Abstract
Background
This meta-analysis aimed to assess the cardiac safety profile of domperidone treatment for the risk of cardiovascular (CV) event and QT prolongation.
Methods
Data from nine studies involving 101,155 patients were used for the analysis of CV event risk, while data from eight studies involving 390 patients were used for the analysis of QT prolongation risk.
Results
Meta-analysis findings suggested a significant increase in CV risk under domperidone as compared to no treatment for domperidone doses of >30 mg/day (OR: 3.14, 95% CI, 1.191 to 8.304, p = 0.021), no significant increase in QT prolongation event rates with domperidone (3.54%, 95% CI, 1.73% to 7.10%) and a significantly lower CV risk for domperidone than for metoclopramide (OR: 0.63, 95% CI, 0.58 to 0.70, p < 0.001).
Conclusions
The present meta-analysis indicates that domperidone treatment may not be associated with an overall CV event risk increase at doses ≤30 mg/day and does not result in QT prolongation.
Keywords: Domperidone, metoclopramide, dosage, cardiovascular risk, sudden cardiac death, ventricular arrhythmia, QT prolongation, meta-analysis
Introduction
Domperidone (DMP), a peripheral dopamine D2-receptor antagonist with prokinetic and antiemetic properties, has been broadly prescribed for nausea and vomiting, gastroparesis and gastroesophageal reflux disease and stimulation of breast milk production.1–4
DMP and metoclopramide (MCP) are dopamine antagonists with a strong affinity for dopamine receptors in the central as well as peripheral nervous systems, and specifically the gastrointestinal tract, and thus they act as antiemetics at the chemoreceptor trigger zone and as prokinetics in the upper gastrointestinal tract to accelerate gastric emptying.5 Although MCP readily crosses the blood-brain barrier leading to central nervous system side effects in up to 40% of patients ranging from somnolence to extrapyramidal symptoms, DMP poorly penetrates the blood-brain barrier but maintains a powerful antiemetic effect at the chemoreceptor trigger zone level as well as its peripheral prokinetic properties.5 Accordingly, DMP is considered a safer alternative to MCP in patients with intolerance to MCP treatment or those requiring long-term therapy for upper gastrointestinal motility problems in whom nausea and vomiting are prominent.5
Although the safety profile of DMP is more acceptable than MCP and cisapride,3 hazardous cardiovascular (CV) adverse effects are considered likely because of its narrow therapeutic index.6,7
The putative mechanism by which DMP delays cardiac repolarization and prolongs the QT interval is considered to involve blockage of IKr, the rapid component of the delayed rectifier potassium current,8 via inhibiting the potassium efflux channel, human ether-a-go-go-related gene, leading to a prolongation of cardiac repolarization.8–11 A prolonged QT interval is considered a predictive, noninvasive risk factor for sudden cardiac death (SCD) since a delay in ventricular repolarization can lead to a more chaotic cardiac depolarization/repolarization cycle and can provoke arrhythmias with high risk of SCD, such as ventricular fibrillation and torsade de pointes.5,12
Published data on prolongation of QT interval and potential adverse CV events such as ventricular arrhythmias (VAs) and/or SCD under DMP treatment are largely limited to case reports and case-control studies alongside inconsistent findings.2,4,5,7,10,13–32 Therefore, a need for further investigation with large clinical trials is emphasized to address the safety profile of DMP more appropriately.2,5
Until such a study is published, to evaluate this serious claim, we aimed with the present meta-analysis to assess the association between different doses of DMP and CV events and QT prolongation risk in comparison to MCP.
Materials and methods
Search strategy and study selection
The PubMed database was searched using the following terms: (a) “domperidone” AND at least one of the terms “cardiovascular, QT, death, arrhythmia” and (b) “domperidone” AND “metoclopramide” AND “cardiovascular,” in titles and abstracts limited to articles published in English and studies in humans. The literature was searched from inception to June 30, 2018. The authors evaluated the title and abstract of each article separately. The full text of 115 articles was reached.
Study selection
We included studies that met the following criteria: (1) a clinical study published as an original article; (2) exposure of interest was oral DMP treatment, (3) outcome of interest was a CV event or QT prolongations, and (4) available odds ratio (OR) with 95% confidence interval (CI) (or data to calculate these) was provided. Exclusion criteria were: (1) preclinical studies, (2) case reports and meta-analyses, and (3) lack of sufficient information on CV events or QT interval measurement.
Publication bias
Potential publication bias was explored using visual inspection of Begg’s funnel plot asymmetry, classic fail-safe N analysis, Begg’s rank correlation and Egger’s weighted regression tests.33,34
Heterogeneity assessment
The I2 statistics of Higgins and Thompson was used to assess heterogeneity among studies.35 I2 values of 25%, 50% and 75% represent low, moderate and high heterogeneity, respectively.36 The presence of heterogeneity across studies was defined using Cochran’s Q test with a 0.10 significance level.37
Quality of studies
The quality of studies was assessed using Jadad scoring38 for clinical studies, using the Newcastle-Ottawa Scale for Case-Control Studies for case-control studies, and using the Newcastle-Ottawa Scale for Cohort Studies for single-arm clinical studies.39,40
Jadad scoring has three items with a score range of 0 to 5. The first item is related to randomization (0, nonrandomized; 1, randomized but the sequence of the randomization was not reported; 2, randomized appropriately). The second item is related to double-blinding (0, not double-blinded; 1, double-blinded but the details were not reported; 2, appropriate double-blinding techniques were performed). The third item is related to withdrawals and dropouts (0, number and reasons for withdrawals were not stated; 1, number and reasons for withdrawals were stated). An a priori cutoff value for Jadad score to include the studies was not set.38
The Newcastle-Ottawa Scale for Case-Control studies is based on the selection of case and controls, comparability of cases and controls and ascertainment of exposure, while the Newcastle-Ottawa Scale for Cohort studies is based on the selection of cohorts, comparability of cohorts and assessment of outcome. “High”-quality choices are identified with a star, with a maximum of one star for each item within the “Selection” and “Exposure/Outcome” categories and a maximum of two stars for “Comparability.” For case-control studies a statement of no history of disease or incident and demonstration that outcome of interest was not present at start of study earns a star, whereas for cohort studies, adequacy of follow-up of cohorts is based on assessment of the follow-up of the exposed and nonexposed cohorts to ensure that losses are not related to either the exposure or the outcome.39,40
Statistical methods
ORs with a 95% CI for CV event risk were presented based on calculated risk (using raw data provided in each study) as well as adjusted risk obtained from original data provided in each study. Fixed-effects and random-effects models were applied to all comparisons to determine corresponding overall effect sizes and related CIs when heterogeneity was absent or evident, respectively. Only random-effects model findings are presented here since there was no significant difference between both models for all variables except for DMP vs MCP. For analysis of QT prolongation risk, event rates, difference between pretreatment and posttreatment means for QT interval values and Hedge’s g, value with 95% CIs were used. All statistical analyses were performed with Comprehensive Meta-Analysis, v.2.2.064 (www.Meta-Analysis.com, USA). Summary statistics are expressed as mean ± standard deviation (SD), n (%) and 95% CI, where appropriate. A two-sided p < 0.05 was considered statistically significant.
