in fasted state and an increased duodenal vitamin D receptor expression.
Keywords: Bile salts, duodenal permeability, functional dyspepsia, vitamin D receptor
Key summary
1. Summarize the established knowledge on this subject.
Functional dyspepsia is a common functional gastrointestinal disorder characterized by increased intestinal permeability and low-grade inflammation.
The factors causing these alterations are still unknown.
Likely candidates are genetic factors, stress and luminal factors, such as bile salts.
2. What are the significant and/or new findings of this study?
Patients with functional dyspepsia display a decreased fasted duodenal bile salt concentration and an increased vitamin D receptor protein expression in the duodenal mucosa.
Introduction
Functional dyspepsia (FD) is a common gastrointestinal disorder defined by the Rome III criteria as the presence of one or more of the following recurrent or persistent symptoms, namely postprandial fullness, early satiation, epigastric pain and epigastric burning.1 These symptoms originate from the gastroduodenal region without evidence of a structural disease at routine investigations.1
Moreover, FD seems to be a heterogeneous disorder in which diverse pathophysiological mechanisms are involved.2 Over the last few decades, mainly gastric abnormalities have been considered to underlie dyspeptic symptom generation.2 More recent evidence also points toward involvement of duodenal abnormalities in the pathogenesis of FD.2 Early reports showed the presence of duodenal mucosal low-grade inflammation in patients with FD.2 Moreover, impaired duodenal barrier function may represent another pathophysiological mechanism in FD, which is probably related to low-grade inflammation.3
The etiology of the latter alterations in FD is unknown but likely candidates are genetic factors, stress and duodenal luminal factors, including bile salts (BS).4 BS have been shown to be involved in the pathophysiology of irritable bowel syndrome (IBS).5 Furthermore, it is well established that the majority of patients with FD report onset or worsening of symptoms after a meal, which coincides with the release of BS into the duodenum.6 In addition, increased duodenal acid exposure, acid-suppressive therapy or an alteration in the microbiome, which are often present in FD, can provoke changes in the BS pool.7 BS can activate five BS receptors, namely transmembrane G-protein-coupled receptor 5 (TGR5), farnesoid X receptor (FXR), pregnane X receptor (PXR), constitutive androstane receptor (CAR) and vitamin D receptor (VDR). These receptors play an important role in diverse processes, for instance in the regulation of BS transport and metabolism, inflammation and immunity.8,9
We aimed to evaluate the luminal BS content and receptor expression in patients with FD and healthy volunteers (HVs). To this end, we hypothesized that patients with FD display an altered duodenal BS concentration and/or composition or receptor expression, which may contribute to the pathogenesis of FD.
Materials and methods
Participants
Patients meeting Rome III criteria for FD were recruited from the outpatient clinic of the Department of Gastroenterology at the University Hospitals Leuven or via a mailing list and advertisement.3 The symptom pattern was confirmed by a questionnaire designed by our group to identify patients with FD according to the Rome III criteria.10 HVs were recruited via a mailing list. Exclusion criteria for all participants were: symptoms or history of gastrointestinal disease (only for HVs), celiac disease, diabetes mellitus, allergy/atopy, coagulation disorders/anticoagulant therapy and first-degree relatives with celiac disease, Crohn’s disease or type I diabetes mellitus. All drugs potentially affecting the BS pool and gastrointestinal permeability were discontinued at least two weeks before the gastroscopy. A Rome III questionnaire was used to evaluate IBS comorbidity. Psychosocial comorbidity was assessed by the Patient Health Questionnaire 9 (PHQ-9), and the Visceral Sensitivity Index (VSI) questionnaire was used to evaluate gastrointestinal-specific anxiety. The dyspepsia symptom score (DSS) was used to evaluate the dyspeptic symptom intensity over the preceding three months in patients with dyspepsia.11 This protocol conformed to the 1975 Declaration of Helsinki and was approved by the Leuven University Hospitals Ethics Committee (s56910; 18 December 2015). All participants approved and signed the informed consent before inclusion in this study.
