Abstract
Background
Several noninvasive biomarkers are available for diagnosing liver fibrosis stage and predicting hepatocellular carcinoma (HCC) development in patients with chronic hepatitis and liver cirrhosis. However, these biomarkers are not sufficiently accurate. Recently, von Willebrand factor (VWF) has been related to angiogenesis and apoptosis. Furthermore, VWF is associated with hepatic spare ability and HCC.
Objective
We aimed to determine whether VWF is a potential biomarker for liver fibrosis and HCC development.
Methods
Two hundred and twelve patients with chronic hepatitis B and C were recruited. VWF antigen (VWF: Ag) levels in each patient were determined via enzyme-linked immunosorbent assay. Univariable and multivariable analyses were used to determine the risk factor of HCC.
Results
The VWF: Ag levels were higher in patients with severe liver fibrosis stage and/or HCC development than in those without. The area under the curve of VWF: Ag for diagnosis of severe liver fibrosis stage was 0.721. Multivariable analysis showed that only VWF: Ag was a predictive biomarker for HCC development.
Conclusions
VWF: Ag is related to liver fibrosis and may be useful for predicting HCC development. VWF is a potentially useful biomarker to diagnose severe liver fibrosis and predict HCC development.
Keywords: VWF, HCC, chronic hepatitis, liver cirrhosis, angiogenesis
Introduction
Chronic hepatitis prognosis is affected by the occurrence of liver cirrhosis (LC) and hepatocellular carcinoma (HCC).1,2 Evaluating liver fibrosis and predicting HCC development and/or diagnosis of HCC in the early stage may improve prognosis. Liver biopsy is useful to evaluate liver fibrosis, but is associated with several risks, such as bleeding and infection.3 Thus, a noninvasive and simple examination to evaluate liver fibrosis progression should be developed. Many biomarkers have been developed for evaluating liver fibrosis progression, including hyaluronic acid and type IV collagen 7S.3 However, these biomarkers are not sufficient for correctly diagnosing liver fibrosis because they are influenced by other factors, such as inflammation, renal dysfunction and collagen disease.4
There are 780,000 new cases of HCC and 750,000 annual deaths due to HCC worldwide.5 Although many biomarkers6 including α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP) have been developed, they are not useful for predicting and diagnosing early-stage HCC. HCC can be diagnosed at an early stage through imaging techniques. However, a method for the prediction of HCC development has not yet been established. HCC usually develops in patients with severe liver fibrosis and/or elderly patients. However, it also develops in many patients with mild-to-moderate liver fibrosis and/or young patients.
von Willebrand factor (VWF)7,8 is a multimeric glycoprotein with a signal peptide, propeptide and mature VWF portion, which consists of 2050 amino acids. It is synthesized in vascular endothelial cells (ECs) and released into the plasma as unusually large VWF multimers.9 Every VWF monomer contains many specific domains with a particular function, such as the A1 domain, which binds to platelet GPIb receptor. VWF is released into the plasma from a damaged blood vessel, binds to collagen on the damaged vascular ECs, binds to platelets and promotes blood coagulation.7–9 VWF is involved in the mechanisms of hemostasis, such as platelet adhesion and aggregation.
The quantitative and/or qualitative abnormalities of VWF lead to von Willebrand disease (VWD),10,11 which involves bleeding tendencies such as subcutaneous bleeding, mucosal bleeding including nosebleeds, menorrhagia, and gastrointestinal bleeding. VWF may be involved in angiogenesis as VWD is associated with angiodysplasia;12 endoscopic examination shows that many angioectasias are related to bleeding in the gastrointestinal tract. The lack of VWF promotes angiogenic processes, as vascular EC proliferation is increased in the absence of VWF in vitro and the density of the vasculature is increased in VWF-deficient mice.13–15
VWF antigen (VWF: Ag) is associated with hepatic spare ability and HCC, as VWF: Ag is increased in patients with LC in accordance with the progression of LC16 and HCC;17 this pathophysiology is related to angiogenesis.18,19 VWF: Ag levels are associated with the severity of malignant tumours.20 In the current study, we investigated the relationship between VWF: Ag and liver fibrosis or hepatocarcinogenesis, and attempted to determine whether VWF: Ag is a potentially useful biomarker to evaluate liver fibrosis and predict HCC development.
