Abstract
Background
Barrett’s oesophagus affects patients’ quality of life and may be a psychological burden due to the threat of developing an oesophageal adenocarcinoma.
Objective
Assessing the oesophageal adenocarcinoma risk perceived by non-dysplastic Barrett’s oesophagus patients and its association with quality of life, illness perception and reflux symptoms.
Methods
This cross-sectional questionnaire study included 158 Barrett’s oesophagus non-dysplastic patients aged 18–75 years. Based on their annual and lifetime oesophageal adenocarcinoma risk estimations measured with the Magnifier Scale, patients were classified as overestimating or underestimating. Associations between the groups where assed on demographics, reflux symptoms and results of the Outcomes Study Short-Form-36 (SF-36) and the Brief Illness Perception Questionnaire (B-IPQ).
Results
The annual oesophageal adenocarcinoma risk was overestimated by 41%. Overestimating patients had lower means on the SF-36 domains: bodily pain (annual p = 0.007 and lifetime p = 0.014), general health (annual p = 0.011 and lifetime p = 0.014), vitality (annual p = 0.030), physical functioning (lifetime p = 0.028), worse illness perception (total score p = 0.001) and significantly more reflux symptoms.
Conclusions
Overestimation of the oesophageal adenocarcinoma risk by Barrett’s oesophagus patients was associated with decreased quality of life and worse illness perceptions, which is most likely caused by symptoms of dyspepsia and reflux. These symptoms should be adequately treated, and patients may be in need of extra support and specific information about their oesophageal adenocarcinoma risk.
Keywords: Barrett’s oesophagus, quality of life, reflux, functional dyspepsia, perceived cancer risk
Study highlights
1. What is current knowledge?
- Barrett’s is a premalignant condition.
- Barrett’s patients have decreased quality of life.
- Patients tend to overestimate their oesophageal adenocarcinoma risk.
2. What is new here?
- Overestimating the risk of developing an oesophageal adenocarcinoma is associated with decreased quality of life.
- Overestimating the risk of developing an oesophageal adenocarcinoma is associated with more symptoms of reflux and dyspepsia.
- Overestimating the risk of developing an oesophageal adenocarcinoma is associated with worse illness perceptions.
Introduction
Barrett oesophagus (BO) is a premalignant condition involving a metaplastic transformation of the lower oesophageal lining from squamous to intestinal epithelium, which is caused by gastroesophageal reflux disease.1,2 BO is associated with an increased risk of an oesophageal adenocarcinoma (OAC). The relative risk of OAC in persons with non-dysplastic BO is 30–125 times higher than that of the general population; however, their absolute risk is low (approximately 0.5% per year).3
A recent systematic literature review found that BO is associated with a significant decrease in quality of life (QoL), measured via both generic and disease-targeted instruments. In addition, patients with BO are at risk for psychological consequences such as depression, anxiety and stress.
These negative effects of BO on QoL and psychological health may be related to the patient’s perception of the risk of developing OAC.4 Nevertheless, a study of 92 US patients with BO who were undergoing endoscopic surveillance found that 68% of the patients overestimated their annual risk of developing OAC, and 38% overestimated their lifetime cancer risk.5 Likewise, a European study found that 20% of BO patients overestimated their numeric annual OAC risk.6 However, to date it is unknown whether the OAC risk perceived by BO patients is associated with QoL and illness perception.
To better understand the possible psychological burden due to the threat of developing an OAC, the aim of this study was to assess the OAC risk perceived by patients with non-dysplastic BO in an endoscopic surveillance programme and to associate these perceived OAC risks with illness perception and QoL.
Materials and methods
Patients
A cross-sectional questionnaire study was performed by recruiting patients from a prospective database in an endoscopic BO surveillance programme at the Catharina Hospital, Eindhoven, The Netherlands, a tertiary referral centre for surveillance and endoscopic treatment of BO. Patients were invited to participate between November 2016 and January 2017, at a time independent of their gastroscopy.