Experimental methods
Western blot
Upper gastroduodenoscopy was performed by an experienced endoscopist to obtain two biopsy samples from the second part of the duodenum with biopsy forceps (Radial Jaw3, outside diameter 2.2 mm; Boston Scientific, Natick, MA, USA).3 The biopsy samples were snap-frozen in liquid nitrogen. Protein extracts (15 µg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane.3 Membranes were incubated overnight with primary antibodies: rabbit anti-CAR (1:1000, 186869 Abcam, Cambridge, UK), rabbit anti-TGR5 (1:2000, 72608 Abcam), mouse anti-VDR (1:1000, 89626 Abcam), rabbit anti-PXR (1:1000, 85451 Abcam), and goat anti-FXR (0.3 µg ml−1, 51970 Abcam). Secondary antibodies used were peroxidase-conjugated goat anti-rabbit immunoglobulin (Ig)G, goat anti-mouse IgG (both 1:5000, 31460 and 31430, respectively; Thermo Scientific, Leuven, Belgium) or donkey anti-goat IgG (1:5000, 97110 Abcam). As a protein-loading control, all blots were stained with mouse anti-vinculin (1:5000, V9131 Sigma-Aldrich, Overijse, Belgium). Bands were quantified by densitometry using ImageQuant LAS 4000 mini and ImageQuant TL 1D gel analysis software V8.1. Fold change was determined relative to the average of the healthy group.3
Duodenal fluid aspirations
After the gastroduodenoscopy, an aspiration catheter was placed in the second part of the duodenum under fluoroscopic control. Duodenal fluids were aspirated and collected every 15 minutes during one hour in a fasted state and 1.5 hours after an oral liquid meal (Fortimel Energy®; Nutricia, Zoetendaal, The Netherlands, 150 kcal per 100 ml with 5.9 g proteins, 18.4 g carbohydrates and 5.8 g lipids, pH6.7). Duodenal fluids were centrifuged at 4℃ at maximum speed for five minutes and stored at −20℃ until BS analysis by a Thermo Fisher Scientific liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) system. The pH of every sample was measured with a pH-Meter 766 Calimatic at room temperature (23.5℃). The fasting and fed BS concentration and pH were calculated as the average of all time points in fasted and fed state, respectively. BS are expressed as absolute concentrations (mM) and as percentages of total BS (%). Total BS is the molar sum of all measured BS.
BS analysis
Concentration of taurocholic acid (TC), glycocholic acid (GC), taurodeoxycholic acid (TDC), glycodeoxycholic acid (GDC), tauroursodeoxycholic acid (TUDC), glycoursodeoxycholic acid (GUDC), taurochenodeoxycholic acid (TCDC), glycochenodeoxycholic acid (GCDC), cholic acid (C), deoxycholic acid (DC), chenodeoxycholic acid (CDC) and lithocholic acid (LC) for each participant and time point was measured with LC-MS/MS by the Drug Delivery and Disposition group (KU Leuven, Leuven, Belgium).12
Statistical analysis
Data were analyzed using GraphPad prism 7.04 and values were considered significantly different when p < 0.05. Two-tailed unpaired t tests or Mann–Whitney U tests, depending on data distribution, were performed to analyze between-group differences. Pearson or Spearman correlation coefficient was used, where appropriate, for correlation analysis. Two-way repeated-measures analysis of variance (ANOVA) was used to compare BS concentrations and percentages between groups. Finally, correction for multiple testing was performed with the Bonferroni correction. Data were presented as mean ± SEM or as median (interquartile range).
Results
Study population
In total, we recruited 25 patients with FD and 25 HVs (Table 1). Table 2 summarizes the grading of dyspeptic symptoms in the patient group. Six patients were classified as epigastric pain syndrome (EPS), and 10 patients as postprandial distress syndrome (PDS); seven patients belong to the EPS-PDS overlap group and two patients did not fill out the questionnaires. In patients, the PHQ-9 depression score was 5.5 (2.75–9) and the VSI score was 34 (21–45). Based on previously published criteria,13 two patients were assumed to have postinfectious FD; 19 patients fulfilled criteria for comorbid IBS. Twelve patients who were taking proton pump inhibitors discontinued the intake at least two weeks prior to the start of the study.
Table 1.
Demographics and clinical characteristics of the participants.
| Characteristic | Healthy volunteers | Patients with functional dyspepsia |
|---|---|---|
| Gender, female:male | 12:13 (48% female) | 18:7 (72% female) |
| Age, years (range) | 24.6 ± 1.2 (18–45) | 38.3 ± 2.9 (18–57) a |
| Dyspeptic symptoms, Dyspepsia symptom score | 1 (0–2) | 11 (8–13)b |
| Body mass index, kg/m2 | 22.9 ± 0.6 | 23.5 ± 0.8 |
Data are mean ± SEM or median (interquartile range); N = 25 for both groups.
p < 0.05. bp < 0.0001.
Table 2.
