Abstract
Background and aims
Hp(2–20), a Helicobacter pylori-derived peptide interacting with N-formyl peptide receptors (FPRs), accelerates the healing of gastric injury in rats. Whether Hp(2–20) affects the recovery of inflamed colonic mucosa is unknown. We evaluated whether Hp(2–20) accelerated the healing of 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis and explored the mechanism(s) underlying any such effect.
Methods
Fifteen rats underwent rectal administration of Hp(2–20) 250–500 µg/kg/day, or of its control peptide Hp1 for 10 days, following induction of colitis with TNBS. Macroscopic and histological damage was quantified using predetermined injury scores. FPR1, COX-2, TNF-α, TGF-β, HB-EGF and tissue transglutaminase (t-TG) messenger RNA (mRNA) expression in colonic tissue was determined by quantitative polymerase chain reaction; FPR1, TNF-α and COX-2 protein levels by Western blotting.
Results
(1) Hp(2–20) accelerated healing of TNBS-induced colitis compared to controls consistently with the expression of FPRs in colonic mucosa; (2) TNBS upregulated mRNA mucosal expression of COX-2, TNF-α, TGF-β, HB-EGF and t-TG and (3) this, with the exception of HB-EGF, was significantly counteracted by Hp(2–20).
Conclusions
Hp(2–20), an FPR agonist, accelerates the healing of TNBS-induced colitis in the rat. This effect is associated with a significant reduction in colonic tissue levels of COX-2, TGF-β, TNF-α and t-TG. We postulate that FPR-dependent pathways may be involved in the repair of inflamed colonic mucosa.
Keywords: Hp(2–20), TNBS colitis, rat, healing, formyl peptide receptors
Introduction
Helicobacter pylori (H. pylori) is a microaerophilic, spiral-shaped, gram-negative bacterium that colonizes the human stomach. H. pylori infects 45% of the world population and is the major causative agent of gastroduodenal disorders, being associated with an increased risk of distal gastric cancer and primary gastric mucosa-associated lymphoid tissue.1–7
Hp(2–20) is a 19-amino acid peptide derived from the N-terminal region of H. pylori ribosomal protein L1, which promotes proliferation of gastrointestinal epithelial cells in vitro, stimulates the migration and invasion of gastric cells,8 upregulates vascular endothelial growth factor (VEGF) expression and accelerates the healing of gastric mucosa in vivo.8,9 Hp(2–20) peptide also exerts antimicrobial and immunomodulatory effects.10 We demonstrated that it is able to promote basophil and eosinophil chemotaxis11,12 in gastric mucosa.
The biological effects of Hp(2–20) are mediated through the interaction with N-formyl peptide receptors (FPRs), which are seven transmembrane G protein-coupled receptors. Three FPRs have been identified in humans, each encoded by a different gene: FPR1, FPR2 and FPR3.13,14 Human FPRs have a high affinity for N-formyl peptides and are expressed on the cell membranes of the host immune cells (e.g. neutrophils, monocytes). FPRs are overexpressed during bacterial infections, favoring the migration of macrophages to the site of bacterial invasion.14 Human FPRs are also expressed on nonimmune cells such as intestinal adenocarcinoma cells.9,15 Babbin et al. demonstrated that N-formyl-Met-Leu-Phe (fMLF), an N-formyl synthetic peptide that mimics the activity of bacterial N-formyl peptides, acts as an FPR agonist, accelerating the healing of injured intestinal epithelial cell monolayers,9 thus suggesting that FPRs may play a role in the recovery of injured intestinal mucosa. Similarly, it has been demonstrated that human FPRs exert a key role in the healing of several other epithelial tissues including nasal mucosa,16 retinal pigment epithelium,17 lung,18,19 and skin.20 Altogether, previous data support the evidence that FPRs might exert a homeostatic role sustaining resolution of inflammation and wound healing in several types of epithelia.13,21–23
Whether Hp(2–20) is effective in the recovery of inflamed colonic mucosa is not known. The aim of this study was therefore to evaluate whether Hp(2–20) accelerates the healing of 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis in the rat and to explore the mechanism(s) underlying any such effect.
Methods
Peptides
Hp(2–20) synthetic peptide (NH2-AKKVFKRLEKLFSKIQNDK-COOH) and Hp1 (NH2-AKKVFKRLELLFSKIQNDK-COOH) were synthesized and high-performance liquid chromatography purified (>95%) by Primm srl, Italy, and stored as lyophilized solids at +4℃ and at –20℃ when solubilized. The K to L substitution of Hp1 disrupts the α-helical structure of the Hp(2–20) peptide.