Patients were eligible if aged between 18 and 75, and if they had prevalent non-dysplastic BO for longer than 6 months. BO was defined as red columnar lined oesophagus (>1 cm) above the proximal margins of the gastric folds on the gastroscopy, the histological presence of intestinal metaplasia in at least one biopsy, and the absence of dysplasia or OAC. Patients had to be able to read and understand the Dutch informed consent and the questionnaires.
Patients were excluded if they had a history of BO endoscopic treatment or a surgical oesophageal resection, if their life expectancy was less than 5 years or if they were to undergo a gastroscopy within 1 week of inclusion. Patients who did not respond after 4 weeks received a one-time postal reminder.
Questionnaires
Patients were asked to complete a questionnaire including demographic and clinical items, i.e. age, sex, marital status, employment status, educational level, duration of BO and comorbidity.
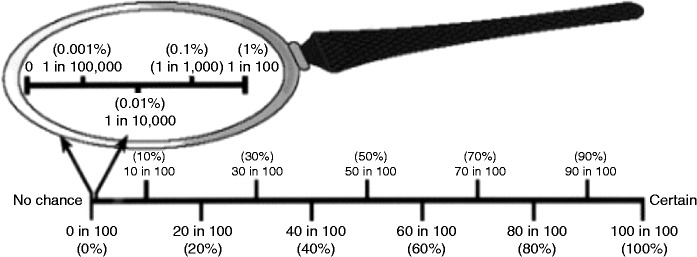
Perceived OAC risk was measured with the Magnifier Scale. This scale, which is presented in Figure 1, features a magnifying glass to represent probabilities between 0 and 100% on a logarithmic scale. This is a validated scale to assess the perceived cancer risk on a low probability range (<1%).7 The Magnifier Scale left of the line allows precise estimation of risks < 1%. The questionnaire provided the patients with the average OAC risk in the general population of 0.002% per person-year. Patients were asked to indicate their estimation of their annual and lifetime risks of developing OAC by placing an ‘X’ in the magnifying glass or on the line.
Figure 1.
The magnifying glass scale. Reprinted with permission from Woloshin et al.7
The perceived OAC risk was further assessed with two additional questions: ‘How do you perceive your own risk of developing oesophageal carcinoma in the next year?’ and ‘How do you perceive your own lifetime risk of developing oesophageal carcinoma?’. These questions were assessed using a seven-point Likert scale with the responses ‘none’, ‘very small’, ‘small’, ‘neither small nor large’, ‘large’, ‘very large’ or ‘certain’.
Generic QoL was measured with the Outcomes Study Short Form-36 (SF-36). This widely used questionnaire has been validated for measuring generic QoL in multiple disease states.8,9 The SF-36 measures health status in eight domains: physical functioning, social functioning, physical role functioning, emotional role functioning, vitality, bodily pain, mental health and general health. Scores on the SF-36 range from 0–100 on each dimension and on the summary scales, with higher scores indicating better QoL.
Cognitive and emotional representations of BO were assessed with the Brief Illness Perception Questionnaire (B-IPQ). A recent meta-analysis showed that the scales of this questionnaire had good concurrent validity and predictive validity.10–12 The B-IPQ uses a nine single-item scale approach and each item is scored on a 0–10 scale. Five of the items assess cognitive illness perceptions, two items assess emotional perceptions and one item assesses illness comprehensibility. A higher score reflects greater perceived threat of the illness. The causal scale is an open-ended response item that asks patients to list the three most important self-perceived causal factors of BO.
The presence of reflux symptoms was measured with the Gastro Esophageal Reflux Disease Questionnaire (GerdQ). This validated, self-administered six-item questionnaire uses a four-point Likert scale (0–3) to score the frequency of four positive predictors of gastroesophageal reflux disease (GERD): heartburn, regurgitation, sleep disturbance due to reflux symptoms and use of over-the-counter medication. Furthermore, it uses a reversed Likert scale (3–0) for two negative predictors of GERD (epigastric pain and nausea), resulting in a total GerdQ score range of 0–18. A score higher than eight reflects the potential presence of GERD.13,14
Statistical analysis
The cohort was divided into two groups according to their perception of developing OAC, as indicated on the Magnifier Scale. First, a dichotomous variable was created for the annual OAC risk overestimate group and for the underestimate group. Patients who perceived their annual risk to be greater than twice the annual OAC risk of 0.5% per year (>1%) were considered overestimating. A patient was considered underestimating their annual OAC risk when perceiving the OAC risk to be < 0.025%.