Materials and methods
Study design
This retrospective observational study included patients with chronic hepatitis types B and C who underwent liver biopsy from October 2002 to October 2010. We initially investigated the relationship between liver fibrosis stage and characteristics of patients. Subsequently, we studied the relationship between HCC development during the observation period and characteristics of patients at the time of liver biopsy. The eligibility criteria were age ≥20 years and positive results for hepatitis B surface antigen or hepatitis C virus antibody. The exclusion criteria were history of HCC at the time of liver biopsy and <100 days of observation period. All subjects gave written informed consent prior to participation in the study. The study protocol was approved by the Ethics Committee of Nara Medical University (project number: 471) on 4 December 2011 and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Patients
Three hundred and fifteen patients underwent liver biopsy at our hospital from February 2002 to October 2010. We enrolled 212 patients according to the study criteria. All patients had no thrombosis. Twenty-seven patients developed HCC during the observation period. Six patients who developed HCC achieved sustained virological response for hepatitis C or showed disappearance of hepatitis B virus by interferon, direct-acting antiviral or nucleoside analogue therapy. The 132 patients without HCC achieved sustained virological response for hepatitis C or showed disappearance of hepatitis B virus by interferon, direct-acting antiviral or nucleoside analogue therapy. Twenty-seven patients died during the observation period (3 patients died due to liver failure; 3 patients, HCC; 1 patient, oesophageal varix rupture; and 20 patients, disease other than liver disease).
Evaluation
Liver tissues were fixed in 10% formalin and embedded in paraffin. The paraffin tissues were cut into 5 µm-thick sections. We used haematoxylin and eosin staining and azan staining to evaluate liver fibrosis stage. Liver fibrosis stage was diagnosed according to new the Inuyama classification (F0, no fibrosis; F1, portal fibrosis widening; F2, portal fibrosis widening with bridging fibrosis; F3, bridging fibrosis plus lobular distortion; and F4, LC) of chronic hepatitis proposed by the Inuyama Symposium.21 After liver biopsy, follow-up evaluation was performed. The patients underwent blood examination, including AFP and/or DCP, every 3–4 months and ultrasound examination, dynamic computed tomographic scanning and/or dynamic magnetic resonance imaging every 4–6 months. The surveillance and diagnosis of HCC was performed in accordance with the clinical practice guidelines for HCC 2013 by The Japan Society of Hepatology.22
Determination of VWF: Ag levels
Blood samples were obtained from patients at the time of liver biopsy. These samples were stored in plastic tubes containing 0.38% sodium citrate. Platelet-poor plasma was prepared by centrifugation at 3000 × g for 15 min at 4℃ and stored in aliquots at –80℃ until analysis. Plasma VWF: Ag was measured by sandwich enzyme-linked immunosorbent assay using a rabbit polyclonal anti-human VWF antibody (Dako, Denmark), and the normal level of VWF: Ag was 100 ± 53%.
Statistical analysis
The differences between the paired and unpaired groups were analyzed using the Mann–Whitney U-test and the Steel–Dwass test. Correlations were calculated with Spearman’s rank test. Categorical data were analyzed using Fisher’s exact test. Univariate and multivariate analyses were performed to determine the risk factors for HCC development. The following 14 variables were analyzed for potential covariates that are risk factors for HCC development at the time of sample collection: age, sex, hepatitis virus, albumin, aspartate aminotransferase, alanine aminotransferase, prothrombin time, platelet count, AFP, DCP, hyaluronic acid, type IV collagen 7S, liver fibrosis stage and VWF: Ag. The Cox proportional hazards regression analysis was applied to determine independent risk factors for HCC development using age, albumin, aspartate aminotransferase, platelet count, hyaluronic acid, liver fibrosis stage and VWF: Ag. These factors had a p value < 0.2 in the univariate analysis. Multivariate analysis using a Cox proportional hazard regression model, with stepwise selection of variables based on the Akaike information criterion, was also performed. Data are expressed as mean ± SD. A two-tailed p value < 0.05 was considered significant. Analyses were carried out using EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0). EZR is a modified version of R commander (version 1.6-3) that includes statistical functions that are frequently used in biostatistics.23
Results
Clinical characteristics of patients
The patient characteristics are shown in Table 1. The mean age of patients with chronic liver injury at liver biopsy was 57.7 ± 12.0 years. Twenty-five patients had chronic hepatitis type B and 187 patients had chronic hepatitis type C. The study population comprised 100 men and 112 women. The average observation period after liver biopsy was 3052.9 ± 1154.6 (range: 136–5551) days. The average age of the patients who developed HCC was 66.1 ± 7.2 years at the time of liver biopsy. HCC development period was 2541.1 ± 1088.4 (range: 647–4265) days. Age, AFP, hyaluronic acid, type IV collagen 7S and fibrosis 4 (Fib4) index were higher in patients with severe fibrosis (F3 and F4) than in patients with absent to moderate liver fibrosis (F0 to F2). Albumin, prothrombin time and platelet count were lower in patients with severe fibrosis than in patients with absent to moderate liver fibrosis (Table 2). Age, aspartate aminotransferase, AFP, hyaluronic acid, Fib4 index and liver fibrosis stage were higher in patients who developed HCC during the observation period than in patients without HCC at the time of liver biopsy. Albumin was lower in patients who developed HCC during the observation period than in patients without HCC (Table 3).