TNBS-induced colitis
Male Wistar rats, weighing 200 ± 21 g, were obtained from Harlan Italy and were housed in a temperature-controlled environment with a 12-hour light-dark cycle, and given free access to regular laboratory chow diet and water for at least one week. All animals received human care and the study protocol was approved by the Committee of Laboratory Animals of Federico II University according to institutional guidelines. Rats then were given a single enema of 0.5 ml TNBS solution (12% in 50% ethanol-water), via a rubber catheter inserted 8 cm from the anus under light anesthesia.24
Experimental design
To evaluate the effect of Hp(2–20) on TNBS-induced colitis, 15 rats were divided into three groups (n = 5 in each group). They were divided as follows: control group, rats with colitis induced by TNBS and then treated with Hp1 control peptide (500 µg/kg/day); TNBS + low-dose Hp(2–20), rats with TNBS-induced colitis then treated with rectal administration of Hp(2–20) 250 µg/kg/day; TNBS + high-dose Hp(2–20), rats with TNBS-induced colitis then treated with rectal administration of Hp(2–20) 500 µg/kg/day. Finally, five rats were treated only with TNBS vehicle (normal untreated rats). Hp(2–20) was administered rectally for 10 days, starting three days after induction of colitis with TNBS. This procedure did not require the animals to be anesthetized or restrained; the small diameter of the catheter (about 2 mm) and the small volume injected (0.5 ml) made the procedure rapid and painless in conscious rats. On the tenth day, the animals were sacrificed, the colon was collected and, after macroscopic evaluation, part of the tissue was fixed in 10% formalin and part was snap-frozen in liquid nitrogen and then stored at –80℃.
Evaluation of macroscopic and histological damage
Macroscopic and histological damage was quantified using predetermined injury scores. Macroscopic damage evaluation was carried out by graduating the damage on a scale from 0 to 5 on the basis of the following parameters: hyperemia without ulcers, fibrosis, and ulceration of different extension (Table 1).24 Formalin-fixed colonic tissue was embedded in paraffin, and hematoxylin/eosin-stained sections were assessed histologically by light microscopy by a pathologist blinded to the treatment. According to previous reports,25,26 four parameters, each scored on a 0–5 scale of severity, were considered (Table 2). The final total histological score (from 0 to 20) was determined by adding up the single scores. Colonic inflammation was graded as low (1–5), moderate (6–10) or severe (11–20).
Table 1.
Macroscopic damage score.
| Macroscopic features | Score |
|---|---|
| Normal mucosa | 0 |
| Hyperemia | 1 |
| Fibrosis | 2 |
| Ulcerations <1 cm | 3 |
| Ulcerations >1 cm and <2 cm | 4 |
| Ulcerations >2 cm | 5 |
Table 2.
Histological damage score.
| Histological features | Score |
|---|---|
| Extent of ulceration | 0–5 |
| Submucosal infiltration | 0–5 |
| Crypt abscesses | 0–5 |
| Wall thickness | 0–5 |
Immunohistochemical evaluation
Tissue sections were incubated with fresh 3% hydrogen peroxide in methanol for 20 minutes and then washed with phosphate-buffered saline (pH 7.4). The sections were incubated with 1% normal blocking serum for 30 minutes and then with goat polyclonal immunoglobulin G (IgG) anti-FPR1 (Santa Cruz Biotechnology) for 60 minutes at room temperature. The secondary antibody was biotinylated anti-goat IgG (Vector BA9500). Immunoreactive protein was detected by development with the ABC Vectastain kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s instructions. Negative controls were obtained omitting the primary antibody. The sections, counterstained with hematoxylin, were then dehydrated, mounted, and observed under a light microscope.
Protein extraction and Western blot analysis
Frozen rat colon mucosa samples were homogenized in radio-immunoprecipitation lyses buffer (0.1% sodium dodecyl sulfate (SDS), 0.5% deoxycholate, 1% Nonidet, 100 mM NaCl, 10 mM Tris-HCl (pH 7.4)) containing a protease inhibitor cocktail (Sigma, St. Louis, MO, USA), 0.5 mM dithiotreitol, and 0.5% phenylmethylsulfonyl fluoride. After 30 minutes at 4℃, tissue lysates were clarified by centrifugation at 14,000 rpm for 10 minutes at 4℃. The cleared tissue lysates were collected and stored at –80℃ and protein concentration of each sample was determined by Bradford assay (Coomassie brilliant blue protein assay; Bio-Rad, Melville, NY, USA). The antibodies used in this study were the following: (1) goat polyclonal IgG anti-cyclooxygenase-2 (anti-COX-2) (UPSTATE 07-693); (2) goat polyclonal IgG anti-tumor necrosis factor alpha (anti-TNF-α) (sc1349 Santa Cruz Biotechnology); (3) goat polyclonal IgG anti-FPR1 (Santa Cruz Biotechnology); and (4) mouse monoclonal anti-Actin (sc58323 Santa Cruz Biotechnology). The secondary antibodies were biotinylated anti-goat IgG (Vector BA9500) and biotinylated anti-mouse IgG (Vector BA200) as appropriate. Total protein extracts were subjected to SDS-polyacrylamide gel electrophoresis (10% and 7% polyacrylamide) under reducing conditions. After electrophoresis, proteins were transferred to nitrocellulose membrane (pure nitrocellulose membrane, 0.45 m Bio-Rad Laboratories); complete transfer was assessed using prestained protein standards (Invitrogen LC5925). To block nonspecific binding sites, the membranes were treated for one hour with blocking solution: 5% milk in Tris-NaCl-Tween (TNT) (10 mM Tris pH 8, 150 mM NaCl and 0.05% Tween-20), and then were incubated overnight at 4℃ with the primary antibody: (1) anti-COX-2 (diluted 1:500); (2) anti-TNF-α (diluted 1:200) in TNT (0.05% Tween-20) 20% fetal calf serum (FBS); (3) anti-FPR1 (diluted 1:2000) in TNT (0.1% Tween-20) 5% milk; and (4) anti-Actin (diluted 1:1000) in TNT (0.05% Tween-20) 20% FBS. After washing with TBS, membranes were incubated for two hours (at room temperature) with the appropriate biotinylated secondary antibody. Immunoreactive proteins were detected by development with the ABC Vectastain kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s instructions.
