Abstract
Background
Esophageal shortening (ES) might be observed during high-resolution manometry (HRM), in particular after the rapid drink test (RDT). We aimed to assess its diagnostic value in patients referred for HRM.
Methods
HRM of patients without previous esophagogastric surgery or endoscopic treatment was retrospectively reviewed using the Chicago Classification v3.0. ES and pan-esophageal pressurization were analyzed during the RDT (200-ml free drinking in a sitting position).
Results
A total of 2141 cases (1291 females, mean age 54 years) were reviewed. During the RDT, ES occurred in 4% and pan-esophageal pressurization in 14% of patients. ES was almost exclusively encountered in patients with impaired esophagogastric junction relaxation or major disorders of peristalsis. Among 31 patients with ES and no definite diagnosis of achalasia, 19 had follow-up and 13 (68%) changed diagnostic category: two adenocarcinoma of the cardia, and 11 cases of atypical achalasia. The positive predictive value of ES for a significant esophageal disorder was 95%.
Conclusion
ES is rarely observed during the RDT. When present, it is associated with major motility disorders, especially achalasia. When the diagnostic criteria for achalasia are not fulfilled, further complementary examinations should be performed to rule out incomplete forms of achalasia or an infiltrative process of the cardia.
Keywords: Achalasia, dysphagia, esophageal motility disorder, human, predictive positive value
Key summary
Summarize the established knowledge on this subject
The rapid drink test (RDT) is of interest to evaluate esophageal clearance during esophageal high-resolution manometry.
Esophageal shortening after the RDT occurs very rarely in controls.
Esophageal shortening is frequently associated with achalasia.
What are the significant and/or new findings of this study?
In a large retrospective cohort of patients undergoing esophageal high-resolution manometry, esophageal shortening during the rapid drink test was mainly associated with impaired esophagogastric junction relaxation or major disorders of peristalsis.
In the rare cases when esophageal shortening is present and associated with minor manometric abnormalities or normal manometry, a complementary workup should be performed to rule out incomplete forms of achalasia or an infiltrative process of the cardia.
Introduction
Esophageal shortening, as a result of longitudinal muscle contraction, has been described both in health and disease. It was first described in animals and humans during normal primary peristalsis.1–3 In particular, Edmundowicz and Clouse first described the occurrence of esophageal shortening preceding the onset of contraction waves in the distal segment of the esophageal body.3 Esophageal shortening was then demonstrated to have an important role in the occurrence of transient lower esophageal sphincter (LES) relaxations (TLESRs), in gastroesophageal reflux disease (GERD) and in eliciting heartburn.4–8 In a study with concomitant fluoroscopy and high-resolution manometry (HRM), the onset of esophageal shortening preceded manometric evidence of retrograde flow.6 Furthermore, some studies have suggested that prolonged contractions of the longitudinal muscle of the esophagus may be associated with noncardiac chest pain.9,10 Esophageal shortening was also described in patients with achalasia and major disorders of peristalsis such as esophageal spasm. In this setting esophageal shortening, mostly occurring a few seconds after swallowing, might play an important role in emptying the esophagus.11–13
Using concomitant esophageal HRM and high-frequency intraluminal ultrasound imaging, Mittal et al. demonstrated that esophageal shortening seen as an upward LES lift during HRM was a surrogate marker for longitudinal muscle contraction.14
In the last decade esophageal HRM has become the gold standard for the diagnosis of esophageal motor disorders.15
The latest iteration of the Chicago Classification (CC) for esophageal motility disorders demonstrated an improvement in the identification of motor disorders with higher symptom burden compared to previous versions.16,17
Provocative tests were developed to enhance diagnostic accuracy in patients with suspected motility disorders.18–20 The rapid drink test (RDT, consisting of 200-ml free drinking in a sitting position) and test meal can be used during esophageal HRM. They represent more physiological conditions than single water swallows and thus improve the diagnosis yield of HRM.21,22
The RDT might be useful to depict a significant obstruction at the level of the esophagogastric junction (EGJ). In this case, pan-esophageal pressurization occurs during the RDT. This pan-esophageal pressurization was frequently noticed in patients with achalasia.23,24 Esophageal shortening can also be observed after the RDT.