Frequency of severity grading by dyspepsia symptom score for each of the nine dyspeptic symptoms in patients with functional dyspepsia (N = 24).
| Patients | Absent | Mild | Moderate | Severe |
|---|---|---|---|---|
| Discomfort | 6 | 5 | 13 | 0 |
| Postprandial fullness | 2 | 9 | 13 | 0 |
| Bloating | 4 | 5 | 13 | 2 |
| Epigastric pain | 9 | 4 | 10 | 1 |
| Early satiety | 7 | 7 | 10 | 0 |
| Nausea | 6 | 9 | 7 | 2 |
| Vomiting | 22 | 1 | 1 | 0 |
| Belching | 7 | 7 | 8 | 2 |
| Epigastric burning | 6 | 11 | 6 | 1 |
Less acidic duodenal environment after food intake in FD
Duodenal aspirates were obtained from 17 patients and 21 HVs, as the positioning of the catheter in the duodenum was impossible in 12 participants. During fasting, the pH of the duodenal aspirates from patients and HVs was similar (pH 6.9 ± 0.3 vs pH 6.8 ± 0.2; p = 0.61). In HVs, the duodenal aspirate pH decreased significantly after food intake (pH 6.8 ± 0.2 vs pH 6.0 ± 0.1; p = 0.01). In patients, no significant difference in pH was found between fasted and fed state (pH 6.9 ± 0.3 vs pH 6.5 ± 0.1; p = 0.16). HVs displayed a lower pH than patients after meal intake (pH 6.0 ± 0.1 vs pH 6.5 ± 0.1; p = 0.002).
Patients with FD displayed decreased fasting BS concentrations
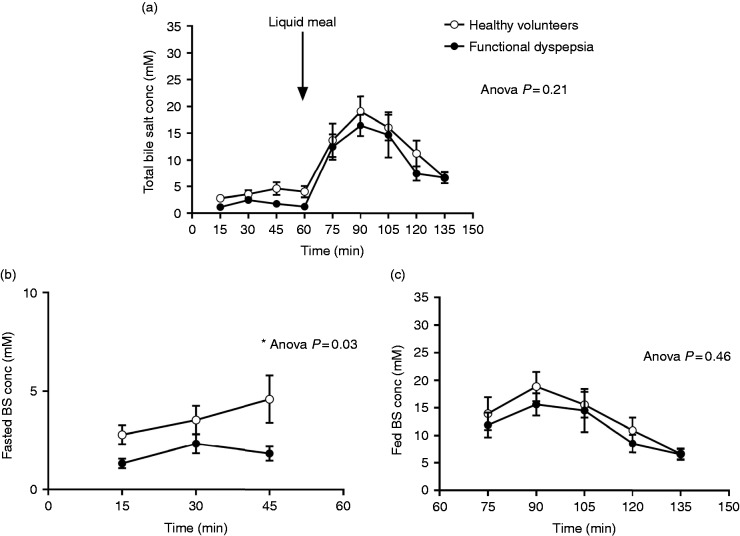
Total BS over time were similar in patients and HVs (7.1 ± 2.0 mM vs 9.1 ± 2.0 mM; ANOVA p = 0.21; Figures 1(a) and 2). However, total BS were decreased in patients compared to HVs during fasting (1.8 ± 0.3 mM vs 3.6 ± 0.5 mM; ANOVA p = 0.03; Figure 1(b)). During fed state, total BS were similar in both groups (11.4 ± 1.7 mM vs 13.2 ± 2.1 mM; ANOVA p = 0.46; Figure 1(c)). BS peak concentrations occurred both in HVs (18.8 ± 2.7 mM vs 3.6 ± 0.5 mM; p < 0.0001) and in patients (14.5 ± 2.0 mM vs 1.8 ± 0.3 mM; p < 0.0001) 30 minutes after liquid meal intake. These total BS peak concentrations were similar between patients and HVs (p = 0.3). TUDC and GUDC were present in small amounts in human intestinal fluid, while GC and GCDC were the most abundant BS. Unconjugated BS and LC were present at or below the detection limit in aspirated intestinal fluids. The pooled concentrations of secondary BS (GDC, TDC, GUDC and TUDC) were similar in HVs and patients (Figure 2(b)). On the other hand, the pooled concentrations of primary BS (GC, TC, GCDC and TCDC) were decreased in patients compared to HVs during fasting (1.3 ± 0.2 mM vs 2.9 ± 0.4 mM; ANOVA p = 0.03; Figure 2(a)). Interestingly, the ratio of primary over secondary BS was lower in patients compared to HVs during fed state (3.7 ± 0.1 vs 5.2 ± 0.2; ANOVA p = 0.04; Figure 2(c)). When we compared the individual BS between patients and controls, GC, GCDC and TCDC concentrations were decreased in patients with FD during fasting. Also, GDC percentages were increased and TCDC percentages were decreased during fasting in patients with FD compared to HVs. However, none of these differences of individual BS remained significant after correction for multiple testing. Health state but not gender significantly determined the variation in total BS during fasting (ANOVA p = 0.03 with 12.9% of total variation and ANOVA p = 0.1 with 5.7% of total variation, respectively). In addition, no correlation was found between age and BS concentration in both groups (p = 0.5, Spearman = −0.2 in FD; p = 0.7, Spearman = 0.09 in HVs). Finally, IBS comorbidity had no effect on BS concentrations in patients with FD and HVs.