RNA extraction, reverse transcription-polymerase chain reaction (RT-PCR) analysis and real-time PCR
Gene expression of COX-2, TNF-α, transforming growth factor beta (TGF-β), heparin-binding epidermal growth factor (HB-EGF), tissue transglutaminase (t-TG) and FPR1 was evaluated by quantitative RT-PCR analysis. Total RNA was extracted using an RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) from colonic tissue. The purity of total RNA was assessed using a NanoDrop_ND-100 spectrophotometer at 260 nm. Two micrograms of total RNA were used in the first-strand complementary DNA (cDNA) synthesis by TaqMan® Reverse Transcription Reagents (Applied Biosystems, Branchburg, NJ, USA). The cDNA was diluted with RNase-free water for a final volume of 200 µl and stored at –20℃ until used. COX-2, TNF-α, TGF-β, HB-EGF, t-TG and FPR1 messenger RNA (mRNA) expression levels were analyzed by Taq-Man® Gene Expression Assays (Applied Biosystems). Quantitative real-time PCR was carried in triplicate using preoptimized primer/probe mixture and TaqMan universal PCR master mix (Applied Biosystems) on a StepOne™ Real-Time PCR System (Applied Biosystems). The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) housekeeping gene was used as an endogenous control for normalization of gene expression assays. We analyzed the relative gene expression data using the delta-delta threshold cycle (Ct) method. The sample values represent X-fold differences from a control sample (given a designated value of 1) within the same experiment. The assay identification codes (Assay ID) for each gene are as follows: COX-2 (Rn01483828-m1), TNF-α (Rn00562055-m1), TGF-β (Rn00572010-m1), HB-EGF (Rn00564075-m1), t-TG (Rn00571440-m1), FPR1 (Rn01441684-s1) and GAPDH (Rn 01775763-g1).
Statistical analysis
Statistically significant differences among the three groups of rats were determined with one-way analysis of variance followed by the Tukey’s multiple comparison test. Data were expressed as mean ± standard deviation (SD), and p < 0.05 was considered statistically significant.
Results
Macroscopic and histological damage
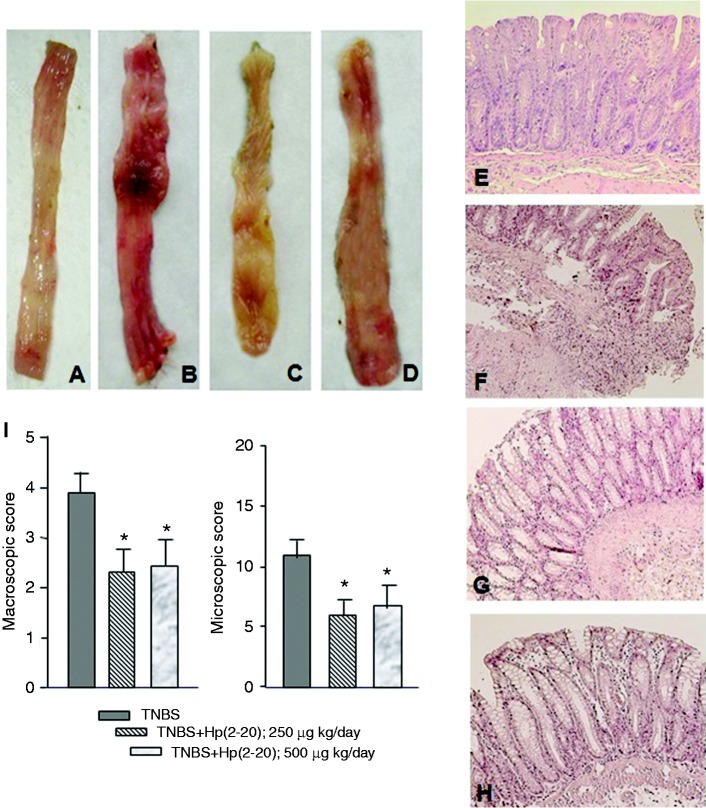
The normal macroscopic and microscopic appearance of normal rat colon is reported in Figure 1(a) and (e), respectively. TNBS induced visible colonic damage extending the entire colon length with an increase in thickness and damage to the superficial epithelium accompanied by hyperemia and ulcerations (Figure 1(b)). No statistically significant differences were detected between the TNBS alone and the TNBS + Hp1 administration in terms of colonic rat damage evaluation (not shown). Rectal administration of Hp(2–20), 250 µg or 500 µg/kg/day, for 10 days, ameliorated macroscopic (Figure 1(c) and (d)) and microscopic appearance of the rat colon (Figure 1(f)–(h)) and decreased the extent of macroscopic damage and ulcerations induced by TNBS as compared to Hp1-treated animals. The low-dose Hp(2–20) allowed the administration of a total of 2.5 mg/rat, an amount comparable to that administered to rats after indomethacin gastric injury.8 The administration over 10 days, instead of the single administration chosen to counteract indomethacin-induced gastric injury,8 was preferred to cover the slower induction of tissue injury sustained by TNBS compared to the rapid injury induced by indomethacin.27
Figure 1.