So far provocative tests and esophageal shortening are not taken into account in the CC of esophageal motor disorders. Very few data are available about the occurrence and significance of esophageal shortening among patients undergoing esophageal HRM.
The aim of this study was to assess the occurrence of esophageal shortening during or after the RDT in a cohort of patients referred for esophageal HRM and to determine its diagnostic value.
Methods
Patients
Patients referred for esophageal HRM from October 2011 to February 2017 in one single center were retrospectively included. Exclusion criteria were: (1) patients under 18 years of age; (2) previous thoracic or esophagogastric surgery; (3) previous endoscopic treatment for esophageal motor disorders (pneumatic dilation, botulinum toxin (Botox) injection, per-oral endoscopic myotomy (POEM)); and (4) incomplete HRM protocol (fewer than seven analyzable 5-ml water swallows or absence of RDT). In case of several HRM studies in one single patient, only the first one was taken into account. According to French law, this kind of retrospective analysis of data obtained during clinical evaluation of patients does not require approval by an ethics review board. Patients were informed prior to HRM that their clinical data could be used for clinical research, after anonymization. They had the option of signing a document indicating their refusal to participate, in which case their files were not used for the study.
Clinical data
Demographic data (age, gender and body mass index (BMI)) as well as previous medical and surgical history were collected the day of the HRM. Systematic questionnaires were used to evaluate GERD symptoms, (gastroesophageal reflux disease questionnaire (GERD-Q) score25) and dysphagia (Eckardt score26).
Among patients with esophageal shortening during the RDT, results of complementary examinations including upper gastrointestinal endoscopy, esophageal biopsies, computed tomography scan, endoscopic ultrasonography, barium esophagogram and impedance planimetry (EndoFLIP™, Crospon, Galway, Ireland) were reviewed if available.
Esophageal HRM
All patients underwent esophageal HRM (ManoScan™, Medtronic, Boulogne-Billancourt, France) after overnight fasting. The probe (consisting of 36-sensor solid-state pressure sensors) was introduced transnasally and positioned to record pressure variations from the hypopharynx to the stomach with at least three intragastric sensors. The standardized protocol consisted of 10 5-ml water swallows realized at 30-second intervals in the supine position. The patient was then asked to sit up and the RDT was performed: The patient was instructed to drink 200 ml of water as quickly as possible.22,24
HRM studies were retrospectively analyzed using ManoView™ v3.0 software (Medtronic, Minneapolis, MN, USA). The 10 5-ml swallows were assessed according to the CC v3.0 and esophageal motility disorders diagnosed using the hierarchical algorithm proposed for the interpretation of HRM studies.16 With regard to the standard classification, some HRM recordings did not fit with the diagnosis of achalasia, most frequently because of a low (or normal) integrated relaxation pressure (IRP). We defined an incomplete form of achalasia based on the results of one or several additional techniques: IRP during RDT above 12 mmHg as proposed by Ang et al.,23 thickening of the esophageal muscle on EUS, clear barium stasis at five minutes after barium swallow, or decreased EGJ distensibility on impedance planimetry.27
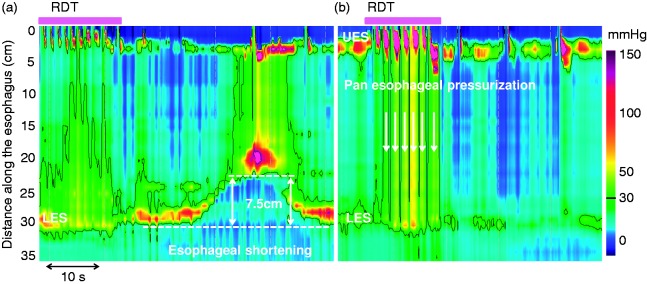
During the RDT esophageal shortening was defined as an upward lift of the pressure band indicative of the LES.28 The ManoView basic smart mouse tool was used to measure the distance between the baseline position of the LES just prior to the RDT and its maximal axial position during the RDT or within 60 seconds after the beginning of the RDT. A threshold of more than 1 cm was used to define esophageal shortening (Figure 1(a)).22 As esophageal shortening has recently been added to the manometric criteria for the identification of TLESRs, cases occurring in this setting were excluded.29 The occurrence of pan-esophageal pressurization defined as a homogeneous increase of intraesophageal pressure greater than 30 mmHg was evaluated during the RDT24 (Figure 1(b)).