Secondly, a dichotomous variable was created for the lifetime OAC risk over- and underestimate group. To classify patients as over- or underestimating their lifetime OAC risk, the average life expectancy was first calculated for each subject based on sex, age and the average life expectancy according to the Central Agency for Statistics in the Netherlands.15 Then, the expected lifetime risk was calculated for each patient with the following formula: expected lifetime OAC risk = average life expectancy × 0.5%. Overestimation of a lifetime OAC risk was defined as a lifetime risk estimated as 10% higher than the calculated expected lifetime OAC risk. If subjects estimated their lifetime OAC risk to 10% lower than the calculated lifetime OAC risk, they were classified as underestimating.
The results are presented as mean with SD or as median with interquartile range (IQR), as appropriate. Subjects with missing values on the Magnifier Scale were excluded. Missing values on the GerdQ, B-IPQ were not used for analysis. Differences between the demographics of both groups were identified with the Pearson χ2 test or Fisher’s exact test, as appropriate. Bivariate analyses were performed to detect differences between the annual and lifetime overestimate and underestimate groups in terms of QoL, illness perceptions and GerdQ, using the Student’s t-test or Mann–Whitney U test (depending on normality) for continuous variables, and the χ2 test or Fisher’s exact test for categorical or ordinal variables. All tests were two-tailed.
Spearman’s rho test was used to determine the correlation between the outcomes of the Magnifier Scale and the response rating scale. The level of significance was set at a p-value of p < 0.05. Data management and analysis were performed using SPSS (IBM version 23). All authors had access to the study data, and they all reviewed and approved the final manuscript.
Results
After screening a total of 383 patient files, 233 patients were found eligible and were invited to participate in this study. In total, 170 patients (73%) signed informed consent and returned the questionnaire, and 158 patients (68%) completed the questionnaire sufficiently for analysis. Of the study population, patients were predominantly men (77%), the mean age of patients was 62.7 (36–76) years and the median time since BO diagnosis was 79 (6–383) months. The demographic and clinical baseline characteristics are shown in Table 1.
Table 1.
Demographic and clinical baseline characteristics.
| Annual risk |
Lifetime risk |
|||||
|---|---|---|---|---|---|---|
| Underestimate n = 93 (59%) | Overestimate n = 65 (41%) | p | Underestimate n = 42 (26%) | Overestimate n = 40 (25%) | p | |
| Male sex, N (%) | 75 (80.6) | 46 (70.8) | 0.15 | 35 (83.3) | 32 (80.0) | 0.78 |
| Age in years, mean (SD) | 63.0 (9.1) | 62.2 (8.9) | 0.61 | 58.9 (9.5) | 60.8 (9.5) | 0.37 |
| Time since Barrett diagnosis in months, median (IQR) | 75.0 (6–383) | 95.0 (7–319) | 0.46 | 68.5 (6–205) | 96.5 (7–319) | 0.05 |
| Marital status, N (%) | 0.10 | 0.21 | ||||