Figure 1.
Total bile salt concentration (BS conc) in healthy volunteers (HVs) and patients with functional dyspepsia. (a) This graph shows the total BS conc per time point during 2.5 hours in HVs and patients. During the first hour, the participants fast. During the last 1.5 hour, the participants are in fed state after an oral liquid meal intake. The BS conc in function of time are similar in patients and HVs. (b) The average total BS conc during fasting is lower in the patient group compared to the control group. (c) After food intake, the average total BS conc is similar in both groups. The p values were calculated with two-way analysis of variance.
Figure 2.
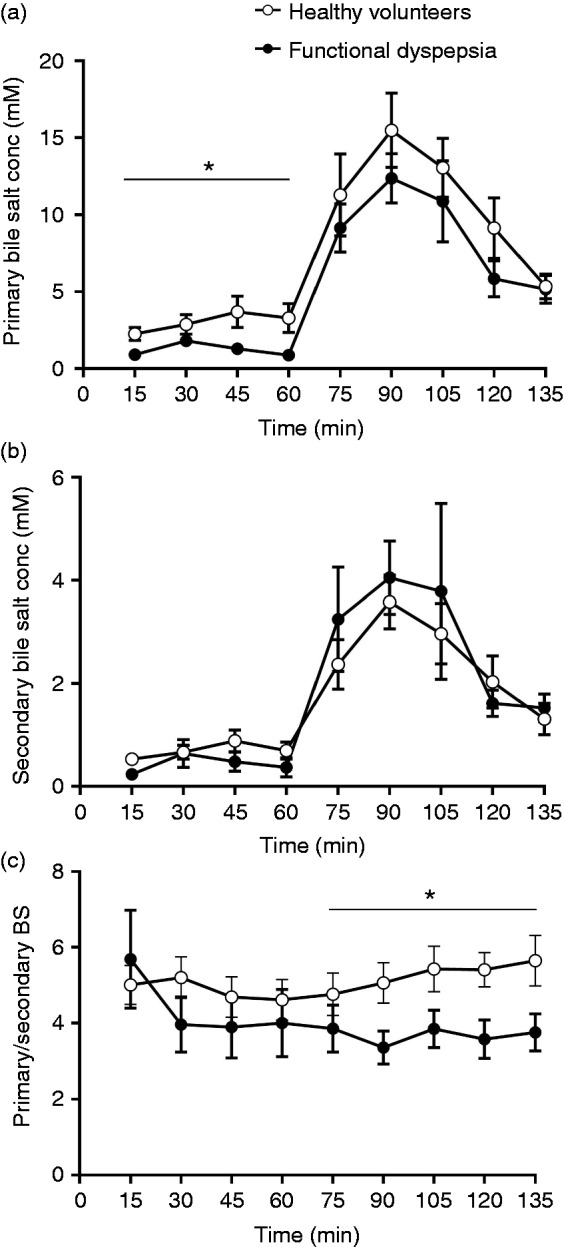
Primary and secondary bile salt concentrations (BS conc) in healthy volunteers (HVs) and patients with functional dyspepsia (FD). (a) The primary BS conc are lower in patients with FD compared to HVs during fasting. (b) The secondary BS conc are similar in both groups. (c) The primary to secondary BS ratio is decreased in patients with FD compared to HVs during fed state. The p values were calculated with two-way analysis of variance.
Patients with FD displayed duodenal VDR upregulation
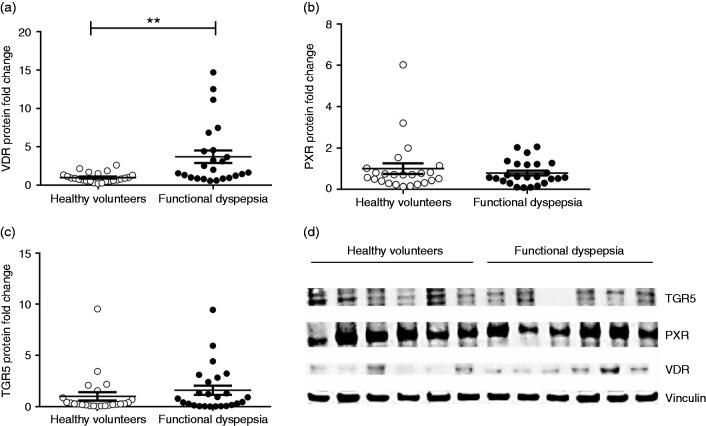
An increased duodenal VDR protein expression was shown in patients with FD compared to HVs (3.7 ± 1.0-fold; p < 0.0005; Figure 3(a)). Duodenal PXR and TGR5 expression did not differ significantly between patients and HVs (0.8 ± 0.2-fold; p = 0.9 and 1.6 ± 0.6-fold; p = 0.4, respectively; Figure 3(b) and (c)). No or very low CAR and FXR protein expression could be detected in duodenal biopsies in both groups. Furthermore, health state and gender significantly determined the variation in VDR expression (p = 0.0003 with 24.2% of total variation and p = 0.02 with 8.6% of total variation, respectively); male (N = 9) as well as female (N = 15) patients with FD had higher expression compared to male (N = 12) and female (N = 12) HVs (5.7 ± 1.6-fold; p = 0.004 and 2.5 ± 0.8-fold; p = 0.003, respectively).