Table 1.
Characteristics of patients.
| Variable | Patients (n = 212) |
|---|---|
| Age (year) | 57.7 ± 12.0 |
| Sex (male/female) | 100/112 |
| Hepatitis B/C | 25/187 |
| Albumin (g/dL) | 4.25 ± 0.39 |
| Aspartate aminotransferase (IU/L) | 53.6 ± 53.1 |
| Alanine aminotransferase (IU/L) | 67.2 ± 72.9 |
| Prothrombin time (%) | 99.3 ± 14.3 |
| Platelet count (×104/mm3) | 18.5 ± 17.4 |
| AFP (ng/ml) | 16.6 ± 53.4 |
| DCP (mAU/ml) | 21.3 ± 7.2 |
| Hyaluronic acid (ng/ml) | 115.4 ± 163.8 |
| Tyep IV collagen 7S (ng/ml) | 25.7 ± 56.2 |
| Fib4 index | 2.72 ± 2.07 |
| Liver fibrosis (stage) | 1.70 ± 0.98 |
| VWF: Ag (%) | 141.4 ± 91.5 |
The data are expressed as mean ± SD.
AFP: α-fetoprotein; DCP: des-γ-carboxy prothrombin; Fib4 index: The fibrosis 4 index; VWF: Ag: von Willebrand factor antigen.
Table 2.
Clinical data of patients between mild to moderate liver fibrosis and severe liver fibrosis.
| Variable | None to moderate liver fibrosis (F0–2, n = 153) | Severe liver fibrosis (F3–4, n = 59) | p value |
|---|---|---|---|
| Age (year) | 56.6 ± 12.3 | 61.1 ± 10.4 | p < 0.05 |
| Sex (male/female) | 68/85 | 32/27 | NS |
| Hepatitis B/C | 19/134 | 6/53 | NS |
| Albumin (g/dL) | 4.3 ± 0.35 | 4.1 ± 0.44 | p < 0.05 |
| Aspartate aminotransferase (IU/L) | 49.9 ± 55.1 | 62.3 ± 30.4 | NS |
| Alanine aminotransferase (IU/L) | 63.1 ± 55.1 | 74.9 ± 50.2 | NS |
| Prothrombin time (%) | 101.3 ± 14.6 | 92.7 ± 11.0 | p < 0.05 |
| Platelet count (×104/mm3) | 19.7 ± 19.3 | 14.3 ± 6.9 | p < 0.05 |
| AFP (ng/ml) | 6.1 ± 9.4 | 21.0 ± 31.0 | p < 0.05 |
| DCP (mAU/ml) | 21.0 ± 6.7 | 23.5 ± 8.5 | NS |
| Hyaluronic acid (ng/ml) | 73.8 ± 76.2 | 224.2 ± 265.1 | p < 0.05 |
| Type IV collagen 7S (ng/ml) | 21.6 ± 53.1 | 31.1 ± 51.5 | p < 0.05 |
| Fib4 index | 2.50 ± 1.85 | 4.77 ± 2.76 | p < 0.05 |
| Liver fibrosis (stage) | 1.24 ± 0.48 | 3.29 ± 0.46 | p < 0.05 |
| VWF: Ag (%) | 129.6 ± 87.4 | 185.3 ± 95.2 | p < 0.05 |
The data are expressed as mean ± SD.
AFP: α-fetoprotein; DCP: des-γ-carboxy prothrombin; F0: no fibrosis; F1: portal fibrosis widening; F2: portal fibrosis widening with bridging fibrosis; F3: bridging fibrosis plus lobular distortion; F4: liver cirrhosis; Fib4 index: The fibrosis 4 index; VWF: Ag: von Willebrand factor antigen.
Table 3.