Results
Included studies
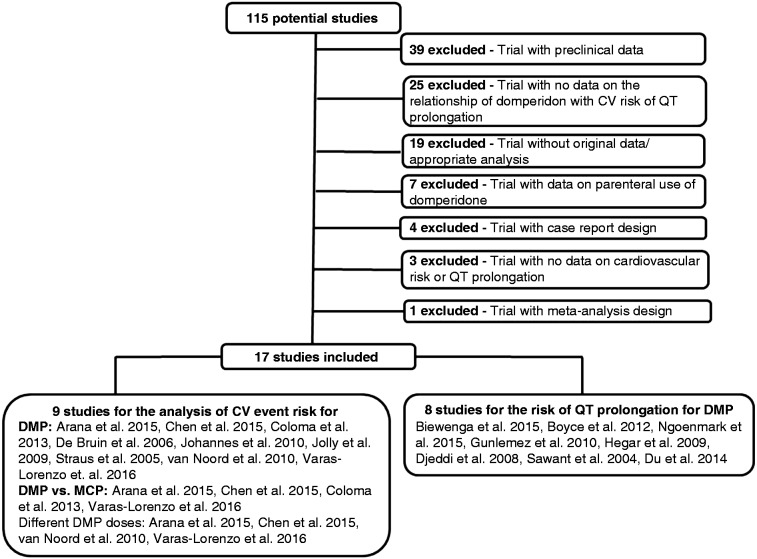
Of 115 studies initially selected, 98 studies were excluded because of inclusion of preclinical data (n = 39), lack of data on the relationship of DMP with CV risk or QT prolongation (n = 25), lack of original data or appropriate statistical analysis (n = 19), inclusion of data on parenteral use of DMP (n = 7), case reports (n = 4), no data on CV risk or QT prolongation (n = 3) and meta-analysis (n = 1). Of 17 studies included in the meta-analysis, data from nine studies17–25 were used to analyze DMP-related risk of a CV event, as well as for CV risk related to DMP vs MCP17–19,25 and related to different doses of DMP.17,18,23,25 Data from the remaining eight studies3,26,32 were used to analyze DMP-related risk of QT prolongation. Figure 1 illustrates the corresponding flow diagram of the study selection.
Figure 1.
Flow diagram of study selection.
CV: cardiovascular; DMP: domperidone; MCP: metoclopramide.
Data on quality of studies are provided in Table 1.
Table 1.
Characteristics of studies included in the meta-analysis.
| Domperidone and metoclopramide – CV event
risk | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (year) | Country | Study design | Age (year) (mean±SD) | Treatments | included in meta-analysis for CV event risk
related to |
Cardiac outcome parameter(s) | ||||||
| DMP | MCP | DMP vs. MCP | DMP doses | |||||||||
| Arana et al. (2015)13 | UK | Case controla Crossover | 44.0±23.0 | DMP (<30 / 30 / >30 mg/day) & MCP | + | + | + | + | SCD | |||
| Varas-Lorenzo et al. (2016)21 | ||||||||||||
| Chen et al. (2015)14 | China | Crossover | 60.8±19.1 | DMP (≤30 / >30 mg/day) & MCP | + | + | + | + | VA and SCD | |||
| Coloma et al. (2013)15 | EU | Case-control | All ages | DMP & MCP | + | + | +b | - | AMI | |||
| De Bruin et al. (2007)16 | NL | Case-control | 47.5±26.8 59.6±21.7 | DMP | + | - | - | - | CA | |||
| Johannes et al. (2010)17 | USA | Case-control | 79.4 | DMP | + | - | - | - | VA and SCD | |||
| Jolly et al. (2009)18 | UK | Case-control | 67.6±12.4 | DMP | + | - | - | - | SCD | |||
| Straus et al. (2005)19 | NL | Case-control | 71±13 69±13 | DMP | + | - | - | - | SCD | |||
| van Noord et al. (2010)20 | NL | Case-control | 66.3± 13.9 72.5±14.1 | DMP (<30 / 30 / >30 mg/day) | + | - | - | + | VA and SCD | |||
|
Domperidone – QT prolongation risk
| ||||||||||||
| Author (year) | Country | n | Study design | Mean Age | Age group | QT definition |
QT value
|
QTc prolongation (>500 msc) | ||||
|
Pre-tx
|
Post-tx | Difference | ||||||||||
| Mean | SD | Mean | SD | Mean | n | |||||||
|
| ||||||||||||
| Biewenga et al. (2015)22 | Belgium | 44 | TQT | 43.5 y | 18-55 y | QTcP | 408.0c | 32.2 | 400.8 | 31.0 | -7.2 | 0 |
| Boyce et al. (2012)23 | UK | 24 | TQT | 26.6 y | 18-39 y | QTcF | 401.4 | 31.7 | 407.3 | 31.5 | 5.8 | 0 |
| Ngoenmak et al. (2016)3 | Thailand | 22 | clinical | 8.5 mo | <2 y | QTcB | 411.0 | 34.6 | 395.5 | 38.2 | -15.5 | 2 |
| Gunlemez et al. (2010)24 | Turkey | 40 | clinical | 32 day | Premature | QTcB | 370.0 | 30.0 | 380.0 | 30.0 | 10.0 | 2 |
| Hegar et al. (2009)25 | Belgium | 10 | clinical | 5.6 mo | Infant | QTcB | 404.0 | 18.0 | 402.0 | 20.0 | -2.0 | 0 |
| Djeddi et al. (2008)26 | France | 31 | clinical | 18 day | Newborn | QTcB | 373.2 | 4.8 | 387.2 | 5.1 | 14.0 | 1 |
| Sawant et al. (2004)27 | India | 27 | clinical | 35.0 y | 18-60 y | QT | 320.0 | 55.0 | 310.0 | 46.0 | -10.0 | 0 |
| Du et al. (2014)28 | China | 192 | clinical | 41.3 y | 18-65 y | QT | - | - | - | - | - | 0 |
| Quality assessment | ||||||||||||
| Quality assessment category |
||||||||||||
|
|
Selection | Comparability | Outcome | |||||||||
| Case-control studiesd | ||||||||||||
| Arana et al. (2015)13/ Varas-Lorenzo et al. (2016)21 | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ | |||||||||
| Chen et al. (2015)14 | ⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ | |||||||||
| Coloma et al. (2013)15 | ⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ | |||||||||
| De Bruin et al. (2007)16 | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ | |||||||||
| Johannes et al. (2010)17 | ⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ | |||||||||
| Jolly et al. (2009)18 | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ | |||||||||
| Straus et al. (2005)19 | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ | |||||||||
| van Noord et al. (2010)20 | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ | |||||||||
| Single-arm studiese | Selection | Comparability | Outcome | |||||||||
|
| ||||||||||||
| Ngoenmak et al. (2016)3 | ⋆⋆⋆ | - | ⋆⋆⋆ | |||||||||
| Gunlemez et al. (2010)24 | ⋆⋆⋆ | - | ⋆⋆⋆ | |||||||||
| Djeddi et al. (2008)26 | ⋆⋆⋆ | - | ⋆⋆⋆ | |||||||||
| Clinical trialsf | Randomization | Double-blinding | Withdrawals and dropouts | |||||||||
|
| ||||||||||||
| Biewenga et al. (2015)22 | 2 | 2 | 1 | |||||||||
| Boyce et al. (2012)23 | 2 | 2 | 1 | |||||||||
| Hegar et al. (2009)25 | 2 | 0 | 1 | |||||||||
| Sawant et al. (2004)27 | 1 | 0 | 0 | |||||||||
| Du et al. (2014)28 | 2 | 2 | 1 | |||||||||
AMI: Acute myocardial infarction; CA: Cardiac arrest; DMP: Domperidone; Eu: Europe; MCP: Metoclopramide; NL: Netherlands; SCD: Sudden cardiac death; UK: United Kingdom; VA: Ventricular arrhythmia; TQT: thorough QT/QTc; tx: treatment,
Included in the meta-analysis, bcalculated from original data provided in the study, cValues in italic refer to data not provided in the publication but calculated using the other data available in the study, dNewcastle-Ottawa Scale for Case-Control Studies, eNewcastle-Ottawa Scale for Cohort Studies, f Jadad score for clinical trials
CV event risk
Characteristics of studies
Characteristics of studies17,25 selected for the analysis of DMP/MCP-related CV event risk are presented in Table 1. All studies were retrospective except two studies (one with crossover and the other is a case control in a small group of patients). Cardiac outcome analysis was based both on VA and SCD in three studies,18,21,24 while on SCD per se in four studies,17,22,23,25 acute myocardial infarction risk in one study19 and cardiac arrest accompanied with or without death in one study20 (Table 1).
