Abstract
Cytokines are important molecules that regulate the ontogeny and function of the immune system. They are small secreted proteins usually produced upon activation of cells of the immune system, including lymphocytes and myeloid cells. Many cytokines have been described, and several have been recognized as pivotal players in immune responses and in human disease. In fact, several anticytokine antibodies have proven effective therapeutics, especially in various autoimmune diseases. In the last 15 years, new cytokines have been described, and many remain poorly understood. Among the most recent cytokines discovered are interleukins-30 (IL-30) to IL-40. Several of these are members of other cytokine superfamilies, including several IL-1 superfamily members (IL-33, IL-36, IL-37, and IL-38) as well as several new members of the IL-12 family (IL-30, IL-35, and IL-39). The rest (IL-31, IL-32, IL-34, and IL-40) are encoded by genes that do not belong to any cytokine superfamily. Our aim of this review was to present a concise version of the information available on these novel cytokines to facilitate their understanding by members of the immunological community.
Keywords: : interleukins, cytokine receptors, inflammation
Introduction
Cytokines are small secreted proteins produced by cells of the immune system, usually upon immune cell activation. Cytokines regulate immune and inflammatory responses, and many of them play critical roles in the development of the immune system. The original cytokines included interleukin-1 (IL-1) and IL-2, which received their interleukin designation at the Second International Lymphokine Conference that was held in Interlaken, Switzerland in 1979. Since then, the number of interleukins has grown significantly. However, even though they are molecules of extreme importance in immunology, their numbers have made it difficult even for immunologists to keep pace with the discovery and characterization of these cytokines. Indeed, among them, there are some that have become “superstars” of immunology (eg, IL-17A), while others languish in relative obscurity (IL-11) as judged by the number of scientific papers documenting studies with these cytokines.
By the onset of the 21st century, the number of interleukins has reached IL-30 and kept on increasing. There are currently 40 ILs named. Consequently, the last 10 are among the ones that have relatively few studies reported on them. Part of the problem is that it is obviously difficult to take time to peruse the literature to obtain enough information on these newer interleukins. The purpose of this review is therefore to compile and present essential information on these important new interleukins to facilitate their understanding within the immunology community.
One important aspect we should discuss is the evolution of cytokines. In general, gene families formed through evolution from an original ancestral gene that duplicated and specialized, giving rise to a family of genes that encode structurally related proteins that may then develop specialized functions (Zlotnik and Yoshie 2012). The chemokines are an excellent example of this process, but it also applies to other cytokine families. Some examples include IL-2, which is evolutionarily related to IL-15 and to IL-21, while IL-4 and IL-13 are the results of gene duplication (indeed, their genes are still located adjacent to each other in the human genome). There are several genes of the IL-10 family, but some of the largest interleukin superfamilies include IL-1 and IL-12, with several members each. In fact, within the IL-30 to IL-40 cohort there are several IL-1 superfamily members (IL-33, IL-36, IL-37, and IL-38) as well as several new members of the IL-12 family (IL-30, IL-35, and IL-39). Moreover, the human genome contains an estimated 10% of genes encoding secreted proteins, so another prediction is that there remain new cytokines to be identified. Within the IL-30 to IL-40 cohort, these are represented by IL-31, IL-32, IL-34, and IL-40. The latter interleukins are generally more difficult to identify (because they do not share structural/genetic similarities to other cytokines), so they were generally identified through screening of genomic databases or other bioinformatics-based screening methods.
The growing use of gene arrays and other gene expression analysis methods has also helped expand the universe of interleukins. A certain interleukin may become “popular” through key studies published in the literature, but gene expression studies do not depend on the “popularity” of a particular cytokine. They will faithfully reflect the levels of expression of a particular transcript in a given cell/tissue independently of their popularity in the literature. We expect that many of the interleukins we discuss in this review will show up in gene array studies, which will help understand their functions and become better known. Personally, we found this exercise revealing, as it became clear that several of these molecules exhibit very interesting functions, which strongly suggest that they may be important potential targets for future therapeutics. In fact, anticytokine (or anticytokine receptor) antibodies already represent important therapeutics [antitumor necrosis factor-α (TNF-α), anti-IL-17A, and anti-IL-23].