Clinical data of patients with or without hepatocellular carcinoma development.
| Variable | HCC not development (n = 185) | HCC development (n = 27) | p value |
|---|---|---|---|
| Age (year) | 56.3 ± 12.3 | 66.1 ± 7.2 | p < 0.05 |
| Sex (male/female) | 85/100 | 15/12 | NS |
| Hepatitis B/C | 24/161 | 1/26 | NS |
| Albumin (g/dL) | 4.3 ± 0.32 | 4.1 ± 0.48 | p < 0.05 |
| Aspartate aminotransferase (IU/L) | 47.7 ± 30.4 | 64.5 ± 36.9 | p < 0.05 |
| Alanine aminotransferase (IU/L) | 61.1 ± 55.6 | 62.8 ± 31.2 | NS |
| Prothrombin time (%) | 98.6 ± 14.5 | 92.9 ± 9.7 | NS |
| Platelet count (×104/mm3) | 18.7 ± 20.3 | 12.7 ± 5.4 | NS |
| AFP (ng/ml) | 7.8 ± 11.0 | 31.4 ± 47.0 | p < 0.05 |
| DCP (mAU/ml) | 20.8 ± 4.9 | 21.5 ± 7.5 | NS |
| Hyaluronic acid (ng/ml) | 103.8 ± 159.2 | 175.2 ± 148.3 | p < 0.05 |
| Type IV collagen 7S (ng/ml) | 24.7. ± 54.6 | 9.1 ± 4.5 | NS |
| Fib4 index | 2.43 ± 1.83 | 4.66 ± 2.52 | p < 0.05 |
| Liver fibrosis (stage) | 1.66 ± 0.94 | 2.42 ± 1.03 | p < 0.05 |
| VWF: Ag (%) | 137.9 ± 86.9 | 207.3 ± 107.9 | p < 0.05 |
The data are expressed as mean ± SD.
AFP: α-fetoprotein; DCP: des-γ-carboxy prothrombin; Fib4 index: The fibrosis 4 index; VWF: Ag: von Willebrand factor antigen.
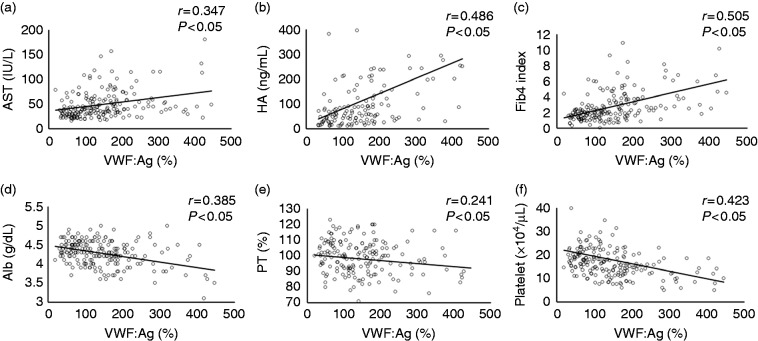
Relationship between VWF: Ag and liver fibrosis and laboratory examinations
The plasma levels of VWF: Ag increased according to the progression of liver fibrosis and were directly correlated with liver fibrosis stage (Figure 1). VWF: Ag levels were directly correlated with aspartate aminotransferase (Figure 2(a)), hyaluronic acid (Figure 2(b)) and Fib4 index (Figure 2(c)). VWF: Ag was inversely correlated with albumin (Figure 2(d)), prothrombin time (Figure 2(e)) and platelet count (Figure 2(f)). Other parameters including type IV collagen 7S were not correlated with VWF: Ag. VWF: Ag level was higher in patients with severe fibrosis (F3 and F4) than in patients with absent to moderate liver fibrosis (F0 to F2) (Table 2). The area under the curve (AUC) of VWF: Ag for the diagnosis of severe liver fibrosis stage was 0.721, which has moderate accuracy in the receiver operating characteristic curve. The AUCs were not different between VWF: Ag and hyaluronic acid (0.796), IV collagen 7S (0.744) and Fib4 index (0.755). The AUCs were also not different between VWF: Ag and other biomarkers and/or parameters including age, albumin, aspartate aminotransferase, prothrombin time, platelet count and AFP (data not shown).
Figure 1.
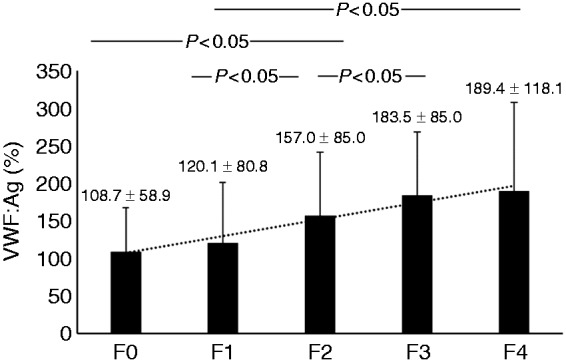
Relationship between von Willebrand factor antigen levels and liver fibrosis stage.