Figure 3.
Expression of bile salt receptors in the duodenal mucosa. Duodenal transmembrane G-protein-coupled receptor 5 (TGR5), pregnane X receptor (PXR), vitamin D receptor (VDR) and housekeeping gene vinculin protein expression is evaluated by Western blot. Results are normalized to the housekeeping protein, vinculin, and protein fold change is determined relative to the mean value of the control group. (a) Patients with functional dyspepsia display increased duodenal VDR protein expression compared to healthy volunteers (HVs). ((b) and (c)) Duodenal PXR and TGR5 expression does not differ significantly between patients and HVs. N = 25 for both groups; **p < 0.01. (d) Representative Western blot of six HVs and six patients with FD.
Duodenal VDR expression correlated with secondary BS concentrations in HVs
We evaluated the association between VDR expression and BS concentrations in fasted and fed state in patients and volunteers by correlation analysis (Table 3). A positive correlation was found between duodenal VDR expression and secondary BS concentration (Spearman = 0.54; p = 0.01), and a negative correlation was seen between VDR expression and the ratio of primary to secondary BS (Pearson = −0.55; p = 0.01) after food intake in HVs. When we pooled the results from both groups, a positive correlation was found between VDR expression and secondary BS concentration after food intake (Spearman = 0.36; p = 0.03). However, none of the correlations remained significant after correction for multiple testing.
Table 3.
Correlation between duodenal vitamin D receptor (VDR) protein expression and bile salts.
| Correlation with duodenal VDR protein expression | Control group |
Patient group |
All participants |
|||
|---|---|---|---|---|---|---|
| p value | Correlation coefficient | p value | Correlation coefficient | p value | Correlation coefficient | |
| Fasted state | ||||||
| Total bile salts | 0.44 | 0.19 | 0.46 | −0.19 | 0.14 | −0.25 |
| Primary bile salts | 0.40 | 0.21 | 0.66 | −0.11 | 0.19 | −0.22 |
| Secondary bile salts | 0.25 | 0.28 | 0.08 | −0.44 | 0.32 | −0.17 |
| Primary/secondary bile salts | 0.09 | −0.40 | 0.53 | 0.17 | 0.97 | −0.01 |
| Fed state | ||||||
| Total bile salts | 0.64 | 0.11 | 0.79 | 0.08 | 0.96 | −0.01 |
| Primary bile salts | 0.91 | −0.03 | 0.45 | 0.21 | 0.60 | −0.09 |
| Secondary bile salts | 0.01 | 0.54 | 0.61 | 0.14 | 0.03 | 0.36 |
| Primary/secondary bile salts | 0.01 | −0.55 | 0.46 | 0.21 | 0.51 | −0.11 |
No correlation remains significant after Bonferroni correction for multiple testing.
BS concentrations correlated positively with epigastric burning
Several correlations were found between BS concentrations and dyspeptic symptoms (Table 4). With correction for multiple testing, the positive correlation between epigastric burning and BS concentrations in patients during fasting and fed state remained significant.
Table 4.