Figure 1.
Esophageal shortening and pan-esophageal pressurization during the rapid drink test (RDT). The pink boxes above the pressure topography plots indicate the period with repetitive swallows during RDT. (a) Esophageal shortening is defined as an elevation of the lower esophageal sphincter (LES) greater than 1 cm (7.5 cm on this example; the dashed lines indicate the lower border of the LES). (b) Pan-esophageal pressurization is defined as a homogeneous pressurization > 30 mmHg between the upper esophageal sphincter (UES) and the LES (indicated with vertical white arrows).
Statistical analysis
Quantitative data were expressed as median (range) and qualitative data as percentage. Patients were first divided into groups according to the diagnosis of esophageal motor disorders and then compared according to the presence of esophageal shortening during the RDT. Between groups, quantitative data were compared using analysis of variance and qualitative data were compared using chi-square test or Fisher exact test when appropriate. A p value < 0.05 was considered statistically significant.
Results
Patient characteristics
Among 3531 consecutive esophageal HRM, 2141 patients fulfilled the inclusion criteria and were included in the study. The mean age was 54 years (18–93) and there were more females (1291, 60%) than males. GERD symptoms and dysphagia were the most frequent symptoms of presentation (34% and 26%, respectively). Patient characteristics are described in Table 1.
Table 1.
Patient characteristics.
| Characteristics, n = 2141 | |
| Mean age (range), years | 54 (18–93) |
| Gender, n (%) | 1291 females (60%) |
| Median BMI (range), kg/m2 | 25 (14–57) |
| Dominant symptom | |
| Dysphagia, n (%) | 547 (26%) |
| GERD symptoms, n (%) | 749 (35%) |
| Oropharyngeal dysphagia and/or ENT symptoms, n (%) | 241 (11%) |
| Cough, asthma, pneumonia, n (%) | 150 (7%) |
| Chest pain, n (%) | 137 (6%) |
| Abdominal symptomsa, n (%) | 162 (8%) |
| Belching, n (%) | 44 (2%) |
| Connective tissue disease, myositis, n (%) | 84 (4%) |
| Miscellaneousb, n (%) | 27 (1%) |
| Median Eckardt score (range) | 2 (0–12) |
| Score ≥ 3c, n (%) | 961 (45%) |
| Median GERD-Q score (range) | 7 (0–18) |
| Score ≥ 9d, n (%) | 809 (38%) |
BMI: body mass index; GERD: gastroesophageal reflux disease; ENT: ear, nose and throat; GERD-Q: gastroesophageal reflux disease questionnaire.
Abdominal symptoms included nausea, vomiting, subocclusion and constipation.
Miscellaneous dominant symptoms included hiccup, halitosis, no symptoms (evaluation before bariatric surgery), suspicion of rumination, food impaction, vocal cord granuloma and fever.
An Eckardt score ≥ 3/12 defines achalasia-type symptom severity.24
A GERD-Q score ≥ 9 is in favor of pathological GERD.23
Esophageal motor disorders according to the CC v3.0
The distribution of esophageal motility disorders is summarized in Table 2. Overall an abnormal HRM was present in 1032 (48%) patients. A minor disorder of peristalsis was noticed in 669 patients (65%). Among patients with impaired EGJ relaxation and major disorders of peristalsis, achalasia was the most frequent disorder (138 patients (38%)).