CV event risk—DMP
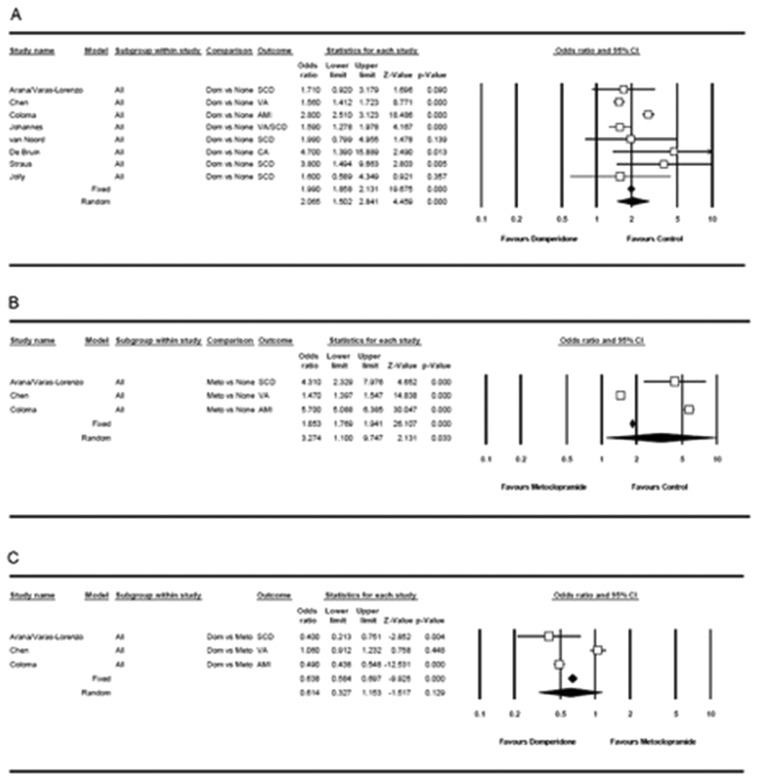
A meta-analysis of 101,155 patients (33,432 in the CV event group (1873 on DMP) and 67,723 in the control group (1751 on DMP)) from nine studies17–25 suggested a 1.56 to 4.70 times higher rate of DMP use in patients with a CV event than those without a CV event, and suggested a significant increase in CV risk under DMP treatment (OR: 2.07, 95% CI, 1.50 to 2.84, p < 0.001 with a random-effects model; Q = 68.883, I2 = 89.8% for adjusted risk) (Table 2, Figure 2(a)).
Table 2.
Meta-analysis of CV event risk related to DMP, MCP and DMP vs MCP treatment; DMP-CV event risk according to DMP dose.
| Cardiac outcome | CV event (n) |
Adjusted risk |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Present |
Absent |
OR | 95% LCL | 95% UCL | Z | p | ||||
| Total | On DMP/MCP | Total | On DMP/MCP | |||||||
| DMP vs no treatment | ||||||||||
| Arana et al. (2015)17 | SCD | 3239 | 28 | 12,572 | 52 | 1.71 | 0.92 | 3.18 | 1.696 | 0.090 |
| Varas-Lorenzo et al. (2016)25 | ||||||||||
| Chen and Hsiao (2015)18 | VA | 25,356 | 1644 | 25,356 | 1148 | 1.56 | 1.41 | 1.72 | 8.771 | <0.001 |
| Coloma et al. (2013)19 | AMI | – | – | – | – | 2.80 | 2.51 | 3.12 | 18.486 | <0.001 |
| De Bruin et al. (2007)20 | CA | 140 | 7 | 560 | 15 | 4.70 | 1.39 | 15.89 | 2.490 | 0.013 |
| Johannes et al. (2010)21 | VA/SCD | 1608 | 169 | 6428 | 481 | 1.59 | 1.28 | 1.98 | 4.167 | <0.001 |
| Jolly et al. (2009)22 | SCD | 1010 | 6 | 3030 | 12 | 1.60 | 0.59 | 4.35 | 0.921 | 0.357 |
| Straus et al. (2005)23 | SCD | 775 | 9 | 6297 | 15 | 3.80 | 1.49 | 9.66 | 2.803 | 0.005 |
| van Noord et al. (2010)24 | SCD | 1304 | 10 | 13,480 | 28 | 1.99 | 0.80 | 5.0 | 1.478 | 0.139 |
| Overall | RE model | 33,432 | 1873 | 67,723 | 1751 | 2.07 | 1.50 | 2.84 | 4.459 | <0.001 |
|
Heterogeneity test
|
Q | I 2 | Tau | Tau 2 | p | |||||
| 68.883 | 89.8 | 0.353 | 0.124 | <0.001 | ||||||
| MCP vs no treatment | ||||||||||
| Arana et al. (2015)17 | SCD | 3239 | 37 | 12,572 | 44 | 4.31 | 2.33 | 7.98 | 4.652 | <0.001 |
| Varas-Lorenzo et al. (2016)25 | ||||||||||
| Chen and Hsiao (2015)18 | VA | 25,356 | 1316 | 25,356 | 932 | 1.47 | 1.40 | 1.55 | 14.838 | <0.001 |
| Coloma et al. (2013)19 | AMI | – | – | – | – | 5.70 | 5.09 | 6.39 | 30.047 | <0.001 |
| Overall | RE model | 28,595 | 1353 | 37,928 | 975 | 3.274 | 1.10 | 9.75 | 2.131 | 0.033 |
|
Heterogeneity test
|
Q | I 2 | Tau | Tau 2 | p | |||||
| 463.06 | 99.6 | 0.947 | 0.898 | <0.001 | ||||||
| DMP vs MCP | ||||||||||
| Arana et al. (2015)13 | SCD | – | – | – | – | 0.40 | 0.21 | 0.75 | –2.852 | 0.004 |
| Varas-Lorenzo et al. (2016)25 | ||||||||||
| Chen and Hsiao (2015)18 | VA | 25,356 | 1644 | 25,356 | 1316 | 1.06 | 0.91 | 1.23 | 0.758 | 0.448 |
| Coloma et al. (2013)19 | AMI | – | – | – | – | 0.49 | 0.44 | 0.55 | –12.531 | <0.001 |
| Overall | FE model | 25,356 | 1644 | 25,356 | 1316 | 0.64 | 0.58 | 0.70 | –9.925 | <0.001 |
| RE model | 25,356 | 1644 | 25,356 | 1316 | 0.61 | 0.33 | 1.15 | –1.517 | 0.129 | |
|
Heterogeneity test
|
Q | I 2 | Tau | Tau 2 | p | |||||
| 67.23 | 97.0 | 0.529 | 0.280 | <0.001 | ||||||
| DMP daily dose | ||||||||||
| <30 mg | ||||||||||
| Arana et al. (2015)17 | SCD | 806 | 4 | 4503 | 10 | 1.96 | 0.44 | 8.75 | 0.882 | 0.378 |
| Varas-Lorenzo et al. (2016)25 | ||||||||||
| van Noord et al. (2010)24 | SCD | 1304 | 2 | 13,480 | 10 | 1.24 | 0.19 | 8.11 | 0.225 | 0.822 |
| Overall | 2110 | 6 | 17,983 | 20 | 1.64 | 0.51 | 5.29 | 0.830 | 0.407 | |
| 30 mg | ||||||||||
| Arana et al. (2015)17 | SCD | 817 | 15 | 4528 | 35 | 1.48 | 0.69 | 3.18 | 1.004 | 0.316 |
| Varas-Lorenzo et al. (2016)25 | ||||||||||
| van Noord et al. (2010)24 | SCD | 1304 | 4 | 13,480 | 15 | 1.02 | 0.23 | 4.47 | 0.026 | 0.979 |
| Overall | 2121 | 19 | 18,008 | 50 | 1.37 | 0.69 | 2.70 | 0.903 | 0.366 | |
| >30 mg | ||||||||||