Correlation between clinical factors and total bile salts (N = 15).
| Patients with functional dyspepsia |
||||
|---|---|---|---|---|
| Clinical factors | Fasted state |
Fed state |
||
| p value | Correlation coefficient | p value | Correlation coefficient | |
| VSI | 0.09 | 0.43 | 0.27 | 0.30 |
| PHQ-9 | 0.19 | 0.34 | 0.03 | 0.56 |
| DSS | 0.004 | 0.66 | 0.21 | 0.34 |
| Weight loss | 0.14 | 0.38 | 0.77 | 0.08 |
| Discomfort | 0.31 | 0.26 | 0.49 | 0.19 |
| Postprandial fullness | 0.64 | 0.12 | 0.29 | −0.29 |
| Bloating | 0.07 | 0.45 | 0.49 | 0.19 |
| Epigastric pain | 0.01 | 0.59 | 0.08 | 0.47 |
| Early satiation | 0.30 | −0.26 | 0.33 | −0.27 |
| Nausea | 0.10 | 0.41 | 0.13 | 0.41 |
| Vomiting | 0.71 | 0.15 | 0.40 | −0.31 |
| Belching | 0.09 | 0.43 | 0.89 | 0.04 |
| Epigastric burning | <0.0001 | 0.83 | 0.0004 | 0.80 |
| Sternal burning | 0.03 | 0.53 | 0.43 | 0.23 |
Only the symptom epigastric burning remains significant after correction for multiple testing. DSS: dyspepsia symptom score; PHQ-9: Patient Health Questionnaire 9; VSI: Visceral Sensitivity Index.
Discussion
In the last few years, there has been an increasing interest in duodenal alterations in patients with FD.2–4,14 In the present paper, we report decreased total duodenal BS concentrations during fasting and increased duodenal VDR expression in FD compared to controls.
The analysis of duodenal fluids showed a similar fasting pH in patients with FD and HVs. After meal intake, however, the pH of duodenal aspirates from HVs decreased, while the pH of duodenal aspirates from patients remained similar to fasting. In line with our results in HVs, Wuyts et al. reported a pH of duodenal aspirates from HVs in fasted and fed states of 7.5 and 6.3, respectively.15 The higher postprandial pH in patients with FD is in apparent conflict with the results from a previous study by Lee et al., in which reduced duodenal acid clearance and increased late postprandial duodenal acid exposure was reported in a selected group of dyspeptic patients with prominent nausea.16 The different results may be related to differences in patient selection, meal choice, method of pH measurement and especially study design, with a shorter postprandial measurement time in the present study compared to the 24-hour duodenal pH recording in the study by Lee and colleagues in which differences occurred mainly after the second postprandial hour.
Our main finding was decreased total BS concentration during fasting in patients with FD. According to the literature, dietary components and intestinal inflammation can provoke changes in the intestinal microbiome and the microbiome can lead to changes in the BS pool.8,17 BS on the other hand can influence the intestinal microbiome by their antibacterial properties and inhibit the intestinal bacterial endotoxin absorption by their anti-inflammatory properties.8,19,18 Decreased BS levels are linked to increased bacterial load and inflammation.19 In view of this, our results suggest that decreased BS levels may contribute to duodenal changes such as increased permeability and (low-grade) inflammation, possibly through increased translocation of bacterial products.3 On the other hand, our statistically significant data do not necessarily indicate that this decreased duodenal BS concentration has a significant biological effect in patients with FD. Further research is necessary to establish to which extent an altered luminal BS composition plays a role in the pathophysiology of FD. Treatment trials with administration of exogenous BS (ursodeoxycholic acid, obeticholic acid) may be required to fully address this issue.
In the present study, we demonstrated decreased primary BS concentrations and a shifted primary to secondary BS ratio in patients with FD. Primary BS are more hydrophilic than secondary BS. Hydrophilic BS have cytoprotective effects, whereas hydrophobic BS have cytotoxic effects.8 Therefore, the hydrophobic nature of the BS pool is linked with pathological conditions.19 This finding of a decreased primary to secondary BS ratio in patients with FD may contribute to impaired duodenal barrier function.3,14 In addition, we report no effect of gender and age on BS levels. Conflicting data have been reported on male and female plasma BS concentrations.20 Our data, however, concern luminal BS concentrations.
Furthermore, duodenal VDR expression increased almost four-fold in our patient group. VDR regulates BS metabolism and synthesis and is involved in the action of secondary BS.8,18 VDR is also involved in cell differentiation, inhibition of proliferation and immunoregulation.21 Kong et al. demonstrated that VDR can protect the gastrointestinal tract from BS toxicity and preserve mucosal barrier homeostasis.21 Vitamin D3 is a strong VDR ligand that inhibits proinflammatory cytokine production, alters the gastrointestinal microbiome and increases bacterial diversity.8,22–24 These actions of vitamin D explain its positive interference in inflammatory disorders.25 It is conceivable that the upregulation of VDR in FD is part of a mucosal defense against low-grade inflammation, as has been observed in other epithelia.26,27 However, there are two alternate explanations for duodenal VDR upregulation in FD. First, VDR expression shows great variability in the duodenum.28 Inconsistent location of biopsy sites may thus induce variability. However, biopsy preservation was performed from approximately the same position in the second part of the duodenum, indicating that this is unlikely to underlie a significantly increased VDR expression in FD compared to HVs. Second, VDR upregulation may be due to a vitamin D deficiency present in the patient group. In view of this, evaluation of the vitamin D status of patients with FD is important, but was beyond the scope of this study.