Table 2.
Distribution of high-resolution manometry diagnosis according to the Chicago Classification v3.0.
| Motility disorder | Number (percentage) | |
|---|---|---|
| Type 1 achalasia | 14 (0.7%) | Impaired EGJ relaxation |
| Type 2 achalasia | 88 (4%) | |
| Type 3 achalasia | 36 (2%) | |
| EGJ outflow obstruction | 66 (3%) | |
| Distal esophageal spasm | 17 (0.8%) | Major disorders of peristalsis |
| Jackhammer esophagus | 54 (3%) | |
| Absent contractility | 88 (4%) | |
| Ineffective esophageal motility | 659 (31%) | Minor disorders of peristalsis |
| Fragmented peristalsis | 10 (0.5%) | |
| Normal manometry | 1109 (52%) | Normal |
EGJ: esophagogastric junction.
Esophageal shortening during the RDT
Esophageal shortening occurred in 82 patients (4%): In only eight cases did the shortening occur during the RDT; it occurred after the RDT in the remaining 74 patients. Pan-esophageal pressurization occurred in 292 patients (14%) (Table 3). The median shortening was 25 mm (range, 15–75 mm).
Table 3.
Occurrence of esophageal shortening and pan-esophageal pressurization according to the Chicago Classification v3.0 diagnosis.
| Motility disorder | Esophageal shortening + PEP | Esophageal shortening alone | PEP alone | No PEP, no esophageal shortening | p value |
|---|---|---|---|---|---|
| Achalasia, n (%) | 49 (35%) | 2 (1%) | 75 (54%) | 12 (9%) | p < 0.0001a p < 0.0001b |
| EGJ outflow obstruction, n (%) | 11 (17%) | 3 (5%) | 26 (39%) | 26 (39%) | p < 0.0001a p < 0.0001b |
| Diffuse esophageal spasm, n (%) | 3 (18%) | 1 (6%) | 11 (65%) | 2 (12%) | p < 0.0001a p < 0.0001b |
| Jackhammer esophagus, n (%) | 1 (2%) | 2 (4%) | 21 (39%) | 30 (56%) | p = 0.0025a p < 0.0001b |
| Absent contractility, n (%) | 3 (3%) | 0 (0%) | 4 (4%) | 81 (92%) | p = 0.0096a p = 0.2106b |
| Ineffective esophageal motility, n (%) | 1 (0.2%) | 3 (0.4%) | 20 (3%) | 635 (96%) | |
| Fragmented peristalsis, n (%) | 0 (0%) | 0 (0%) | 2 (20%) | 8 (80%) | |
| Normal, n (%) | 1 (0.1%) | 2 (0.2%) | 64 (6%) | 1042 (94%) | |
EGJ: esophagogastric junction; PEP: pan-esophageal pressurization.
A p value vs minor disorders of peristalsis or normal for esophageal shortening (alone or in combination with PEP).
A p value vs minor disorders of peristalsis or normal for PEP (alone or in combination with esophageal shortening).
Patients with esophageal shortening were older than those without (60 years (19–86) vs 54 (18–93), p < 0.001) and were more likely men (62% vs 39%; p < 0.0001). They also complained more frequently of dysphagia than those without (83% vs 23%, p < 0.0001) and presented a higher Eckardt score (5 (0–11) vs 2 (0–12), p < 0.0001) as well. No difference was noticed regarding BMI (24 kg/m2 (17–40) vs 25 (14–57), p = 0.163) or GERD-Q score (9 (0–15) vs 7 (0–18), p = 0.597). Finally pan-esophageal pressurization during the RDT was more frequent among patients with esophageal shortening than among those without (84% vs 11%, p < 0.0001).