| No relationship | 4 (4.3) | 4 (6.2) | 0 (0) | 3 (7.5) | ||
| Married/living together | 83 (89.2) | 57 (87.7) | 39 (92.9) | 34 (85.0) | ||
| Divorced | 1 (1.1) | 0 (0.0) | 1 (2.4) | 0 (0.0) | ||
| Widow/widower | 5 (5.4) | 4 (6.2) | 2 (4.8) | 3 (7.5) | ||
| Education, N (%) | 0.71 | 0.35 | ||||
| <High school | 29 (31.2) | 25 (38.5) | 9 (21.4) | 12 (30.0) | ||
| High school | 30 (32.3) | 21 (32.3) | 13 (31.0) | 16 (40.0) | ||
| Bachelor’s/university | 33 (35.5) | 19 (29.2) | 19 (45.2) | 12 (30.0) | ||
| Missing value | 1 (1.1) | 0 (0.0) | 1 (2.4) | 0 (0.0) | ||
| Employment status, N (%) | 0.97 | 0.65 | ||||
| Employed | 43 (46.2) | 30 (46.2) | 25 (59.5) | 24 (60.0) | ||
| Unemployed | 9 (9.7) | 8 (12.3) | 5 (11.9) | 3 (7.5) | ||
| Retired | 39 (41.9) | 26 (40.0) | 11 (26.2) | 13 (32.5) | ||
| Missing value | 2 (2.2) | 1 (1.5) | 1 (2.4) | 0 (0.0) | ||
| Total comorbidity, mean (SD) | 2.39 (1.82) | 2.6 (1.92) | 0.43 | 1.98 (1.71) | 2.6 (1.68) | 0.089 |
| Missing value, N (%) | 3 (2.8) | |||||
| Having a friend or family member with cancer, N (%) | 14 (15.2) | 23 (35.4) | 0.003 | 7 (16.7) | 16 (40.0) | 0.019 |
| Missing value | 1 (0.93) | |||||
Demographic and clinical baseline characteristics in patients with non-dysplastic Barrett’s oesophagus who underestimated or overestimated their annual and lifetime oesophageal adenocarcinoma risk. The lifetime risk was estimated correctly by 49%, this group was not used for analysis. A p-value < 0.05 was considered significant.
IQR: interquartile range.
Perceived cancer risk
Annual OAC risk was overestimated by 65 of the 158 included patients (41%) and underestimated by 93 (59%). One patient estimated his annual risk correctly at 0.5%. The lifetime OAC risk was overestimated by 40 patients (25.1%) and correctly estimated by nearly one-half of the patients (48.4%). No significant differences were found between the groups in terms of demographic characteristics. In the overestimate groups, there were significantly more patients who had a friend or family member with cancer at the time of study participation (annual p = 0.003 and lifetime p = 0.019).
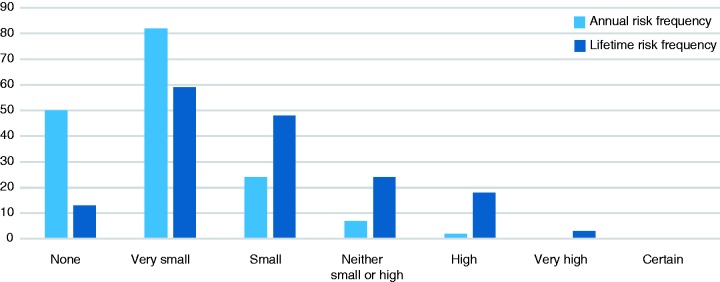
The annual risk perception on the response rate scale is presented in Figure 2. Risk perception on the Magnifier Scale significantly correlated with the OAC risk perception response rating scale (Rs = 0.58, p = <0.001 for annual risk and R = 0.66, p = < 0.001 for lifetime risk).
Figure 2.
Perceived oesophageal adenocarcinoma risk for annual and lifetime risk scores on a response-rate Likert scale in patients with non-dysplastic Barrett’s oesophagus.
GERD symptoms
Overall, 88% of patients stated that they used the PPI as prescribed by their doctor. As shown in Table 2, the overestimate group reported significantly more symptoms of reflux and functional dyspepsia. However, the groups showed no significant differences in the total means of the GerdQ. There were significantly more scores above eight (p = 0.027) in the lifetime overestimate group, suggesting the presence of GERD.
Table 2.
Dyspepsia and reflux symptoms.