Effect of Hp(2–20) on 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis. (a) Macroscopic appearance of normal rat colon. (b) Macroscopic appearance of rat colon with TNBS-induced colitis. The colon appeared thickened and inflamed. (c) Macroscopic appearance of colon in rats with TNBS-colitis following rectal administration of Hp(2–20) 250 µg/kg/day. (d) Macroscopic appearance of colon in rats with TNBS-colitis following rectal administration of Hp(2–20) 500 µg/kg/day. (e) Histology of normal rat colon. (f) Histological appearance of colon in rats with TNBS-induced colitis (control group). Mucosa is ulcerated with an abundant inflammatory infiltrate in the submucosa; also, the colonic wall is thickened. (g) Histological appearance of colon in rats with TNBS-induced colitis following rectal administration of Hp(2–20) at dosage of 250 µg/kg/day. (h) Histological appearance of colon in rats with TNBS-induced colitis following rectal administration of Hp(2–20) at dosage of 500 µg/kg/day. In (g) and (h) treatment with Hp(2–20) significantly reduced mucosal inflammation compared to the control group. (i) Quantitative evaluation of macroscopic and microscopic damage. Values are means for five rats per group, with standard deviations represented by vertical bars. *Mean value was significantly different from that of the control group (p < 0.05).
Quantitatively, Hp(2–20) 250 µg/kg/day and 500 µg/kg/day caused an approximately 35% and 40% decrease of macroscopic damage, respectively, compared with the control group (p < 0.05, Figure 1(i)). Hp(2–20) treatment also caused a 50% decrease in the histological damage with a significant reduction in the extent of inflammatory infiltrate (p < 0.05, Figure 1(i)) compared to control rats. From a clinical point of view, rats with colitis showed a relative but not significant weight loss, poorly formed stools and occasionally streaks of blood in the stool. All these parameters improved during treatment with Hp(2–20) (data not shown).
FPR1 expression in rat colonic mucosa
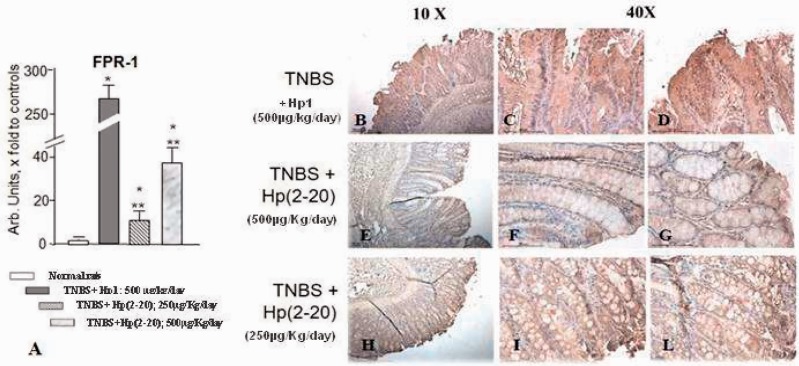
To corroborate the hypothesis of a role of FPRs in the beneficial effect exerted by Hp(2–20) at the colonic level, we first analyzed the expression levels of FPR1 in rat colonic tissues. As shown in Figure 2(a), we were able to detect significant levels of FPR1 mRNA in colonic mucosa of rats. Interestingly, TNBS treatment significantly increased FPR1 expression levels, whereas Hp(2–20) reversed such an effect (Figure 2(a)).
Figure 2.
Effect of Hp(2–20) on FPR1 mRNA expression. (a) FPR1 mRNA expression in normal (i.e. untreated) rats, in rats with TNBS-colitis treated with Hp1 peptide and in rats with TNBS-colitis treated with Hp(2–20) as assessed by RT-PCR (*p < 0.05 vs normal rats; **p < 0.05 vs TNBS-colitis Hp1-treated rats); ((b)–(l)) FPR1 protein expression as assessed by immunohistochemistry in rats with TNBS-colitis treated with Hp1 ((b)–(d)), in rats with TNBS-induced colitis followed by treatment with Hp(2–20) 500 µg/kg/day ((e)–(g)) and in rats with TNBS-induced colitis followed by treatment with Hp(2–20) 250 µg/kg/day ((h)–(l)). Magnification 10 × and 40 × . FPR1: formyl peptide receptor 1; mRNA: messenger RNA; RT-PCR: reverse transcription polymerase chain reaction; TNBS: 2,4,6-trinitrobenzenesulfonic acid.