Esophageal shortening occurred almost exclusively in patients with impaired EGJ relaxation or major disorders of peristalsis (Table 3). Similarly, pan-esophageal pressurization was more frequent among patients with impaired EGJ relaxation or major disorders of peristalsis. Of note, esophageal shortening occurrence without pan-esophageal pressurization during the RDT was rare (16%, 13 cases, of which five were associated with minor disorders of peristalsis or normal manometry), while pan-esophageal pressurization alone without esophageal shortening was more frequent in various manometric diagnoses (76%) including normal manometry (Table 3).
Esophageal shortening and diagnosis of esophageal disorders
Thirty-one patients with esophageal shortening not fulfilling the diagnostic criteria of achalasia were identified: The 19 cases with follow-up are reported in Table 4. Thirteen (68%) ended up with a modified final diagnosis: incomplete form of achalasia in 11 patients and esophageal adenocarcinoma in two cases. Overall, considering the four cases with esophageal shortening and minor manometric abnormalities of normal manometry without further follow-up as false-positive results of the test, the predictive positive value of this finding for a significant esophageal disorder would be 95%.
Table 4.
Follow-up and final diagnosis of 19 patients with esophageal shortening during rapid drink test and without a definite initial diagnosis of achalasia.
| Motility disorder | PEP | Complementary examination | Final/concomitant diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|
| EGJ outflow obstruction (10 cases) | Yes | EUS (muscular thickening) | Incomplete form of achalasia | Botox | Good |
| Yes | EUS (normal) | EGJ outflow obstruction | Antispasmodic agents | Good | |
| Yes | EUS (muscular thickening) | Incomplete form of achalasia | No follow-up | ||
| Yes | EUS (muscular thickening) | Incomplete form of achalasia | POEM | Good | |
| Yes | CT scan (muscular thickening); barium swallow (stenotic appearance of the cardia) | Incomplete form of achalasia | POEM | Good | |
| Yes | EUS; CT scan | Adenocarcinoma of the cardia | Chemotherapy | Poor | |
| Yes | EndoFLIP™ (reduced EGJ distensibility); EUS (muscular thickening) | Incomplete form of achalasia | POEM | Good | |
| Yes | Incomplete form of achalasia | Pneumatic dilation | No follow-up | ||
| No | CT scan | Breast cancer + gastric metastasis | No follow-up | ||
| No | EndoFLIP™, EUS (normal) | EGJ outflow obstruction | |||
| Diffuse esophageal spasm (two cases) | No | Diffuse esophageal spasm | Botox | Poor | |
| Yes | EUS (normal); barium swallow (normal) | Diffuse esophageal spasm | No follow-up | ||
| Absent contractility (three cases) | Yes | CT scan, EUS (periesophageal adenopathy); second HRM: achalasia | Incomplete form of achalasia | POEM | Good |
| Yes | Barium swallow (barium esophageal stasis) | Incomplete form of achalasia | Pneumatic dilation | No follow-up | |
| Yes | Incomplete form of achalasia | Heller myotomy | No follow-up | ||
| Ineffective esophageal motility (three cases) | Yes | Barium swallow (stenotic appearance of the cardia); second HRM: achalasia | Achalasia | POEM | Good |
| No | Barium swallow (barium esophageal stasis); second HRM: achalasia | Incomplete form of achalasia | POEM | No follow-up | |
| No | Barium swallow (chronic intrathoracic gastric volvulus) | Chronic intra-thoracic gastric volvulus | No follow-up | ||
| Normal (one case) | Yes | EUS | Adenocarcinoma of the cardia | Esophageal prosthesis | Poor |
Botox: botulinum toxin injection; CT: computed tomography; EGJ: esophagogastric junction; EUS: endoscopic ultrasonography; PEP: pan-esophageal pressurization; POEM: per-oral endoscopic myotomy; HRM: high-resolution manometry.