| Annual risk Median (SD) |
Lifetime risk Mean (SD) |
|||||
|---|---|---|---|---|---|---|
| Underestimate n = 92 (IQR) | Overestimate n = 63 (IQR) | p | Underestimate n = 42 (IQR) | Overestimate n = 38 (IQR) | p | |
| Heartburn | 0.00 (0.00–0.00) | 0.00 (0.00–2.00) | 0.001 | 0.00 (0.00–0.25) | 0.00 (0.00–2.00) | 0.024 |
| Regurgitation | 0.00 (0.00–0.00) | 0.00 (0.00–1.00) | 0.004 | 0.00 (0.00–0.00) | 0.50 (0.00–2.00) | 0.000 |
| Epigastric pain | 3.00 (3.00–3.00) | 3.00 (1.00–3.00) | 0.033 | 3.00 (3.00–3.00) | 2.00 (1.00–3.00) | 0.000 |
| Nausea | 3.00 (3.00–3.00) | 3.00 (2.00–3.00) | 0.28 | 3.00 (3.00–3.00) | 3.00 (2.00–3.00) | 0.011 |
| Sleeping difficulties | 0.00 (0.00–0.00) | 0.00 (0.00–1.00) | 0.065 | 0.00 (0.00–0.00) | 0.50 (0.00–1.25) | 0.001 |
| Use of counter medication | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.32 | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.23 |
| Total | 6.00 (6.00–7.00) | 6.00 (6.00–9.00) | 0.21 | 6.00 (6.00–7.00) | 6.00 (6.00–9.00) | 0.36 |
| Score > 8, N (%) | 22 (24) | 24 (38) | 0.074 | 7 (17) | 15 (40) | 0.027 |
Gastro Esophageal Reflux Disease Questionnaire scores in patients with non-dysplastic Barrett’s oesophagus who underestimated or overestimated their annual or lifetime OAC risks. There were three patients with missing values; these patients were not used for analysis. A p-value < 0.05 was considered significant.
IQR: interquartile range.
QoL
The results of the SF-36 summary scores are presented in Table 3. Both the annual and the lifetime overestimates group showed significantly lower means on three of the physical domains, namely physical functioning, bodily pain and general health.
Table 3.
Quality of life.
| Annual risk Mean (SD) |
Lifetime risk Mean (SD) |
|||||
|---|---|---|---|---|---|---|
| Underestimate n = 91 | Overestimate n = 64 | p | Underestimate n = 42 | Overestimate n = 39 | p | |
| PF- physical functioning | 84.0 (22.4) | 80.4 (22.9) | 0.024 | 90.5 (19.3) | 83.1 (21.8) | 0.064 |
| RP- role functioning physical | 82.1 (32.8) | 72.6 (39.3) | 0.11 | 85.1 (32.2) | 75.0 (39.3) | 0.14 |
| RE- role functioning emotional | 88.3 (27.8) | 85.4 (31.6) | 0.72 | 85.7 (31.4) | 87.2 (29.2) | 0.92 |
| SF- social functioning | 90.4 (17.9) | 86.7 (21.0) | 0.43 | 93.5 (12.1) | 89.4 (21.0) | 0.24 |
| BP- bodily pain | 82.3 (23.1) | 72.8 (24.7) | 0.004 | 85.9 (20.1) | 73.1 (24.6) | 0.008 |
| MH- mental health | 84.1 (15.6) | 82.5 (16.4) | 0.36 | 83.8 (19.4) | 82.5 (14.2) | 0.62 |
| VT- vitality | 71.3 (22.9) | 66.8 (24.9) | 0.030 | 73.7 (23.7) | 65.3 (20.9) | 0.068 |
| GH- general health | 69.8 (21.6) | 58.19 (20.9) | 0.012 | 71.0 (24.0) | 59.2 (19.1) | 0.015 |
Outcomes Study Short Form-36 scores in patients with non-dysplastic Barrett’s oesophagus who underestimate or overestimate their annual and lifetime risk. There were three missing values; these patients were not used for analyses. A p-value < 0.05 was considered significant.
Illness perception
Patients who overestimated their annual or lifetime OAC risk experienced more symptoms (p = 0.001), had more concerns about their BO (p = 0.000), were more emotionally affected by their BO (p = 0.000), experienced more consequences of the BO (p = 0.000) and were less satisfied with the treatment controlling their BO (p = 0.034). No significant differences were found between the two groups regarding their understanding of BO, their personal control of the disease and their perception of the duration of their BO. The total scores of the illness perception scale were significantly higher/more threatening in the overestimate groups (annual p = 0.000 and lifetime p = 0.000).