Although there is no change in TGR5 and PXR expression, an altered BS composition may lead to an altered activation. TGR5 recognizes TDC, TCDC, TC and taurolithocholic acid and plays a role in preventing inflammation.9 Our results show that TCDC is present in lower levels in patients during fasting. This may suggest that TGR5 is activated to a lesser extent in patients with FD, which may add to the induction/maintenance of duodenal low-grade inflammation. On the other hand, PXR has a role in elimination and detoxification of LC, which strongly binds to PXR.9 LC, however, is not detectable in duodenal fluid. So, we can’t imply whether PXR plays a role in this pathology. Moreover, we demonstrated that being male and having FD is associated with an increased duodenal VDR expression.
Apart from vitamin D, gut microbiome and secondary BS can also regulate VDR.29 Duodenal VDR expression correlates positively with the sum of the secondary BS concentrations in HVs. The correlation suggests that an increase in secondary BS levels may induce duodenal VDR upregulation. However, this correlation is not present in patients, in whom the VDR is already upregulated, potentially as a mucosal protective measure. The pathophysiological relevance of altered duodenal BS to the symptom pattern in FD needs more investigation as we found several correlations, including a positive correlation between BS and epigastric burning. It is conceivable that the altered BS concentration has indirect effects on symptom generation, for instance through altered mucosal integrity, low-grade inflammation and a change in feedback control of gastric emptying and accommodation.2
The strengths of this study are the heterogeneous group of patients representing FD without focusing on a certain subtype, and the use of LC-MS/MS for absolute quantitation of luminal BS. It should be pointed out that the HVs and patients are not gender and age matched, resulting in an older patient group. However, we found no evidence of age- and gender-related changes in bile aspirate composition. In addition, it remains uncertain whether these changes in total BS and VDR expression are a cause of the pathophysiological mechanisms in FD or reflect a consequence of the change in diet in patients who often suffer from postprandial dyspeptic symptoms. Hence, a causal relationship needs to be established in future experiments. Finally, vitamin D blood levels and the marker for BS synthesis need to be measured in patients in future studies to validate the present results.
Overall, we report a decreased duodenal BS concentration during fasting and an increased duodenal VDR expression in FD. This may reveal a tip of a complex interaction between BS, microbiome and VDR underlying the pathophysiological mechanisms in FD. Further research is needed, however, to fully understand the role of BS in the pathophysiology of FD.
Acknowledgments
We thank all volunteers and patients for their participation in the study. We also thank the study nurses of the Gastrointestinal Motility Unit, Leuven University Hospitals.
Declaration of conflicting interests
Jan Tack has given scientific advice to AlfaWassermann, Allergan, Christian Hansen, Danone, Ironwood, Kyowa Kirin, Menarini, Mylan, Novartis, Nutricia, Shionogi, Shire, Takeda, Theravance, Tsumura, Zealand and Zeria Pharmaceuticals; has received research grants or support from Shire, Tsumura and Zeria; and has served on the speakers bureau for Abbott, Allergan, Kiowa Kirin, Menarini, Mylan, Novartis, Shire, Takeda and Zeria. The other authors have nothing to declare.
Funding
This work was supported by a Methusalem grant from Leuven University to Jan Tack (EZX-C9725-METH/14/05). Tim Vanuytsel is a senior clinical investigator, and Hanne Vanheel is a postdoctoral fellow of the Flanders Research Foundation (FWO Vlaanderen).
Ethics approval
This protocol conformed to the 1975 Declaration of Helsinki and was approved by the Leuven University Hospitals Ethics Committee (s56910; 18 December 2015).
Informed consent
All participants approved and signed the informed consent before inclusion in this study.