Discussion
To the best of our knowledge, this study is the first to investigate the yield of esophageal shortening during the RDT in a large population of patients referred for esophageal HRM. The occurrence of esophageal shortening during the RDT was mainly associated with impaired EGJ relaxation or major disorders of peristalsis according to the CC v3.0. It was also associated with pan-esophageal pressurization in most of the cases. When this manometric sign was associated with minor disorders of peristalsis, or with normal manometry, the diagnosis often evolved toward atypical forms of achalasia or neoplasia of the cardia after complementary workup. Finally, the presence of esophageal shortening was associated with more frequent and severe dysphagia.
The RDT is an emerging provocative test and is easy to perform. Previous studies described the potential role of this test to depict significant obstruction at the level of the EGJ and to differentiate patients with achalasia from those with minor motility disorders.21,23,24 Ang et al. defined impaired EGJ relaxation as an IRP during the RDT above 8 mmHg (≥12 mmHg for the diagnosis of achalasia) and/or presence of pan-esophageal pressurization above 30 mmHg. Using this definition, the RDT was considered pathological in 63% of patients with impaired EGJ relaxation, in 7% of major disorders of peristalsis and in 0% of minor disorders of peristalsis or normal manometry.23 Marin and Serra described three different patterns of pressure during the RDT and found that 17% of patients with esophageal symptoms but normal manometry had abnormal responses to the RDT.24 Most recently Marin et al. also described normal responses to the RDT and found only three cases of esophageal shortening >1 cm occurring after the RDT in a series of 90 healthy individuals.22 From our results, the presence of esophageal shortening during the RDT has an excellent positive predictive value (95%) for the diagnosis of a significant esophageal disorder, including some missed cases of esophageal adenocarcinomas. From this, we believe esophageal shortening occurring during or after the RDT should lead to a complementary workup, in the absence of typical and definite manometric signs of achalasia.
Pan-esophageal pressurization during the RDT was frequently associated with esophageal shortening, but the association of both signs did not improve much the diagnostic impact of esophageal shortening. A possible explanation for this association has been described in studies with simultaneous HRM and ultrasound imaging in patients with achalasia, in whom the pressurization of the esophagus was shown to be the result of longitudinal muscle contraction in the distal esophagus.11,30 This contraction causes axial shortening of the esophagus and reduction in the esophageal cross-sectional area. Since the esophagus is closed by the UES and LES, the reduction in esophageal volume leads to an increase in the pressure manifested as pan-esophageal pressurization. This motor pattern seems to be the major mechanism of esophageal emptying in achalasia. The same hypothesis was formulated by Tutuian et al. reporting a case of esophageal shortening and pan-esophageal pressurization in a patient with achalasia.12 The relatively high prevalence of esophageal shortening among patients with achalasia and its association with pan-esophageal pressurization also supports this hypothesis.
This study has several limitations. First, because of the retrospective design of the study, the protocol of complementary examinations was not standardized, and a significant number of patients were lost to follow-up after HRM. The complementary workup when performed was based on the experts’ opinions after the interpretation of HRM. Second, because of the very low prevalence of esophageal shortening among our HRM cohort, comparisons between specific esophageal motor disorders could not be performed. Different diagnoses were merged in larger groups, thus limiting the impact of our conclusions regarding the precise diagnostic yield of esophageal shortening during the RDT.
In conclusion, we demonstrated that esophageal shortening is rarely observed during the RDT. When present, this manometric sign is more likely associated with clinically significant esophageal motor disorders, in particular achalasia, and tends to be associated with more severe symptoms. Thus, the systematic performance of the RDT during HRM and reporting the occurrence of esophageal shortening might be of interest, especially in patients with dysphagia, in the absence of a definite manometric diagnosis of achalasia. We believe that in these patients, the occurrence of esophageal shortening during the RDT should prompt further complementary evaluations to rule out incomplete forms of achalasia. While esophageal shortening is probably a rare phenomenon in case of an infiltrative process of the cardia, the occurrence of such a phenomenon during the RDT should warn the investigator regarding the possibility of this concerning diagnosis.