| Arana et al. (2015)17 | SCD | 810 | 8 | 4498 | 5 | 3.20 | 0.59 | 17.35 | 1.349 | 0.177 |
| Varas-Lorenzo et al. (2016)25 | ||||||||||
| Chen and Hsiao (2015)18 | VA | 25,356 | 192 | 25,356 | 108 | 1.98 | 1.50 | 2.62 | 4.769 | <0.001 |
| van Noord et al. (2010)24 | SCD | 1304 | 4 | 13,480 | 3 | 11.40 | 1.99 | 65.25 | 2.734 | 0.006 |
| Overall | RE model | 27,470 | 204 | 43,334 | 116 | 3.14 | 1.19 | 8.30 | 2.312 | 0.021 |
AMI: acute myocardial infarction; CV: cardiovascular; DMP: domperidone; FE: fixed effects; LCL: lower confidence limit; MCP: metoclopramide; OR: odds ratio; RE: random effects; SCD: sudden cardiac death; UCL: upper confidence limit; VA: ventricular arrhythmia.
Figure 2.
Forest plot displaying adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for the impact of (a) domperidone treatment, (b) metoclopramide treatment, (c) and domperidone vs metoclopramide treatment on cardiovascular event risk.
CV event risk—MCP
A meta-analysis of 66,523 patients (28,595 in the CV event group (1353 on MCP) and 37,928 in the control group (975 on MCP)) from four studies17–19,25 suggested a 1.47 to 5.70 times higher rate of MCP use in patients with a CV event than those without a CV event, and suggested a significant increase in CV risk under MCP treatment (OR: 3.27, 95% CI, 1.10 to 9.75, p = 0.033 with a random-effects model; Q = 463.06, I2 = 99.6% for adjusted risk) (Table 2, Figure 2(b)).
CV event risk—DMP vs MCP
A meta-analysis of DMP vs MCP use in terms of CV event risk revealed the ratio of DMP/MCP use to range from 0.40 to 1.06 in patients with a CV event and suggested a lower CV risk for DMP than for MCP (OR: 0.64, 95% CI, 0.58 to 0.70, p < 0.001 with a fixed-effects model and OR: 0.61, 95% CI, 0.33 to 1.15, p = 0.129 with a random-effects model; Q = 67.23, I2 = 97.0% for adjusted risk) (Table 2, Figure 2(c)).
CV event risk—DMP doses
A meta-analysis of 109,126 patients (19,801 in the CV event group (229 on DMP > 30 mg/day in 204), 79,325 in the control group (186 on DMP > 30 mg/day in 116)) from four studies17,18,24,25 suggested no significant risk of a CV event at doses of <30 mg/day (OR: 1.64, 95% CI, 0.509 to 5.286, p = 0.407) and 30 mg/day (OR: 1.37, 95% CI, 0.693 to 2.699, p = 0.366). These findings suggested a significant increase in CV risk at doses of >30 mg/day (OR: 2.09, 95% CI, 1.59 to 2.75, p < 0.001 with a fixed-effects model and OR: 3.14, 95% CI, 1.19 to 8.30, p = 0.021 with a random-effects model) (Table 2).
Publication bias (DMP-/MCP-CV event risk)
Visual inspection of the funnel plot and Egger regression asymmetry test (intercept (95% CI): 0.292 (−3.88 to 4.46), p = 0.435), classic fail-safe N analysis (n = 426) and Begg-Mazumdar correlation test (Kendall tau b = 0.393, p = 0.087) showed no evidence of publication bias in the analysis between DMP use and CV event risk (See Supplementary Appendix 1(a)).
Visual inspection of the funnel plot and Egger regression asymmetry test (intercept (95% CI): 12.43 (−201.2 to 226.0), p = 0.30), classic fail-safe N analysis (n = 636) and Begg-Mazumdar correlation test (Kendall tau b = 0.333, p = 0.30) showed no evidence of publication bias in the analysis between MCP use and CV event risk (See Supplementary Appendix 1(b)).
Visual inspection of the funnel plot and Egger regression asymmetry test (intercept (95% CI): 0.77 (−125.93 to 127.47), p = 0.48), classic fail-safe N analysis (n = 5) and Begg-Mazumdar correlation test (Kendall tau b = 0.333, p = 0.30) showed no evidence of publication bias in the analysis of DMP vs MCP usage in terms of CV event risk (See Supplementary Appendix 1(c)).
QT prolongation risk
Characteristics of studies
Of the eight studies3,26–32 included in the meta-analysis, two studies26,27 investigated QT/QTc (TQT) studies, and the others3,28–32 were clinical studies of patients with a wide age scale ranging from newborns to adults (Table 1).