References
- 1.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006; 130: 1377–1390. [DOI] [PubMed] [Google Scholar]
- 2.Vanheel H, Farré R. Changes in gastrointestinal tract function and structure in functional dyspepsia. Nat Rev Gastroenterol Hepatol 2013; 10: 142–149. [DOI] [PubMed] [Google Scholar]
- 3.Vanheel H, Vicario M, Vanuytsel T, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut 2014; 63: 262–271. [DOI] [PubMed] [Google Scholar]
- 4.Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 2014; 63: 1293–1299. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M, Choi MG. Review article: Irritable bowel syndrome. Aliment Pharmacol Ther 1997; 11: 3–15. [DOI] [PubMed] [Google Scholar]
- 6.Feinle-Bisset C, Azpiroz F. Dietary and lifestyle factors in functional dyspepsia. Nat Rev Gastroenterol Hepatol 2013; 10: 150–157. [DOI] [PubMed] [Google Scholar]
- 7.Theisen J, Nehra D, Citron D, et al. Suppression of gastric acid secretion in patients with gastroesophageal reflux disease results in gastric bacterial overgrowth and deconjugation of bile acids. J Gastrointest Surg 2000; 4: 50–54. [DOI] [PubMed] [Google Scholar]
- 8.Sipka S, Bruckner G. Immunomodulatory role of bile acids. Int Arch Allergy Immunol 2014; 165: 1–8. [DOI] [PubMed] [Google Scholar]
- 9.Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol 2014; 11: 55–67. [DOI] [PubMed] [Google Scholar]
- 10.Carbone F, Holvoet L, Vandenberghe A, et al. Functional dyspepsia: Outcome of focus groups for the development of a questionnaire for symptom assessment in patients suffering from postprandial distress syndrome (PDS). Neurogastroenterol Motil 2014; 26: 1266–1274. [DOI] [PubMed] [Google Scholar]
- 11.Buckley MJ, Scanlon C, McGurgan P, et al. A validated dyspepsia symptom score. Ital J Gastroenterol Hepatol 1997; 29: 495–500. [PubMed] [Google Scholar]
- 12.Riethorst D, Mols R, Duchateau G, et al. Characterisation of human duodenal fluids in fasted and fed state conditions. J Pharm Sci 2016; 105: 673–681. [DOI] [PubMed] [Google Scholar]
- 13.Tack J, Demedts I, Dehondt G, et al. Clinical and pathophysiological characteristics of acute-onset functional dyspepsia. Gastroenterology 2002; 122: 1738–1747. [DOI] [PubMed] [Google Scholar]
- 14.Ishigami H, Matsumura T, Kasamatsu S, et al. Endoscopy-guided evaluation of duodenal mucosal permeability in functional dyspepsia. Clin Transl Gastroenterol 2017; 8: e83–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wuyts B, Riethorst D, Brouwers J, et al. Evaluation of fasted and fed state simulated and human intestinal fluids as solvent system in the Ussing chambers model to explore food effects on intestinal permeability. Int J Pharm 2015; 478: 736–744. [DOI] [PubMed] [Google Scholar]
- 16.Lee KJ, Demarchi B, Demedts I, et al. A pilot study on duodenal acid exposure and its relationship to symptoms in functional dyspepsia with prominent nausea. Am J Gastroenterol 2004; 99: 1765–1773. [DOI] [PubMed] [Google Scholar]
- 17.Urdaneta V, Casadesús J. Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts. Front Med (Lausanne) 2017; 4: 163–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appleby R, Walters J. The role of bile acids in functional GI disorders. Neurogastroenterol Motil 2014; 26: 1057–1069. [DOI] [PubMed] [Google Scholar]
- 19.Ridlon J, Kang D, Hylemon P, et al. Bile acids and the gut microbiome. Curr Opin Gastroenterol 2014; 30: 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiamoncini J, Curi R, Daniel H. Metabolism of bile acids in the post-prandial state. Essays Biochem 2016; 60: 409–418. [DOI] [PubMed] [Google Scholar]
- 21.Kong J, Zhang Z, Musch M, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 2008; 294: G208–G216. [DOI] [PubMed] [Google Scholar]
- 22.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 2005; 26: 662–687. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JD, Abreu MT. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology 2017; 152: 398–414. [DOI] [PubMed] [Google Scholar]
- 24.Bashir M, Prietl B, Tauschmann M, et al. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur J Nutr 2016; 55: 1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbalho SM, Bechara MD, de Alvares Goulart R, et al. Reflections about inflammatory bowel disease and vitamins A and D. J Med Food 2016; 19: 1105–1110. [DOI] [PubMed] [Google Scholar]
- 26.Ziv E, Koren R, Zahalka MA, et al. TNF-α increases the expression and activity of vitamin D receptor in keratinocytes: Role of c-Jun N-terminal kinase. Dermatoendocrinol 2016; 8: e1137399–e1137399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu S, Liao AP, Xia Y, et al. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol 2010; 177: 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boos A, Riner K, Hässig M, et al. Immunohistochemical demonstration of vitamin D receptor distribution in goat intestines. Cells Tissues Organs 2007; 186: 121–128. [DOI] [PubMed] [Google Scholar]
- 29.Clark A, Mach N. Role of vitamin D in the hygiene hypothesis: The interplay between vitamin D, vitamin D receptors, gut microbiota, and immune response. Front Immunol 2016; 7: 627–627. [DOI] [PMC free article] [PubMed] [Google Scholar]