Acknowledgments
Author contributions are as follows: D.B.: data analysis, manuscript drafting, and approval of final manuscript; S.R.: study concept and design, data acquisition, critical review of content, and approval of final manuscript; A.G.: data acquisition and approval of final manuscript; and F.M.: guarantor of article, study concept and design, data acquisition and analysis, manuscript drafting, and approval of final manuscript.
Declaration of conflicting interests
D.B. and A.G. have nothing to declare. S.R. has served as a consultant for Medtronic and Mayoly Spindler, and has received research support from Crospon and Sandhill Scientific. F.M. has served as a consultant for Medtronic, Laborie and Endostim.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
According to French law, this kind of retrospective analysis of data obtained during clinical evaluation of patients does not require approval by an ethics review board.
Informed consent
Patients were informed prior to HRM that their clinical data could be used for clinical research, after anonymization. They had the option of signing a document indicating their refusal to participate, in which case their files were not used for the study.
References
- 1.Dodds WJ, Stewart ET, Hodges D, et al. Movement of the feline esophagus associated with respiration and peristalsis. An evaluation using tantalum markers. J Clin Invest 1973; 52: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugarbaker DJ, Rattan S, Goyal RK. Swallowing induces sequential activation of esophageal longitudinal smooth muscle. Am J Physiol 1984; 247(5 Pt 1): G515–G519. [DOI] [PubMed] [Google Scholar]
- 3.Edmundowicz SA, Clouse RE. Shortening of the esophagus in response to swallowing. Am J Physiol 1991; 260(3 Pt 1): G512–G516. [DOI] [PubMed] [Google Scholar]
- 4.Kim HI, Hong SJ, Han JP, et al. Specific movement of esophagus during transient lower esophageal sphincter relaxation in gastroesophageal reflux disease. J Neurogastroenterol Motil 2013; 19: 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodds WJ, Dent J, Hogan WJ, et al. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med 1982; 307: 1547–1552. [DOI] [PubMed] [Google Scholar]
- 6.Pandolfino JE, Zhang QG, Ghosh SK, et al. Transient lower esophageal sphincter relaxations and reflux: Mechanistic analysis using concurrent fluoroscopy and high-resolution manometry. Gastroenterology 2006; 131: 1725–1733. [DOI] [PubMed] [Google Scholar]
- 7.Dogan I, Bhargava V, Liu J, et al. Axial stretch: A novel mechanism of the lower esophageal sphincter relaxation. Am J Physiol Gastrointest Liver Physiol 2007; 292: G329–G334. [DOI] [PubMed] [Google Scholar]
- 8.Pehlivanov N, Liu J, Mittal RK. Sustained esophageal contraction: A motor correlate of heartburn symptom. Am J Physiol Gastrointest Liver Physiol 2001; 281: G743–G751. [DOI] [PubMed] [Google Scholar]
- 9.Balaban DH, Yamamoto Y, Liu J, et al. Sustained esophageal contraction: A marker of esophageal chest pain identified by intraluminal ultrasonography. Gastroenterology 1999; 116: 29–37. [DOI] [PubMed] [Google Scholar]
- 10.Mittal RK, Liu J, Puckett JL, et al. Sensory and motor function of the esophagus: Lessons from ultrasound imaging. Gastroenterology 2005; 128: 487–497. [DOI] [PubMed] [Google Scholar]
- 11.Hong SJ, Bhargava V, Jiang Y, et al. A unique esophageal motor pattern that involves longitudinal muscles is responsible for emptying in achalasia esophagus. Gastroenterology 2010; 139: 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tutuian R, Pohl D, Castell DO, et al. Clearance mechanisms of the aperistaltic oesophagus: The “pump gun” hypothesis. Gut 2006; 55: 584–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahrilas PJ, Bredenoord AJ, Fox M, et al. Expert consensus document: Advances in the management of oesophageal motility disorders in the era of high-resolution manometry: A focus on achalasia syndromes. Nat Rev Gastroenterol Hepatol 2017; 14: 677–688. [DOI] [PubMed] [Google Scholar]