DMP-QT prolongation risk: event rates
A meta-analysis of 390 patients from eight studies revealed no significant increase in QT prolongation event rates with DMP use (Table 3, See Supplementary Appendix 2(a)).
Table 3.
Meta-analysis of domperidone-QT prolongation risk.
| Event rate (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author (year)ref | Total n | Patients with QT prolongation | %a | %b | 95% LCL | 95% UCL | Z | p |
| Biewenga et al. (2015)26 | 44 | 0 | 0.00% | 1.11% | 0.07% | 15.43% | –3.156 | 0.002 |
| Boyce et al. (2012)27 | 24 | 0 | 0.00% | 2.00% | 0.12% | 25.13% | –2.724 | 0.006 |
| Ngoenmak et al. (2016)3 | 22 | 2 | 9.09% | 9.09% | 2.28% | 29.96% | –3.105 | 0.002 |
| Du et al. (2014)32 | 192 | 0 | 0.00% | 0.26% | 0.02% | 4.00% | –4.204 | 0.000 |
| Günlemez et al. (2010)28 | 40 | 2 | 5.00% | 5.00% | 1.25% | 17.91% | –4.059 | 0.000 |
| Hegar et al. (2009)29 | 10 | 0 | 0.00% | 4.55% | 0.28% | 44.83% | –2.103 | 0.035 |
| Djeddi et al. (2008)30 | 31 | 1 | 3.23% | 3.23% | 0.45% | 19.64% | –3.346 | 0.001 |
| Sawant et al. (2004)31 | 27 | 0 | 0.00% | 1.79% | 0.11% | 22.96% | –2.808 | 0.005 |
| Overall | 390 | 5 | 1.28% | 3.54% | 1.73% | 7.10% | –8.829 | 0.000 |
| Heterogeneity test |
Q
|
Q
|
Tau
|
Tau
2
|
p
|
|||
| 6.717 | 6.717 | 0.000 | 0.000 | 0.459 | ||||
| QT interval length | ||||||||
| Author (year) |
n | Difference between means | SE | 95% LCL | 95% UCL | Z | p | |
| Biewenga et al. (2015)26 | 44 | –7.20 | 6.31 | –19.57 | 5.17 | –1.141 | 0.254 | |
| Boyce et al. (2012)27 | 24 | 5.90 | 8.54 | –10.84 | 22.64 | 0.691 | 0.490 | |
| Ngoenmak et al. (2016)3 | 22 | –15.50 | 10.29 | –35.68 | 4.68 | –1.506 | 0.132 | |
| Günlemez et al. (2010)28 | 40 | 10.00 | 6.28 | –2.31 | 22.31 | 1.592 | 0.111 | |
| Hegar et al. (2009)29 | 10 | –2.00 | 7.97 | –17.62 | 13.62 | –0.251 | 0.802 | |
| Djeddi et al. (2008)30 | 31 | 14.00 | 1.18 | 11.69 | 16.31 | 11.883 | 0.000 | |
| Sawant et al. (2004)31 | 27 | –10.00 | 12.94 | –35.36 | 15.36 | –0.773 | 0.440 | |
| Overall | 198 | 1.35 | 4.92 | –8.29 | 10.99 | 0.275 | 0.783 | |
| Heterogeneity test |
Q
|
I
2
|
Tau
|
Tau
2
|
p
|
|||
| 25.807 | 76.751 | 10.572 | 111.765 | 0.000 | ||||
| Author (year)ref |
n | Standardized difference between means | SE | 95% LCL | 95% UCL | Z | p | |
| Biewenga et al. (2015)26 | 44 | –0.23 | 0.20 | –0.62 | 0.17 | –1.126 | 0.260 | |
| Boyce et al. (2012)27 | 24 | 0.19 | 0.27 | –0.35 | 0.72 | 0.685 | 0.494 | |
| Ngoenmak et al. (2016)3 | 22 | –0.43 | 0.29 | –1.00 | 0.15 | –1.442 | 0.149 | |
| Günlemez et al. (2010)28 | 40 | 0.33 | 0.22 | –0.09 | 0.76 | 1.549 | 0.121 | |
| Hegar et al. (2009)29 | 10 | –0.11 | 0.42 | –0.93 | 0.72 | –0.250 | 0.802 | |
| Djeddi et al. (2008)30 | 31 | 2.83 | 0.53 | 1.78 | 3.87 | 5.317 | 0.000 | |
| Sawant et al. (2004)31 | 27 | –0.20 | 0.26 | –0.70 | 0.31 | –0.766 | 0.444 | |
| Overall | 198 | 0.23 | 0.26 | –0.28 | 0.74 | 0.886 | 0.376 | |
| Heterogeneity test |
Q
|
I
2
|
Tau
|
Tau
2
|
p
|
|||
| 34.798 | 82.758 | 0.608 | 0.369 | 0.000 | ||||
| Author (year)ref |
n | Standardized paired difference | SE | 95% LCL | 95% UCL | Z | p | |
| Biewenga et al. (2015)26 | 44 | –0.17 | 0.15 | –0.47 | 0.13 | –1.133 | 0.257 | |
| Boyce et al. (2012)27 | 24 | 0.14 | 0.21 | –0.26 | 0.54 | 0.687 | 0.492 | |
| Ngoenmak et al. (2016)3 | 22 | –0.32 | 0.22 | –0.75 | 0.11 | –1.468 | 0.142 | |
| Günlemez et al. (2010)28 | 40 | 0.25 | 0.16 | –0.06 | 0.57 | 1.567 | 0.117 | |
| Hegar et al. (2009)29 | 10 | –0.08 | 0.32 | –0.70 | 0.54 | –0.251 | 0.802 | |
| Djeddi et al. (2008)30 | 31 | 2.13 | 0.33 | 1.50 | 2.77 | 6.564 | 0.000 | |
| Sawant et al. (2004)31 | 27 | –0.15 | 0.19 | –0.53 | 0.23 | –0.769 | 0.442 | |
| Overall | 198 | 0.22 | 0.22 | –0.22 | 0.66 | 0.974 | 0.330 | |
| Heterogeneity test |
Q
|
I
2
|
Tau
|
Tau
2
|
p
|
|||
| 48.913 | 87.733 | 0.549 | 0.301 | 0.000 | ||||
| Author (year)ref |
n | Hedge’s g value | SE | 95% LCL | 95% UCL | Z | p | |
| Biewenga et al. (2015)26 | 44 | –0.22 | 0.20 | –0.61 | 0.17 | –1.126 | 0.260 | |
| Boyce et al. (2012)27 | 24 | 0.18 | 0.26 | –0.34 | 0.70 | 0.685 | 0.494 | |
| Ngoenmak et al. (2016)3 | 22 | –0.41 | 0.28 | –0.97 | 0.15 | –1.442 | 0.149 | |
| Günlemez et al. (2010)28 | 40 | 0.33 | 0.21 | –0.09 | 0.74 | 1.549 | 0.121 | |
| Hegar et al. (2009)29 | 10 | –0.10 | 0.38 | –0.85 | 0.66 | –0.250 | 0.802 | |
| Djeddi et al. (2008)30 | 31 | 2.76 | 0.52 | 1.74 | 3.77 | 5.317 | 0.000 | |
| Sawant et al. (2004)31 | 27 | –0.19 | 0.25 | −0.68 | 0.30 | –0.766 | 0.444 | |
| Overall | 198 | 0.22 | 0.25 | –0.27 | 0.71 | 0.887 | 0.375 | |
| Heterogeneity test |
Q
|
I
2
|
Tau
|
Tau
2
|
p
|
|||
| 34.811 | 82.764 | 0.589 | 0.347 | 0.000 | ||||
LCL: lower confidence limit; OR: odds ratio; SE: standard error; UCL: upper confidence limit.