The plasma levels of von Willebrand factor antigen were F0: 108.7 ± 58.9%, F1: 120.1 ± 80.8%, F2: 157.0 ± 85.0%, F3: 183.5 ± 85.0% and F4: 189.4 ± 118.2%. von Willebrand factor antigen increased according to progression of liver fibrosis and was directly correlated with liver fibrosis stage (r = 0.313, p < 0.05).
F0: no fibrosis; F1: portal fibrosis widening; F2: portal fibrosis widening with bridging fibrosis; F3: bridging fibrosis plus lobular distortion; F4: liver cirrhosis; VWF: Ag: von Willebrand factor antigen.
Figure 2.
Relationship between laboratory data and von Willebrand factor antigen levels.
The plasma levels of von Willebrand factor antigen were directly correlated with (a) aspartate aminotransferase (p < 0.05), (b) hyaluronic acid (p < 0.05) and (c) fibrosis 4 index (p < 0.05). von Willebrand factor antigen levels were inversely correlated with (d) albumin (p < 0.05), (e) prothrombin time (p < 0.05) and (f) platelet count (p < 0.05).
Alb: albumin; AST: aspartate aminotransferase; Fib4 index: fibrosis 4 index; HA: hyaluronic acid; PT: prothrombin time; VWF: Ag: von Willebrand factor antigen.
Advantage of VWF: Ag as predictive biomarker for HCC development
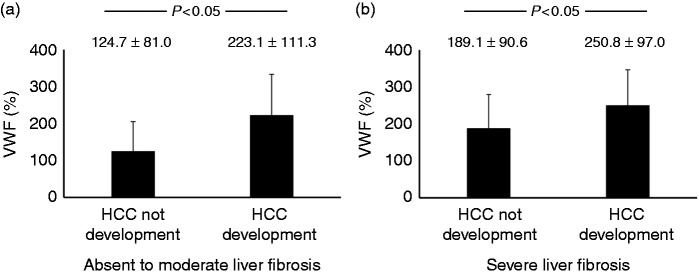
The VWF: Ag was higher in patients who developed HCC during the observation period than in patients without HCC at the time of liver biopsy (Table 3). In addition, the VWF: Ag was higher in patients who developed HCC with absent to moderate liver fibrosis (F0 to F2) and severe fibrosis (F3 and F4) during the observation period than in patients without HCC at the time of liver biopsy (Figure 3(a) and (b)). The VWF: Ag was not correlated with AFP and DCP (not shown). To detect predictive biomarkers for HCC development, we performed univariate analysis. Univariate analysis showed that VWF: Ag, age, albumin, aspartate aminotransferase, platelet count and liver fibrosis stage were useful predictive biomarkers for HCC development, whereas AFP and DCP were not. We also performed multivariate analysis of predictive biomarkers for HCC development. Multivariate analysis showed that only VWF: Ag was a useful predictive biomarker for HCC development (Table 4).
Figure 3.
Relationship between von Willebrand factor antigen levels and hepatocellular carcinoma development.
The von Willebrand factor antigen levels of patients who developed hepatocellular carcinoma with absent to moderate liver fibrosis (F0–2) and severe fibrosis (F3 and F4) during the observation period was higher than that of patients without hepatocellular carcinoma at the time of liver biopsy ((a) and (b)).
F0: no fibrosis; F1: portal fibrosis widening; F2: portal fibrosis widening with bridging fibrosis; F3: bridging fibrosis plus lobular distortion; F4: liver cirrhosis; HCC: hepatocellular carcinoma; VWF: Ag: von Willebrand factor antigen.
Table 4.
Predictive biomarker of hepatocellular carcinoma development.
| Variable | HR (95% CI) | p value |
|---|---|---|
| Unvariable analysis | ||
| Age (per 1-year increase) | 1.090 (1.028–1.156) | <0.001 |
| Sex (male vs. female) | 0.6491 (0.2793–1.508) | 0.3150 |
| Hepatitis virus (B vs. C) | 2.384 (0.3181–17.86) | 0.3979 |
| Albumin (per 1 g/dL decrease) | 0.2547 (0.07188–0.9968) | <0.05 |
| Aspartate aminotransferase (per 1 IU/L increase) | 1.018 (1.018–1.008) | <0.05 |
| Alanine aminotransferase (per 1 IU/L increase) | 1.006 (0.9968–1.014) | 0.2135 |
| Prothrombin time (per 1% decrease)) | 0.9918 (0.974–1.01) | 0.3771 |
| Platelet count (per 104/µL decrease) | 0.8864 (0.8126–0.9670) | <0.001 |
| AFP (per 1 ng/ml increase) | 1.000 (0.9998–1.000) | 0.9271 |
| DCP (per 1 mAU/ml increase) | 1.005 (0.9459–1.067) | 0.8809 |
| Hyaluronic acid (per 1 ng/ml increase) | 1.002 (1.000–1.004) | 0.1559 |
| Type IV collagen 7S (per 1 ng/ml increase) | 0.9892 (0.9586–1.021) | 0.4987 |
| Liver fibrosis (per one-stage increase) | 1.841 (1.256–2.697) | <0.001 |
| VWF: Ag (per 1% increase) | 1.007 (1.004–1.011) | <0.001 |
| Multivariable analysis | ||
| VWF: Ag (per 1% increase) | 1.0070 (1.0010–1.0130) | <0.001 |
The data are expressed as mean ± SD.