By immunohistochemistry, rats treated with TNBS followed by Hp1 for 10 days showed intense FPR1 immunoreactivity present throughout the length of crypts both at the cytoplasmic and extracellular matrix levels in the mucosa of the areas with severe mucosal damage (Figure 2(b)–(d)). Animals that had received Hp(2–20) showed a similar immunohistochemical pattern but FPR1 positivity was much less abundant and decreased in parallel with reduction of the damage severity. The result was evident at both doses of Hp(2–20) administered (Figure 2(e)–(l)).
Hp(2–20)-mediated modulation of mediators in rat colonic mucosa
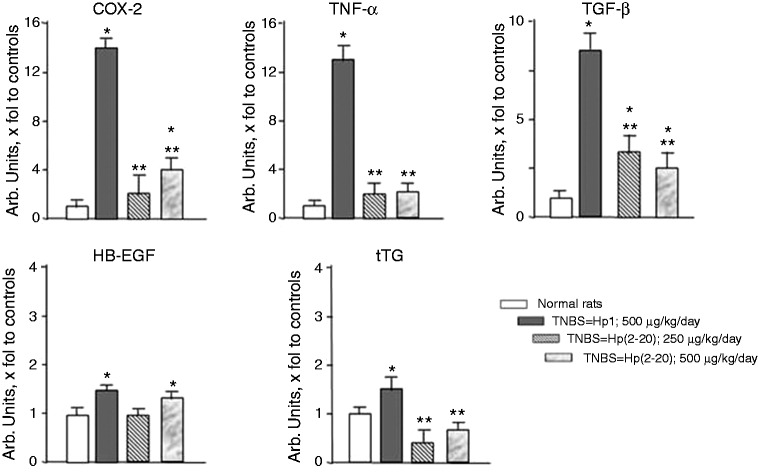
To evaluate whether TNBS caused alteration in selected genes involved in colitis inflammation and mucosal healing, we evaluated mRNA levels of COX-2, TNF-α, TGF-β, HB-EGF, and t-TG.
TNBS increased the mRNA expression of COX-2, TNF-α, TGF-β, HB-EGF, and t-TG compared to normal untreated rats (p < 0.05). Hp(2–20) at both doses significantly counteracted the increase in mRNA expression of COX-2, TNF-α, TGF-β and t-TG brought about by TNBS compared with control Hp1-treated animals (p < 0.05) (Figure 3). No significant effect of Hp(2–20) was observed on HB-EGF (Figure 3).
Figure 3.
Effect of TNBS alone or in combination with Hp(2–20) (250–500 µg/kg/day) on COX-2, TNF-α, TGF-β, HB-EGF and t-TG mRNA expression as assessed by RT-PCR (*p < 0.05 vs normal rats; **p < 0.05 vs TNBS-colitis Hp1-treated rats). COX-2: cyclooxygenase-2; HB-EGF: heparin-binding epidermal growth factor; mRNA: messenger RNA; RT-PCR: reverse transcription polymerase chain reaction; TNBS: 2,4,6-trinitrobenzenesulfonic acid; TNF-α: tumor necrosis factor alpha; TGF-β: transforming growth factor beta; t-TG: tissue transglutaminase.
COX-2 and TNF-α protein levels were evaluated as markers of inflammation in rat colonic mucosa. Protein expression of TNF-α and COX-2 were increased in rats with TNBS-induced colitis (not shown), and this increase was counteracted by Hp(2–20) (Figure 4).
Figure 4.
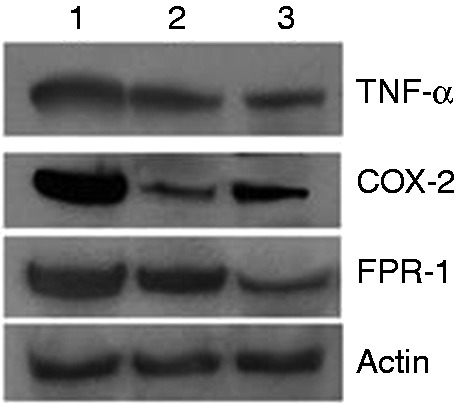
Protein expression of TNF-α, COX-2 and FPR1 at Western blot analysis in TNBS-colitis Hp1-treated rats (1), in rats treated with Hp(2–20) 250 µg/kg/day (2) and in rats treated with Hp(2 20) 500 µg/kg/day (3). COX-2: cyclooxygenase-2; FPR1: formyl peptide receptor 1; TNBS: 2,4,6-trinitrobenzenesulfonic acid; TNF-α: tumor necrosis factor alpha.
Consistently with these results, FPR1 protein expression was regulated by TNBS and Hp(2–20) (Figure 4). In particular, TNBS upregulated FPR1 protein expression (not shown), whereas Hp(2–20) efficiently counteracted this effect.