Calculated based on data presented in the paper. bCalculated by means of a meta-analytical approach.
Visual inspection of the funnel plot and Egger regression asymmetry test (intercept (95% CI): −2.343 (−4.321 to −0.364); p = 0.027), classic fail-safe N analysis (n = 162) and Begg-Mazumdar correlation test (Kendall tau b = −0.107; p = 0.71) showed no evidence of publication bias in the analysis between DMP use and CV event risk based on event rates (See Supplementary Appendix 2(b)).
DMP-QT prolongation risk: QT interval length
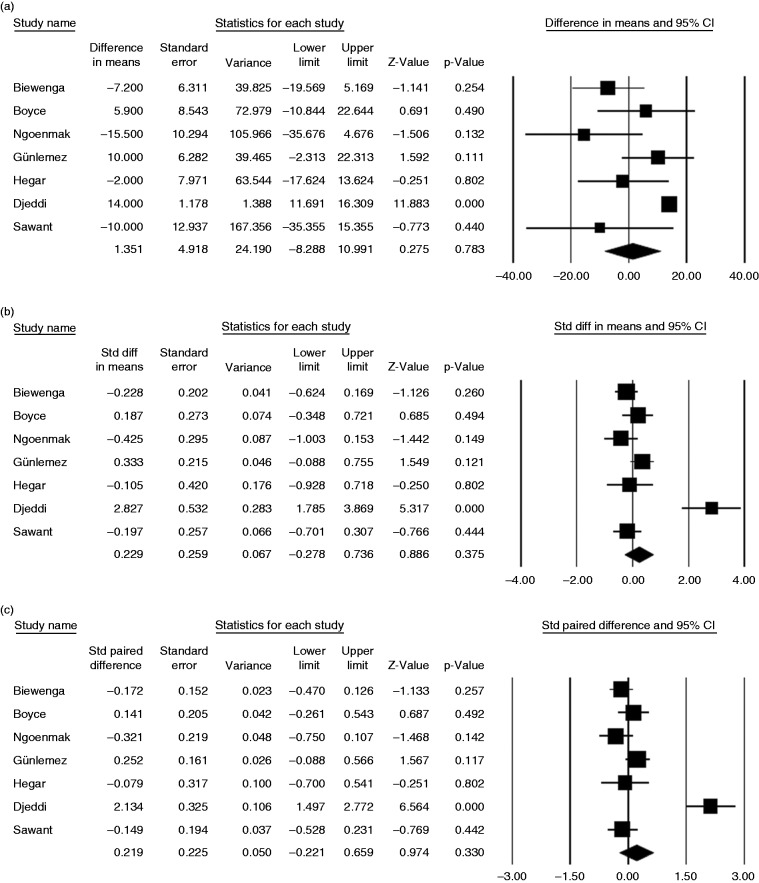
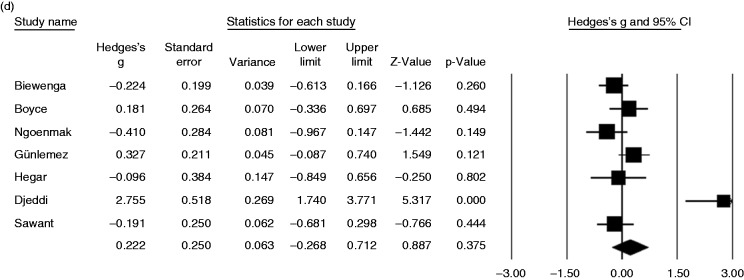
A meta-analysis of 198 patients from eight studies on DMP-QT prolongation risk revealed no significant difference between pretreatment and posttreatment QT interval in patients receiving DMP, based on the difference between means (1.35 ms, 95% CI, 8.29 ms to 10.99 ms, p = 0.783), standardized difference between means (0.23 ms, 95% CI, −0.28 ms to 0.74 ms, p = 0.376), standardized paired difference (0.22 ms, 95% CI, −0.22 ms to 0.66 ms, p = 0.330) and Hedge’s g value (0.22, 95% CI, −0.27 to 0.71, p = 0.375) (Table 3, Figure 3).
Figure 3.
Forest plot displaying (a) difference, (b) standardized difference, (c) paired standardized difference between means of pre- and posttreatment QT interval, and (d) Hedges g value and 95% confidence intervals for the impact of current domperidone treatment on QT interval prolongation.
Heterogeneity was noted for the outcome of the QT prolongation for difference between means (p = 0.000, I2 = 76.8%), standardized difference between means (p = 0.000, I2 = 82.8%), standardized paired difference (p = 0.000, I2 = 87.7%) and Hedge’s g value (p = 0.000, I2 = 82.8%) (Table 3).
For the difference between means, standardized difference between means, standardized paired difference between means and Hedge’s g values, respectively: The visual inspection of the funnel plot and Egger regression asymmetry test (intercept (95% CI): −2.272 (−3.638 to −0.905), p = 0.0079; 4.545 (−2.985 to 12.076), p = 0.18; 5.358 (−4.945 to 15.660), p = 0.24 and 4.959 (−2.678 to 12.597), p = 0.16; respectively), classic fail-safe N analysis (p > 0.05 for each), and Begg-Mazumdar correlation test (Kendall tau b = −0.286, p = 0.37 for the difference between means; Kendall tau b = 0.190; p = 0.55 for others) showed no evidence of publication bias in the analysis between DMP use and the risk for OT prolongation (See Supplementary Appendix 3).
Discussion
The present meta-analysis of studies with DMP and MCP treatments17–19,25 suggested a significantly lower CV event risk with DMP. When DMP treatment was compared with no treatment, a significant increase in CV event risk was suspected under DMP treatment based on adjusted risk in the overall analysis population. However, subgroup analysis of studies with different DMP doses17,18,24,25 confirmed that this seemingly significant increase in CV event risk under DMP treatment occurred only with higher daily doses (>30 mg/day), with no CV event risk attributable to DMP use at regular (≤30 mg/day) daily doses.