AFP: α-fetoprotein; CI: confidence interval; DCP: des-γ-carboxy prothrombin; Fib4 index: The fibrosis 4 index; HR: hazard ratio; VWF: Ag: von Willebrand factor antigen.
Discussion
Most cases of LC and HCC in Japan result from the progression of chronic hepatitis types B and C. Thus, in this study, we investigated these patients.
We previously reported that plasma levels of VWF: Ag increase according to hepatic spare ability decline in patients with LC.16 In this study, VWF: Ag was related to liver fibrosis progression of chronic hepatitis and inversely correlated with albumin, prothrombin time and platelet count. VWF is observed more brightly in hepatic ECs in histological findings according to liver fibrosis progression.24 LC is known to be related to angiogenesis16 and VWF is synthesized in vascular ECs.7–9 VWF suppresses angiogenesis.13–15 These results indicate that VWF: Ag is possibly associated with liver fibrosis progression. However, VWF: Ag was not different from conventional biomarkers and parameters in the diagnosability of severe liver fibrosis stage. Thus, we considered that the usefulness of VWF: Ag in severe liver fibrosis stage was not superior to conventional biomarkers and parameters. VWF: Ag should be used in combination with conventional biomarkers and/or parameters for diagnosing severe liver fibrosis stage. Their combination may increase the diagnosability of liver fibrosis stage.
Angiogenesis plays an important role in HCC development.17 HCC development is related to vascular endothelial growth factor (VEGF) as the VEGF level of patients with HCC, those with metastasis and large tumour size, is higher than that of patients without HCC.25,26 Anti-VEGF therapy can be used to treat HCC.17 VWF suppresses angiogenesis through VEGF; human umbilical vein ECs have been treated by short interfering RNA, which inhibited VWF expression, increased angiogenesis and increased VEGF in vitro.13 In this study, VWF was an independent risk factor to predict HCC development.
Tumour markers, including AFP and DCP, are often used to diagnose and analyse the recurrence and treatment effects of HCC. In a previous study, VWF: Ag levels were higher in patients with HCC than in patients without HCC.17 VWF: Ag showed a different functional mechanism from the conventional tumour markers including AFP and DCP as VWF suppresses angiogenesis. Whether VWF: Ag is a biomarker for recurrence and treatment effects of HCC has not been clarified; thus, we considered that VWF: Ag is not a useful tumour marker. However, our current study showed that VWF: Ag may be a potentially useful biomarker for the prediction of HCC occurrence.
VWF is associated with apoptosis as it reduces tumour metastasis, induces the cell death of tumour cells27,28 and tumour cells have a higher metastatic potential in VWF-deficient mice than in VWF-expressing mice.27 VWF is potentially related to HCC through angiogenesis and apoptosis. The usefulness of predicting HCC development by VWF: Ag may be related to liver fibrosis. VWF may be associated with HCC development through liver fibrosis. Conversely, VWF is related to apoptosis and angiogenesis. LC with HCC had higher VWF: Ag levels than LC without HCC.17 In addition, VWF: Ag levels increase in other malignant tumours including gastric and colon cancers,29–31 and VWF: Ag may become a biomarker of treatment effects in malignant tumours32 not related to liver fibrosis. Our study found that the VWF: Ag levels of patients who developed HCC with absent to moderate liver fibrosis and severe fibrosis were higher than those of patients without HCC. Thus, patients who develop HCC have higher VWF: Ag levels than patients without HCC who have the same liver fibrosis stage. We considered that the difference of VWF: Ag levels between patients who developed HCC and patients without HCC may be related to apoptosis.