Discussion
TNBS-induced colitis is widely used as a model for inflammatory bowel diseases (IBDs) and, in particular, for Crohn’s disease (CD). Rectal administration of TNBS leads to colonic inflammation characterized by ulcerations, hyperemia, edema/congestion of mucosa and infiltration of inflammatory cells in the submucosa with formation of granulomas.24
H. pylori is known to induce gastric pathologic conditions because of the interaction between a number of virulence factors peculiar to the bacterium with host-related and environmental-related factors. Hp(2–20) is a recently identified peptide produced by the bacterium that exerts beneficial effects on gastric mucosa through the interaction with the FPRs.8 We have previously shown that exogenous administration of Hp(2–20) promotes gastric mucosal healing following indomethacin-induced ulceration in the rat by sustaining epithelial cell migration and proliferation, as well as the expression of VEGF.8
The present study is the first that demonstrates the beneficial effect of Hp(2–20) on inflamed colonic mucosa. We have shown that peptide Hp(2–20) significantly accelerates colonic mucosal healing both at the macroscopic and histological levels and that this effect is associated with a significant reduction in colonic tissue levels of inflammatory mediators. We also have evaluated mediators of damage repair (i.e. TGF-β, t-TG and HB-EGF), showing that TGF-β and t-TG are upregulated in a TNBS-colitis group vs TNBS-untreated rats. Treatment with Hp(2–20) at both doses induced a reduction in mucosal damage and decreased TGF-β and t-TG mRNA expression compared to TNBS-colitis rats (i.e. rats treated with TNBS + Hp1 control peptide).
We also observed an upregulation of HB-EGF mRNA in TNBS colitis vs normal rats but we did not find a reduction of HB-EGF expression in Hp(2–20)-treated vs Hp1-treated rats. HB-EGF is an important factor, early involved in the repair of injured gastrointestinal mucosa and leading to migration and proliferation of epithelial cells.28 We may speculate that, because Hp(2–20) intervenes in a highly coordinated series of events that allow the restitution of the tissue, at different time frames, different mediators may be up- or downregulated.
We evaluated the expression of the main component of the rat FPR family (i.e. FPR1) and found that FPR1 is expressed in intestinal mucosa rats. This is in agreement with a report by Babbin et al., who by using immunofluorescence found that FPR1 is expressed on the baso-lateral membrane of crypt epithelial cells.15 No data are available indicating the specificity of the effects of Hp(2–20) on single components of the rat FPR family. Furthermore, no studies are helpful in dissecting the correspondence of rat FPRs to the human FPR family,14 nor in defining the specificity of FPR ligands for rat components of the FPR family. Thus, we decided to verify the expression levels of the main component of the rat FPR family. However, another paper also demonstrated the expression of FPR2 in rat tissues.29 Based on this, we postulate that FPRs may play a role in the process of gastrointestinal mucosal healing and that Hp(2–20) acts as a promoter of mucosal healing by interacting with FPRs. Furthermore, we were also able to detect increased expression of FPR1 in TNBS-treated tissues and an effect of Hp(2–20) in counterbalancing this phenomenon. We hypothesize that the increased FPR1 expression in colonic mucosa of TNBS-treated rats could be ascribed to a protective FPR1-mediated response activated by colonic mucosa in response to injury. In fact, the innate immune receptors exert a critical role in particular in the intestinal mucosa in which they sense commensal and pathologic microbial organisms and serve to maintain epithelial barrier functions.13,21 Consistently with our results, Chen et al. demonstrated that FPR2 was constitutively expressed in mouse colonic epithelial cells and that dextran sulfate sodium (DSS) intake increased the expression of mouse FPR2 in epithelial cells.30 Thus, FPRs’ upregulation occurs in several organisms in response to different injuries, at least at intestinal levels. This suggests a compensatory response activated at epithelial levels to improve the recovery of mucosal integrity. Although to date we have not been able to assess the receptor specificity of Hp(2–20), the reduced expression of FPR1 following Hp(2–20) treatment supports the concept that rat FPR1 could be downregulated by its ligand interaction. However, blockade of specific component(s) of the FPR family prior to Hp(2–20) treatment would better corroborate our hypothesis. Whether intestinal mucosa from CD patients has higher levels of FPRs compared with normal intestine is under evaluation in our lab as the effect of Hp(2–20) on FPR expression in ex-vivo organ culture of human intestinal mucosa.
Whether FPR ligands other than Hp(2–20) could facilitate colonic mucosa restoration following TNBS treatment has not been studied. Consistent with a fostering role of FPRs and their ligands in the improvement of colonic mucosal restoration, however, a number of in vivo studies have demonstrated that: (i) FPR-deficient mice displayed defective colonic healing following DSS treatment;30 (ii) the synthetic peptide mouse Trp-Lys-Tyr-Met-Val-D-Met (WKYMVm), able to bind and activate all the three FPRs with different affinities, decreased DSS-induced bleeding score and colonic mucosa destruction in mice with ulcerative colitis;31 (iii) cationic steroid antimicrobial 13 (CSA13) administration ameliorated colitis-associated intestinal fibrosis in mice by interacting with FPR2. Interestingly, CSA13 displays a nature and function similar to that of antimicrobial peptides such as Hp(2–20).32 Taken together, these reports suggest that FPR ligands, including fMLF, which is the main agonist to FPRs, might be useful in the development of efficient therapeutic agents against chronic intestinal inflammatory diseases.33
The evidence that Hp(2–20) accelerates colonic mucosal healing may partially explain the inverse association between H. pylori infection and IBD. In fact, a large amount of published data show that there is a low incidence of H. pylori infection in patients with IBD compared with normal controls.34,35 These data would have greater value if proven by further epidemiological studies, especially in newly diagnosed patients with IBD. The low incidence of infection in fact may partly be due to the use of metronidazole and/or ciprofloxacin in patients with IBD, especially in the setting of CD.