Hence, findings from the present meta-analysis suggest that DMP may not be associated with an overall CV event risk increase given the potential CV safety of the drug when used at lower doses (≤30 mg/day), while DMP also seems to offer a much more favorable cardiac safety profile than MCP at any dose in accordance with the literature.3
CV risk attributable to DMP has been considered likely to differ with respect to subgroups of exposed individuals (higher in males and older individuals) as well as according to dosage (higher for doses >30 mg/day) of DMP.2,20,21,24,41
These findings seem in accordance with regulatory restrictions introduced by the European Medicines Agency recommending DMP be used at a reduced dose in adults (up to 30 mg/day) and children (up to 0.75 mg/kg/day), for a restricted period (less than one week) and cautiously in high-risk groups such as the elderly (≥60 years).1
Accordingly, our findings seem to indicate an association of DMP treatment with lower CV risk as compared with MCP treatment and a dose-dependent increase in CV event risk with a 2.1 - to 3.1-fold increase in risk at doses >30 mg/day of MCP. This emphasizes a need for close monitoring for cardiac events and electrocardiogram (ECG) changes and adhering to appropriate cardiac risk monitoring protocols5 in patients receiving DMP at daily doses exceeding 30 mg and weighing the risk not only in the context of clinical decisions but also based on alternatives.2
Nonetheless, given that the present meta-analysis was based exclusively on retrospective studies and the lack of studies with a longitudinal, prospective design in the literature, our findings should be interpreted cautiously.
Notably, data from a retrospective chart review of patients with nausea and vomiting on high-dose DMP (80 to 120 mg) from 2009 to 2013 under an investigational new drug protocol revealed that despite very high dosing, DMP had a minimal risk of CV adverse events along with good clinical efficacy.5
Our meta-analysis suggested no significant QT prolongation risk in terms of pre- and posttreatment QT interval values in patients taking DMP.
Similarly, data from a large-scale retrospective chart review revealed prolonged QTc in seven (28.0%) out of 25 patients with a follow-up ECG in a cohort of 66 patients under DMP treatment independent of the daily dose and with no records on palpitations, chest pain, arrhythmias or changes in heart rate and electrolyte disturbances.5
Nonetheless, it should be noted that a significant heterogeneity was evident for the outcome of the difference between pretreatment and posttreatment means of QT interval in the studies included in our meta-analysis. In addition to data on pre- and posttreatment QT interval, measurements as well as event rates were available in only two studies,3,30 necessitating the calculation of these parameters using provided data in five studies,26–29,31 alongside the availability of only event rates with no data on QT measurements in one study.30 Moreover, patients from included studies showed a wide age scale that ranged from newborns to adults, with evidence on QT prolongation >500 ms in only three studies among pediatric patients; in two out of 22 children aged <2 year,3 two out of 40 premature infants28 and one out of 31 newborns,30 respectively.
Hence, while the present meta-analysis revealed no significant QT prolongation with DMP overall, given the individual data from the studies in pediatric patients, our findings seem to emphasize that pediatricians should be aware of potential cardiac side effects of DMP, particularly in case of concurrent risk factors such as prescription of high doses and the presence of concomitant medications that are likely to increase the QT interval or inhibit the P450 enzyme.3,42
Notably, only two studies had a “thorough QT” (TQT) design in our meta-analysis and both revealed no evidence of QTc prolongation (>500 msc) with any DMP regimen along with mean difference between pretreatment and posttreatment QT intervals of −7.2 and 5.8 ms, respectively.26,27
Available data on QT prolongation and cardiac event risk in patients under DMP treatment are largely limited to case reports and case-control studies along with inconsistent findings,2,4,5,7,10,13–32 and CV risk was evaluated in only one meta-analysis of six studies to date, which revealed current DMP use to increase the risk of VA and SCD by 70%.4 Hence, our findings emphasize a need for large clinical trials with a longitudinal, prospective design to better address the CV safety profile of DMP overall and in selected patient groups.2,5 Nonetheless, our findings emphasize that any risks need to be discussed in detail with patients so they are fully informed.
As a limitation, this meta-analysis was based exclusively on retrospective studies, emphasizing a need for a prospective validation to confirm data provided on DMP-related CV risk. Significant heterogeneity was noted for the outcome of the QT prolongation across included studies, which might have resulted from differences in study designs, patient characteristics and sample sizes and likely influenced the accuracy of our analysis and reduced the statistical power regarding DMP-QT prolongation risk assessment. Consistent with inclusion of studies from large clinical databases, however, classical fail-safe N publication bias tests of moderators indicated large numbers of studies were needed to render the effect size nonsignificant for CV event risk and QT prolongation event rate, indicating that the true effect size of these moderators seems significant enough to allow for certainty in the present meta-analysis.
Conclusion
In conclusion, while overall results of the meta-analysis of nine studies suggest an increased CV event risk in patients under DMP treatment, DMP seems to be associated with increased CV event risk only at doses > 30 mg/day and to offer a more favorable cardiac safety profile than MCP regardless of dosage. A meta-analysis of eight studies suggested no significant QT prolongation risk of event rates and pre- and posttreatment difference in mean QT interval values in patients under DMP treatment. Accordingly, the present meta-analysis seems to indicate that DMP treatment may not be associated with an overall CV event risk increase at doses ≤30 mg/day and does not result in QT prolongations. Our findings emphasize the need for close monitoring for cardiac events and ECG changes among those on high-dose DMP along with weighing the risk both in the context of clinical decisions and of alternatives. There is a need for well-designed, longitudinal, prospective studies to better address the CV safety profile of DMP overall and in selected patient groups.
Supplemental Material
Supplemental material for A meta-analysis on the cardiac safety profile of domperidone compared to metoclopramide by Serhat Bor, Mesut Demir, Oktay Ozdemir and Kivanc Yuksel in United European Gastroenterology Journal
Acknowledgments
The authors would like to thank Cagla Ayhan, MD, and Prof Sule Oktay, MD, PhD, from KAPPA Consultancy Training Research Ltd (Istanbul, Turkey), who provided editorial support funded by Neutec Ar-Ge San and Tic AS (Turkey).
Declaration of conflicting interests
S.B. received research grants from Neutec Ar-Ge San and Tic AS (Turkey). The funding source had no role in the design, practice or analysis of this study. The other authors have nothing to declare.
Funding
This work was supported by Neutec Ar-Ge San and Tic AS (Turkey).
Ethics approval
This type of study is exempt from ethics approval.
Informed consent
Not applicable.
References
- 1.EMA. Pharmacovigilance Risk Assessment Committee. Domperidone assessment report. EMA/152501/ 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Domperidone_31/Recommendation_provided_by_Pharmacovigilance_Risk_Assessment_Committee/WC500168926.pdf (accessed 21 February 2017).