Although VWF suppresses angiogenesis and promotes apoptosis, several malignant tumours including HCC increase VWF levels.25,26,29–31 There was a deficiency of large VWF multimers in malignant tumours,33 i.e. the function of VWF may decline. The levels of a disintegrin and metalloproteinase (ADAM) domain-containing protein 28 in malignant tumours also increased.34 ADAMs are a gene family of transmembrane and/or secreted proteins that play an important role in regulating cell phenotypes via effects on cell adhesion, migration, proteolysis and signaling.35 The ADAM family is implicated in the pathophysiology of numerous diseases. ADAM28 cleaves VWF, and promotes the proliferation, invasion and metastasis of malignant tumour cells.34 We speculated that a decline in the function of VWF results in elevated VWF: Ag levels.
Recently, patients with VWD have been treated with VWF replacement therapy.11 The therapy increases VWF levels in the vessel and controls bleeding. In a previous study and the current study, VWF was shown to be related to angiogenesis and apoptosis, and inhibited malignant tumours. We considered that VWF replacement therapy may be a new therapy to suppress HCC development or other malignant tumour development by inhibiting angiogenesis and promoting apoptosis.
Our findings suggested that VWF could reflect potential HCC development. However, this study has some limitations. First, when VWF: Ag is used as a biomarker to diagnose severe liver fibrosis stage and a predictive biomarker for HCC development, several factors influence VWF: Ag levels. VWF is related not only to angiogenesis and apoptosis, but also to haemostasis and inflammation.17 VWF: Ag levels increase in patients with thrombosis and inflammation. Patients with severe liver fibrosis sometimes develop thrombosis or inflammation including portal thrombosis and spontaneous bacterial peritonitis. Second, this study only included patients with chronic hepatitis types B and C. Other chronic liver diseases, including alcoholic hepatitis, nonalcoholic hepatitis, autoimmune hepatitis and primary biliary cholangitis, should be examined in the future.
In conclusion, VWF contributes to HCC development, reflecting angiogenesis and apoptosis. VWF: Ag is an independent risk factor of HCC development and is associated with liver fibrosis. To our knowledge, this study is the first to show that VWF: Ag is associated with liver fibrosis and is a useful predictive biomarker for HCC development. Thus, we anticipate the development of new diagnostic and therapeutic approaches for chronic hepatitis and HCC prediction by using VWF: Ag.
Acknowledgement
This work was completed with the great help of the late Prof. Masahito Uemura and Ms Hisayo Iino.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Ethics approval
The study protocol was approved by the Ethics Committee of Nara Medical University and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Informed consent
All subjects gave informed consent for participation.
References
- 1.Pinter M, Trauner M, Peck-Radosavljevic M, et al. Cancer and liver cirrhosis: Implications on prognosis and management. ESMO Open 2016; 1: e000042–e000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014; 383: 1749–1761. [DOI] [PubMed] [Google Scholar]
- 3.Machado NO. Complications of liver biopsy - risk factors, management and recommendations. In: Takahashi H. (ed). Liver biopsy, London: InTech, 2011, pp. 392–405. [Google Scholar]
- 4.Chin JL, Pavlides M, Moolla A, et al. Non-invasive markers of liver fibrosis: Adjuncts or alternatives to liver biopsy? Front Pharmacol 2016; 7: 159–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization International Agency for Research on Cancer. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012.http://globocan.iarc.fr/Default.aspx (2012).
- 6.Chauhan R, Lahiri N. Tissue- and serum-associated biomarkers of hepatocellular carcinoma. Biomark Cancer 2016; 8: 37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroll MH, Harris TS, Moake JL, et al. von Willebrand factor binding to platelet GpIb initiates signals for platelet activation. J Clin Invest 1991; 88: 1568–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haberichter SL. von Willebrand factor propeptide: Biology and clinical utility. Blood 2015; 126: 1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moake JL, Turner NA, Stathopoulos NA, et al. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J Clin Invest 1986; 78: 1456–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng C, Motto DG, Di Paola J. Diagnostic approach to von Willebrand disease. Blood 2015; 125: 2029–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castaman G, Linari S. Diagnosis and treatment of von Willebrand disease and rare bleeding disorders. J Clin Med 2017; 6: E45–E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franchini M, Mannucci PM. von Willebrand disease-associated angiodysplasia: A few answers, still many questions. Br J Haematol 2013; 161: 177–182. [DOI] [PubMed] [Google Scholar]