The armamentarium in the treatment of IBD includes several drugs such as mesalazine, corticosteroids, antibiotics and immunosuppressive drugs. More recently, biologic agents have been shown to be able to lead to mucosal healing, an important target of IBD therapy. Biologic agents are costly, however, and are not completely without side effects. Therefore, it is highly auspicious to search for agents that alone or in combination with already known agents may prove of use in treating IBD patients. We recently demonstrated that a nutraceutical consisting of a polyphenol extract from apple accelerated the healing of colonic damage brought about by TNBS in rats.36 We here provide evidence that a product deriving from a bacterium that is harmful to the stomach exerts beneficial effects in an inflammatory condition of the gastrointestinal tract, decreasing mucosal damage both at the macroscopic and microscopic level and downregulating the expression of inflammatory mediators. These results increase our knowledge of the pathophysiological mechanisms by which bacterial products accelerate the reparative processes of gastrointestinal mucosa and lead us to postulate that an FPR-dependent pathway may be involved in the repair of inflamed colonic mucosa, thus representing a possible target of intervention. We may therefore envision the possibility that Hp(2–20) in conjunction with other known agents may have a therapeutic role in IBD.
Informed consent
Since this is a study not involving humans, informed consent does not apply to this manuscript.
Ethics approval
On behalf of all of the Authors, AGG hereby certifies that legal and ethical requirements have been met with regards to the humane treatment of animals described in the study.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Go MF. Review article: Natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther 2002; 16(Suppl 1): 3–15. [DOI] [PubMed] [Google Scholar]
- 2.Blaser MJ, Parsonnet J. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Invest 1994; 94: 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehours P, Mégraud F. Helicobacter pylori infection and gastric MALT lymphoma. Rocz Akad Med Bialymst 2005; 50: 54–61. [PubMed] [Google Scholar]
- 4.Romano M, Ricci V, Zarrilli R. Mechanisms of disease: Helicobacter pylori-related gastric carcinogenesis—implications for chemoprevention. Nat Clin Pract Gastroenterol Hepatol 2006; 3: 622–632. [DOI] [PubMed] [Google Scholar]
- 5.Censini S, Stein M, Covacci A. Cellular responses induced after contact with Helicobacter pylori. Curr Opin Microbiol 2001; 4: 41–46. [DOI] [PubMed] [Google Scholar]
- 6.Kersulyte D, Mukhopadhyay AK, Velapatino B, et al. Differences in genotypes of Helicobacter pylori from different human populations. J Bacteriol 2000; 182: 3210–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricci V, Ciacci C, Zarrilli R, et al. Effect of Helicobacter pylori on gastric epithelial cell migration and proliferation in vitro: Role of VacA and CagA. Infect Immun 1996; 64: 2829–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Paulis A, Prevete N, Rossi FW, et al. Helicobacter pylori Hp(2–20) promotes migration and proliferation of gastric epithelial cells by interacting with formyl peptide receptors in vitro and accelerates gastric mucosal healing in vivo. J Immunol 2009; 183: 3761–3769. [DOI] [PubMed] [Google Scholar]
- 9.Babbin BA, Jesaitis AJ, Ivanov AI, et al. Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J Immunol 2007; 179: 8112–8121. [DOI] [PubMed] [Google Scholar]
- 10.Lee DG, Park Y, Kim HN, et al. Antifungal mechanism of an antimicrobial peptide, HP (2–20), derived from N-terminus of Helicobacter pylori ribosomal protein L1 against Candida albicans. Biochem Biophys Res Commun 2002; 291: 1006–1013. [DOI] [PubMed] [Google Scholar]
- 11.de Paulis A, Prevete N, Fiorentino I, et al. Basophils infiltrate human gastric mucosa at sites of Helicobacter pylori infection, and exhibit chemotaxis in response to H. pylori-derived peptide Hp(2–20). J Immunol 2004; 172: 7734–7743. [DOI] [PubMed] [Google Scholar]
- 12.Prevete N, Rossi FW, Rivellese F, et al. Helicobacter pylori HP(2–20) induces eosinophil activation and accumulation in superficial gastric mucosa and stimulates VEGF-alpha and TGF-beta release by interacting with formyl-peptide receptors. Int J Immunopathol Pharmacol 2013; 26: 647–662. [DOI] [PubMed] [Google Scholar]
- 13.Prevete N, Liotti F, Marone G, et al. Formyl peptide receptors at the interface of inflammation, angiogenesis and tumor growth. Pharmacol Res 2015; 102: 184–191. [DOI] [PubMed] [Google Scholar]