- 2.Buffery PJ, Strother RM. Domperidone safety: A mini-review of the science of QT prolongation and clinical implications of recent global regulatory recommendations. N Z Med J 2015; 128: 66–74. [PubMed] [Google Scholar]
- 3.Ngoenmak T, Treepongkaruna S, Buddharaksa Y, et al. Effects of domperidone on QT interval in children with gastroesophageal reflux disease. Pediatr Neonatol 2016; 57: 60–64. [DOI] [PubMed] [Google Scholar]
- 4.Leelakanok N, Holcombe A, Schweizer ML. Domperidone and risk of ventricular arrhythmia and cardiac death: A systematic review and meta-analysis. Clin Drug Investig 2016; 36: 97–107. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz A, Cooper CJ, Alvarez A, et al. Cardiovascular safety profile and clinical experience with high-dose domperidone therapy for nausea and vomiting. Am J Med Sci 2015; 349: 421–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hondeghem LM. Domperidone: Limited benefits with significant risk for sudden cardiac death. J Cardiovasc Pharmacol 2013; 61: 218–225. [DOI] [PubMed] [Google Scholar]
- 7.Fais P, Vermiglio E, Laposata C, et al. A case of sudden cardiac death following domperidone self-medication. Forensic Sci Int 2015; 254: e1–e3. [DOI] [PubMed] [Google Scholar]
- 8.Drolet B, Rousseau G, Daleau P, et al. Domperidone should not be considered a no-risk alternative to cisapride in the treatment of gastrointestinal motility disorders. Circulation 2000; 102: 1883–1885. [DOI] [PubMed] [Google Scholar]
- 9.Green LW, Ottoson JM, García C, et al. Diffusion theory and knowledge dissemination, utilization, and integration in public health. Annu Rev Public Health 2009; 30: 151–174. [DOI] [PubMed] [Google Scholar]
- 10.van Noord C, Eijgelsheim M, Stricker BHC. Drug- and non-drug-associated QT interval prolongation: QT interval prolongation. Br J Clin Pharmacol 2010; 70: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandenberg JI, Perry MD, Perrin MJ, et al. hERG K+ channels: Structure, function, and clinical significance. Physiol Rev 2012; 92: 1393–1478. [DOI] [PubMed] [Google Scholar]
- 12.Ritter JM. Cardiac safety, drug-induced QT prolongation and torsade de pointes (TdP). Br J Clin Pharmacol 2012; 73: 331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi M, Giorgi G. Domperidone and long QT syndrome. Curr Drug Saf 2010; 5: 257–262. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Department of Health and Human Services. U.S. Food and Drug Administration. FDA talk paper: FDA warns against women using unapproved drug, domperidone, to increase milk production, https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm173886.htm (2004, accessed 21 February 2017).
- 15.Rocha CM, Barbosa MM. QT interval prolongation associated with the oral use of domperidone in an infant. Pediatr Cardiol 2005; 26: 720–723. [DOI] [PubMed] [Google Scholar]
- 16.Michaud V, Turgeon J. Domperidone and sudden cardiac death: How much longer should we wait? J Cardiovasc Pharmacol 2013; 61: 215–217. [DOI] [PubMed] [Google Scholar]
- 17.Arana A, Johannes CB, McQuay LJ, et al. Risk of out-of-hospital sudden cardiac death in users of domperidone, proton pump inhibitors, or metoclopramide: A population-based nested case-control study. Drug Saf 2015; 38: 1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen HL, Hsiao FY. Domperidone, cytochrome P450 3A4 isoenzyme inhibitors and ventricular arrhythmia: A nationwide case-crossover study. Pharmacoepidemiol Drug Saf 2015; 24: 841–848. [DOI] [PubMed] [Google Scholar]
- 19.Coloma PM, Schuemie MJ, Trifirò G, et al. Drug-induced acute myocardial infarction: Identifying ‘prime suspects’ from electronic healthcare records-based surveillance system. PLoS One 2013; 8: e72148–e72148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Bruin ML, Langendijk PN, Koopmans RP, et al. In-hospital cardiac arrest is associated with use of non-antiarrhythmic QTc-prolonging drugs. Br J Clin Pharmacol 2007; 63: 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johannes CB, Varas-Lorenzo C, McQuay LJ, et al. Risk of serious ventricular arrhythmia and sudden cardiac death in a cohort of users ofdomperidone: A nested case-control study. Pharmacoepidemiol Drug Saf 2010; 19: 881–888. [DOI] [PubMed] [Google Scholar]
- 22.Jolly K, Gammage MD, Cheng KK, et al. Sudden death in patients receiving drugs tending to prolong the QT interval. Br J Clin Pharmacol 2009; 68: 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straus SM, Sturkenboom MC, Bleumink GS, et al. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J 2005; 26: 2007–2012. [DOI] [PubMed] [Google Scholar]
- 24.van Noord C, Dieleman JP, van Herpen G, et al. Domperidone and ventricular arrhythmia or sudden cardiac death: A population-based case-control study in the Netherlands. Drug Saf 2010; 33: 1003–1014. [DOI] [PubMed] [Google Scholar]
- 25.Varas-Lorenzo C, Arana A, Johannes CB, et al. Improving the identification of out-of-hospital sudden cardiac deaths in a general practice research database. Drugs Real World Outcomes 2016; 3: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biewenga J, Keung C, Solanki B, et al. Absence of QTc prolongation with domperidone: A randomized, double-blind, placebo- and positive-controlled thorough QT/QTc study in healthy volunteers. Clin Pharmacol Drug Dev 2015; 4: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyce MJ, Baisley KJ, Warrington SJ. Pharmacokinetic interaction between domperidone and ketoconazole leads to QT prolongation in healthy volunteers: A randomized, placebo-controlled, double-blind, crossover study. Br J Clin Pharmacol 2012; 73: 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Günlemez A, Babaoğlu A, Arisoy AE, et al. Effect of domperidone on the QTc interval in premature infants. J Perinatol 2010; 30: 50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegar B, Alatas S, Advani N, et al. Domperidone versus cisapride in the treatment of infant regurgitation and increased acid gastro-oesophageal reflux: A pilot study. Acta Paediatr 2009; 98: 750–755. [DOI] [PubMed] [Google Scholar]
- 30.Djeddi D, Kongolo G, Lefaix C, et al. Effect of domperidone on QT interval in neonates. J Pediatr 2008; 153: 663–666. [DOI] [PubMed] [Google Scholar]
- 31.Sawant P, Das HS, Desai N, et al. Comparative evaluation of the efficacy and tolerability of itopride hydrochloride and domperidone in patients with non-ulcer dyspepsia. J Assoc Physicians India 2004; 52: 626–628. [PubMed] [Google Scholar]
- 32.Du Y, Su T, Song X, et al. Efficacy and safety of cinitapride in the treatment of mild to moderate postprandial distress syndrome-predominant functional dyspepsia. J Clin Gastroenterol 2014; 48: 328–335. [DOI] [PubMed] [Google Scholar]
- 33.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med 1998; 17: 841–856. [DOI] [PubMed] [Google Scholar]
- 38.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 39.The Ottawa Hospital Research Institute, Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 28 June 2018).
- 40.The Ottawa Hospital Research Institute, Wells G, Shea B, O’Connell D, et al. Coding manual for case-control studies, http://www.ohri.ca/programs/clinical_epidemiology/nos_manual.pdf (accessed 28 June 2018).
- 41.Marzi M, Weitz D, Avila A, et al. Cardiac adverse effects of domperidone in adult patients: A systematic review [article in Spanish]. Rev Med Chil 2015; 143: 14–21. [DOI] [PubMed] [Google Scholar]
- 42.Cresi F, Marinaccio C, Russo MC, et al. Short-term effect of domperidone on gastroesophageal reflux in newborns assessed by combined intraluminal impedance and pH monitoring. J Perinatol 2008; 28: 766–770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for A meta-analysis on the cardiac safety profile of domperidone compared to metoclopramide by Serhat Bor, Mesut Demir, Oktay Ozdemir and Kivanc Yuksel in United European Gastroenterology Journal