- 13.Starke RD, Ferraro F, Paschalaki KE, et al. Endothelial von Willebrand factor regulates angiogenesis. Blood 2011; 117: 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randi AM, Laffan MA. von Willebrand factor and angiogenesis: Basic and applied issues. J Thromb Haemost 2017; 15: 13–20. [DOI] [PubMed] [Google Scholar]
- 15.Lenting PJ, Casari C, Christophe OD, et al. von Willebrand factor: The old, the new and the unknown. J Thromb Haemost 2012; 10: 2428–2437. [DOI] [PubMed] [Google Scholar]
- 16.Uemura M, Fujimura Y, Ko S, et al. Pivotal role of ADAMTS13 function in liver diseases. Int J Hematol 2010; 91: 20–29. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Wang X, Li S, et al. The role of von Willebrand factor as a biomarker of tumor development in hepatitis B virus-associated human hepatocellular carcinoma: A quantitative proteomic based study. J Proteomics 2014; 106: 99–112. [DOI] [PubMed] [Google Scholar]
- 18.Yoshiji H, Noguchi R, Namisaki T, et al. Combination of sorafenib and angiotensin-II receptor blocker attenuates preneoplastic lesion development in a non-diabetic rat model of steatohepatitis. J Gastroenterol 2014; 49: 1421–1429. [DOI] [PubMed] [Google Scholar]
- 19.Kaji K, Yoshiji H, Ikenaka Y, et al. Dipeptidyl peptidase-4 inhibitor attenuates hepatic fibrosis via suppression of activated hepatic stellate cell in rats. J Gastroenterol 2014; 49: 481–491. [DOI] [PubMed] [Google Scholar]
- 20.Terraube V, Pendu R, Baruch D, et al. Increased metastatic potential of tumor cells in von Willebrand factor-deficient mice. J Thromb Haemost 2006; 4: 519–526. [DOI] [PubMed] [Google Scholar]
- 21.Ichida F, Tsuji T, Omata M, et al. New Inuyama classification; new criteria for histological assessment of chronic hepatitis. Hepatol Res 1996; 6: 112–119. [Google Scholar]
- 22.Kokudo N, Hasegawa K, Akahane M, et al. Evidence-based clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res 2015. DOI: 10.1111/hepr.12464. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko T, Wada H. Diagnostic criteria and laboratory tests for disseminated intravascular coagulation. J Clin Exp Hematop 2011; 51: 67–76. [DOI] [PubMed] [Google Scholar]
- 24.Arimoto J, Ikura Y, Suekane T, et al. Expression of LYVE-1 in sinusoidal endothelium is reduced in chronically inflamed human livers. J Gastroenterol 2010; 45: 317–325. [DOI] [PubMed] [Google Scholar]
- 25.Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): The role of angiogenesis and invasiveness. Am J Gastroenterol 2008; 103: 914–921. [DOI] [PubMed] [Google Scholar]
- 26.Guan Q, Gu J, Zhang H, et al. Correlation between vascular endothelial growth factor levels and prognosis of hepatocellular carcinoma patients receiving radiofrequency ablation. Biotechnol Biotechnol Equip 2015; 29: 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terraube V, Pendu R, Baruch D, et al. Increased metastatic potential of tumor cells in von Willebrand factor-deficient mice. J Thromb Haemost 2006; 4: 519–526. [DOI] [PubMed] [Google Scholar]
- 28.Terraube V, Marx I, Denis CV. Role of von Willebrand factor in tumor metastasis. Thromb Res 2007; 120: S64–S70. [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Sun HJ, Li ZR, et al. Gastric cancer-associated enhancement of von Willebrand factor is regulated by vascular endothelial growth factor and related to disease severity. BMC Cancer 2015; 15: 80–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang WS, Lin JK, Lin TC, et al. Plasma von Willebrand factor level as a prognostic indicator of patients with metastatic colorectal carcinoma. World J Gastroenterol 2005; 11: 2166–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franchini M, Frattini F, Crestani S, et al. von Willebrand factor and cancer: A renewed interest. Thromb Res 2013; 131: 290–292. [DOI] [PubMed] [Google Scholar]
- 32.Marfia G, Navone SE, Fanizzi C, et al. Prognostic value of preoperative von Willebrand factor plasma levels in patients with glioblastoma. Cancer Med 2016; 5: 1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koo BH, Oh D, Chung SY, et al. Deficiency of von Willebrand factor-cleaving protease activity in the plasma of malignant patients. Thromb Res 2002; 105: 471–476. [DOI] [PubMed] [Google Scholar]
- 34.Mochizuki S, Soejima K, Shimoda M, et al. Effect of ADAM28 on carcinoma cell metastasis by cleavage of von Willebrand factor. J Natl Cancer Inst 2012; 104: 906–922. [DOI] [PubMed] [Google Scholar]
- 35.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Asp Med 2008; 29: 258–289. [DOI] [PMC free article] [PubMed] [Google Scholar]