- 14.Ye RD, Boulay F, Wang JM, et al. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev 2009; 61: 119–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babbin BA, Lee WY, Parkos CA, et al. Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J Biol Chem 2006; 281: 19588–19599. [DOI] [PubMed] [Google Scholar]
- 16.Prevete N, Salzano FA, Rossi FW, et al. Role(s) of formyl-peptide receptors expressed in nasal epithelial cells. J Biol Regul Homeost Agents 2011; 25: 553–564. [PubMed] [Google Scholar]
- 17.Zhang XG, Hui YN, Huang XF, et al. Activation of formyl peptide receptor-1 enhances restitution of human retinal pigment epithelial cell monolayer under electric fields. Invest Ophthalmol Vis Sci 2011; 52: 3160–3165. [DOI] [PubMed] [Google Scholar]
- 18.Shao G, Julian MW, Bao S, et al. Formyl peptide receptor ligands promote wound closure in lung epithelial cells. Am J Respir Cell Mol Biol 2011; 44: 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaykhiev R, Beisswenger C, Kandler K, et al. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am J Physiol Lung Cell Mol Physiol 2005; 289: L842–L848. [DOI] [PubMed] [Google Scholar]
- 20.Liu M, Chen K, Yoshimura T, et al. Formylpeptide receptors mediate rapid neutrophil mobilization to accelerate wound healing. PLoS One 2014; 9: e90613–e90613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prevete N, Liotti F, Amoresano A, et al. New perspectives in cancer: Modulation of lipid metabolism and inflammation resolution. Pharmacol Res 2018; 128: 80–87. [DOI] [PubMed] [Google Scholar]
- 22.Prevete N, Liotti F, Illiano A, et al. Formyl peptide receptor 1 suppresses gastric cancer angiogenesis and growth by exploiting inflammation resolution pathways. Oncoimmunology 2017; 6: e1293213–e1293213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prevete N, Liotti F, Visciano C, et al. The formyl peptide receptor 1 exerts a tumor suppressor function in human gastric cancer by inhibiting angiogenesis. Oncogene 2015; 34: 3826–3838. [DOI] [PubMed] [Google Scholar]
- 24.Morris GP, Beck PL, Herridge MS, et al. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989; 96: 795–803. [PubMed] [Google Scholar]
- 25.D’Argenio G, Cosenza V, Sorrentini I, et al. Butyrate, mesalamine, and factor XIII in experimental colitis in the rat: Effects on transglutaminase activity. Gastroenterology 1994; 106: 399–404. [DOI] [PubMed] [Google Scholar]
- 26.Loguercio C, D’Argenio G, Delle Cave M, et al. Direct evidence of oxidative damage in acute and chronic phases of experimental colitis in rats. Dig Dis Sci 1996; 41: 1204–1211. [DOI] [PubMed] [Google Scholar]
- 27.Antoniou E, Margonis GA, Angelou A, et al. The TNBS-induced colitis animal model: An overview. Ann Med Surg (Lond) 2016; 11: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radulescu A, Zhang HY, Chen CL, et al. Heparin-binding EGF-like growth factor promotes intestinal anastomotic healing. J Surg Res 2011; 171: 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang G, Zhang L, Chen X, et al. Formylpeptide receptors promote the migration and differentiation of rat neural stem cells. Sci Rep 2016; 6: 25946–25946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen K, Liu M, Liu Y, et al. Formylpeptide receptor-2 contributes to colonic epithelial homeostasis, inflammation, and tumorigenesis. J Clin Invest 2013; 123: 1694–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SD, Kwon S, Lee SK, et al. The immune-stimulating peptide WKYMVm has therapeutic effects against ulcerative colitis. Exp Mol Med 2013; 45: e40–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu C, Ghali S, Wang J, et al. CSA13 inhibits colitis-associated intestinal fibrosis via a formyl peptide receptor like-1 mediated HMG-CoA reductase pathway. Sci Rep 2017; 7: 16351–16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prevete N, de Paulis A, Sgambato D, et al. Role of formyl peptide receptors in gastrointestinal healing. Curr Pharm Des. Epub ahead of print 15 May 2018. DOI: 10.2174/1381612824666180516102234. [DOI] [PubMed]
- 34.Sonnenberg A, Genta RM. Low prevalence of Helicobacter pylori infection among patients with inflammatory bowel disease. Aliment Pharmacol Ther 2012; 35: 469–476. [DOI] [PubMed] [Google Scholar]
- 35.Zhang S, Zhong B, Chao K, et al. Role of Helicobacter species in Chinese patients with inflammatory bowel disease. J Clin Microbiol 2011; 49: 1987–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Argenio G, Mazzone G, Tuccillo C, et al. Apple polyphenols extract (APE) improves colon damage in a rat model of colitis. Dig Liver Dis 2012; 44: 555–562. [DOI] [PubMed] [Google Scholar]