Discussion
As is already known, BO is a premalignant condition that affects patients’ QoL and it may be a psychological burden due to the threat of developing OAC. This study is the first to show that overestimating the OAC risk is associated with a significantly lower QoL in the physical domains, more reflux and dyspeptic symptoms and worse illness perceptions. These differences were not associated with the number of comorbidities. It is important to point out that in comparison to the QoL results in other BO populations, our study population scored higher overall on all domains of the SF-36.16–18
The association between overestimating the OAC risk, reduced QoL and worse illness perceptions may partly be explained by the presence of more symptoms of reflux and dyspepsia. This is consistent with the study of Shaheen et al.,5 who found that patients overestimating their risk of developing OAC were more likely to have reflux symptoms. A Chinese study found that Health Related Quality of Life in BO patients was strongly associated with presentation of reflux symptoms.19
Patients who overestimated their OAC risk were significant more likely to have a friend or family member with cancer at the time of study participation, hence this factor could most likely have influenced their illness perception. These results are in line with those of previous studies that concluded that a family history of cancer is associated with overestimating one’s own cancer risk.20,21
When assessing the OAC risk perceived by BO patients, previous studies used several instruments other than the Magnifier Scale. A Likert linear number scale was used by Kruyshaar et al.,6 and time trade-off values were used by Gerson et al.16 The linear number scale and the magnifying glass scale are similar in validity, reliability and usability. However, only the magnifying glass scale is validated for eliciting perceptions in the low-probability range (<1%).11 A previous study showed that time trade-off values may be less valid in patients aged over 60.22 Since the average BO population is 60 or older, time trade-off values may not have been appropriate in our study population. In our opinion, by using the Magnifier Scale like Shaheen et al.,5 this study used the best-validated scale available for assessing the perceived OAC risk within the BO population.
In contrast to the results of Shaheen et al.,5 this study showed that the majority underestimated their annual and lifetime OAC risk (68 versus 41%). A possible explanation for this difference might be that there are several culture differences as well as differences in healthcare systems. In contrast to Shaheen et al.,5 our questionnaire provided patients with the average OAC in the general population of 0.002% per person-year. This may have influenced our patients to perceive their OAC risk to be lower on the Magnifier Scale.
A limitation of this cross-sectional study is that although associations are confirmed, no causal factor of overestimating behaviour can be identified. There is a potential bias in patients who experienced psychological stress caused by non-BO-related origins, which may have led to more reflux and dyspeptic symptoms. Also, this was a single-centre study in a BO expert clinic, which implies that our study population may not be representative of the BO population worldwide.
Overall, this study confirms that overestimation of the OAC risk by non-dysplastic BO patients is associated with a decreased QoL and worse illness perception, which is most likely caused by symptoms of dyspepsia and reflux. Providers caring for patients with BO should be aware of the implications of the diagnosis. Patients may be in need of extra support and specific information about their OAC risk. BO patients experiencing reflux-related symptoms should receive adequate treatment.
Further research should be undertaken to investigate the causal factors that influence the OAC risk perceived by BO patients (e.g. patient information and reflux symptoms) in order to improve QoL in this patient group.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Informed consent
Written informed consent was obtained from each patient included in the study.
Ethics approval
The study protocol was approved on 9 September 2016 by the Medical Research Ethics Committees United (MEC-U) reference numberW16.113 and conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a prior approval by the institution s human research committee.