- 14.Mittal RK, Karstens A, Leslie E, et al. Ambulatory high-resolution manometry, lower esophageal sphincter lift and transient lower esophageal sphincter relaxation. Neurogastroenterol Motil 2012; 24: 40–46.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roman S, Huot L, Zerbib F, et al. High-resolution manometry improves the diagnosis of esophageal motility disorders in patients with dysphagia: A randomized multicenter study. Am J Gastroenterol 2016; 111: 372–380. [DOI] [PubMed] [Google Scholar]
- 16.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015; 27: 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel A, Cassell B, Sainani N, et al. Comparison of motor diagnoses by Chicago Classification versions 2.0 and 3.0 on esophageal high-resolution manometry. Neurogastroenterol Motil 2017; 29: e13042–e13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keren S, Argaman E, Golan M. Solid swallowing versus water swallowing: Manometric study of dysphagia. Dig Dis Sci 1992; 37: 603–608. [DOI] [PubMed] [Google Scholar]
- 19.Fornari F, Bravi I, Penagini R, et al. Multiple rapid swallowing: A complementary test during standard oesophageal manometry. Neurogastroenterol Motil 2009; 21: 718–718.e41. [DOI] [PubMed] [Google Scholar]
- 20.Savojardo D, Mangano M, Cantù P, et al. Multiple rapid swallowing in idiopathic achalasia: Evidence for patients’ heterogeneity. Neurogastroenterol Motil 2007; 19: 263–269. [DOI] [PubMed] [Google Scholar]
- 21.Ang D, Misselwitz B, Hollenstein M, et al. Diagnostic yield of high-resolution manometry with a solid test meal for clinically relevant, symptomatic oesophageal motility disorders: Serial diagnostic study. Lancet Gastroenterol Hepatol 2017; 2: 654–661. [DOI] [PubMed] [Google Scholar]
- 22.Marin I, Cisternas D, Abrao L, et al. Normal values of esophageal pressure responses to a rapid drink challenge test in healthy subjects: Results of a multicenter study. Neurogastroenterol Motil 2017; 29: e13021–e13021. [DOI] [PubMed] [Google Scholar]
- 23.Ang D, Hollenstein M, Misselwitz B, et al. Rapid drink challenge in high-resolution manometry: An adjunctive test for detection of esophageal motility disorders. Neurogastroenterol Motil 2017; 29: e12902–e12902. [DOI] [PubMed] [Google Scholar]
- 24.Marin I, Serra J. Patterns of esophageal pressure responses to a rapid drink challenge test in patients with esophageal motility disorders. Neurogastroenterol Motil 2016; 28: 543–553. [DOI] [PubMed] [Google Scholar]
- 25.Jones R, Junghard O, Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther 2009; 30: 1030–1038. [DOI] [PubMed] [Google Scholar]
- 26.Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology 1992; 103: 1732–1738. [DOI] [PubMed] [Google Scholar]
- 27.Ponds FA, Bredenoord AJ, Kessing BF, et al. Esophagogastric junction distensibility identifies achalasia subgroup with manometrically normal esophagogastric junction relaxation. Neurogastroenterol Motil 2017; 29: e12908–e12908. [DOI] [PubMed] [Google Scholar]
- 28.Wang YT, Yazaki E, Sifrim D. High-resolution manometry: Esophageal disorders not addressed by the “Chicago Classification”. J Neurogastroenterol Motil 2012; 18: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roman S, Holloway R, Keller J, et al. Validation of criteria for the definition of transient lower esophageal sphincter relaxations using high-resolution manometry. Neurogastroenterol Motil 2017; 29: e12920–e12920. [DOI] [PubMed] [Google Scholar]
- 30.Mittal RK. Longitudinal muscle of the esophagus: Its role in esophageal health and disease. Curr Opin Gastroenterol 2013; 29: 421–430. [DOI] [PubMed] [Google Scholar]