References
- 1.Barrett NR. Chronic peptic ulcer of the oesophagus and “oesophagitis”. Br J Surg 1950; 38: 175–182. [DOI] [PubMed] [Google Scholar]
- 2.Allison PR, Johnstone AS. The oesophagus lined with gastric mucous membrane. Thorax 1953; 8: 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yousef F, Cardwell C, Galway K, et al. The incidence of esophageal cancer and high-grade dysplasia in Barrett's esophagus: A systematic review and meta-analysis. Am J Epidemiol 2008; 168: 237–249. [DOI] [PubMed] [Google Scholar]
- 4.Crockett SD, Lippmann QK, Dellon ES, et al. Health-related quality of life in patients with Barrett's Esophagus: A systematic review. Clin Gastroenterol Hepatol 2009; 7: 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaheen NJ, Green B, Medapalli RK. The perception of cancer risk in patients with prevalent Barrett's esophagus enrolled in an endoscopic surveillance program. Gastroenterology 2005; 129: 429–436. [DOI] [PubMed] [Google Scholar]
- 6.Kruijshaar ME, Siersema PD, Janssens ACJW, et al. Patients with Barrett's esophagus perceive their risk of developing esophageal adenocarcinoma as low. Gastrointest Endosc 2007; 65: 26–30. [DOI] [PubMed] [Google Scholar]
- 7.Woloshin S, Schwartz LM, Byram S, et al. A new scale for assessing perceptions of chance (a validation study). Med Decis Making 2000; 20: 298–307. [DOI] [PubMed] [Google Scholar]
- 8.McHorney CA, Ware JE, Raczek AE. The MOS 36-item Short-Form Health Survey (SF-36):II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. MedCare 1993; 31: 247–263. [DOI] [PubMed] [Google Scholar]
- 9.Ware JE, Jr, Gandek B, Kosinski M, et al. The equivalence of SF-36 summary health scores estimated using standard and country-specific algorithms in 10 countries: Results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998; 51: 1167–1170. [DOI] [PubMed] [Google Scholar]
- 10.Broadbent E, Petrie KJ, Main J, et al. The brief illness perception questionnaire. J Psychosom Res 2006; 60: 631–637. [DOI] [PubMed] [Google Scholar]
- 11.Weinman J, Petrie KJ. Illness perceptions: A new paradigm for psychosomatics? J Psychosom Res 1997; 42: 113–116. [DOI] [PubMed] [Google Scholar]
- 12.Weinman J, Petrie KJ, Moss-Morris R, et al. The illness perception questionnaire: A new method for assessing the cognitive representation of illness. Psychol Health 1996; 11: 431–446. [Google Scholar]
- 13.Jonasson C, Wernersson B, Hoff DA, et al. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2013; 37: 564–572. [DOI] [PubMed] [Google Scholar]
- 14.Jones R, Junghard O, Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther 2009; 15: 30: 1030–1038. [DOI] [PubMed] [Google Scholar]
- 15.Central Agency for Statistics in the Netherlands. Den haag/heerlen. Jan 2017. Retrieved from the website of the Central agency for Statistics in the Netherlands https://opendata.cbs.nl/statline/#/CBS/nl/dataset/71950ned/table?ts=1535737814109 (accessed Jan 2017).
- 16.Gerson LB, Ullah N, Hastie T, et al. Does cancer risk affect health-related quality of life in patients with Barrett's esophagus? Gastrointest Endosc 2007; 65: 16–25. [DOI] [PubMed] [Google Scholar]
- 17.Eloubeidi MA, Provenzale D. Health-related quality of life and severity of symptoms in patients with Barrett’s esophagus and gastroesophageal reflux disease patients without Barrett’s esophagus. Am J Gastroenterol 2000; 95: 1881–1887. [DOI] [PubMed] [Google Scholar]
- 18.Eloubeidi M, Uslick B, Provenzale D. Health related quality of life (HRQL) in patients with Barrett’s esophagus. Gastroenterology 1997; 112: A14–A14. [DOI] [PubMed] [Google Scholar]
- 19.Lee SW, Lien HC, Chang CS, et al. Health-related quality of life of subjects with Barrett's esophagus in a Chinese population. Plos One 2017; 12: e0190201–e0190201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rini C, Jandorf L, Valdimarsdottir H, et al. Distress among inflammatory bowel disease patients at high risk for colorectal cancer: A preliminary investigation of the effects of family history of cancer, disease duration, and perceived social support. Psychooncology 2008; 17: 354–362. [DOI] [PubMed] [Google Scholar]
- 21.Butler KM, Rayens MK, Wiggins AT, et al. Association of smoking in the home with lung cancer worry, perceived risk, and synergistic risk. Oncol Nurs Forum 2017; 44: E55–E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolan P, Gudex C, Kind P, et al. The time trade-off method: Results from a general population study. Health Econ 1996; 5: 141–154. [DOI] [PubMed] [Google Scholar]