Interestingly, the discovery of a new cytokine used to be a highly significant event in immunology. However, several of the novel cytokines described herein have not received significant attention when they were first reported. This is likely due to a phenomenon of “interleukin fatigue,” ie, a belief that cytokine biology is mostly a done deal and not an “up and coming” topic in immunology. This may be related to the fact that most cytokines were discovered by an older generation of immunologists, and cytokine discovery and characterization are not very popular topics among young investigators. Another problem is that even when a new cytokine is discovered, there are no reagents available. This situation underscores the difficulty of studying the biology of new interleukins. However, in spite of these difficulties, it is apparent that the discovery of these new interleukins will open many new fields of research based on their very interesting biology.
Interleukin-30
IL-30, represented by the p28 subunit of IL-27, is part of the IL-12 cytokine family. IL-27, discovered by Pflanz and others (2002), is a heterodimer composed of Epstein-Barr virus (EBV)-induced gene 3 (Ebi3) subunit noncovalently associated with the smaller p28 subunit (IL-27p28) (Pflanz and others 2002). IL-30, the p28 subunit of IL-27, remains functional even in the absence of Ebi3 (Crabe and others 2009; Shimozato and others 2009; Stumhofer and others 2010). IL-12 family cytokines have emerged as critical regulators of immunity, which regulate both proinflammatory (IL-12 and IL-23) and anti-inflammatory (IL-27, IL-35, and IL-39) immune cell responses through their influence on T and B cell fates. The IL-12 family is unique in having the only heterodimeric cytokines, and this promiscuous pairing of alpha and beta chains is speculated to contribute to the dual role of inflammatory and anti-inflammatory cytokines (Vignali and Kuchroo 2012).
IL-27 was initially thought to be a proinflammatory cytokine (Cox and others 2011), but is now considered an immunoregulatory cytokine (Stumhofer and Hunter 2008; Pot and others 2011; Wojno and Hunter 2012). IL-27 is secreted by activated antigen-presenting cells such as macrophages and dendritic cells (Pflanz and others 2002; Pflanz and others 2004). Treatment of murine and human monocytes with Toll-like receptor (TLR) agonists, such as lipopolysaccharide (LPS) or CpG oligodeoxynucleotides, and interferon-γ (IFN-γ) results in the production of IL-30. Once secreted, IL-27 binds to IL-27R alpha or T cell cytokine receptor signaling through WSX-1 and glycoprotein 130 (gp130) to induce signal transducer and activator of transcription 1 (STAT1) and STAT3 in monocytes (Pflanz and others 2004; Guzzo and others 2010). Recent studies have suggested that IL-30 may also signal through WSX-1 and gp130, but it may require a binding partner such as cytokine-like factor (CLF-1) or soluble IL-6 receptor (sIL-6Ra) (Crabe and others 2009; Stumhofer and others 2010; Garbers and others 2013).
More information is known about IL-27 than IL-30; however, they have been suggested to share overlapping roles. IL-27 has been shown to play an anti-inflammatory role in models of autoimmunity such as autoimmune encephalomyelitis and collagen-induced arthritis (Batten and others 2006; Niedbala and others 2008). IL-27 downregulates the immune response by inhibiting IL-2 and IL-17A expression, while promoting IL-10 production (Villarino and others 2003; Owaki and others 2006; Villarino and others 2006; Fitzgerald and others 2007; Stumhofer and others 2007). Additional studies have shown that IL-30 plays an anti-inflammatory role similar to IL-27. IL-30 is able to suppress IL-17A production, although at a much lower level than IL-27 (Stumhofer and others 2006).
Recent studies have identified IL-30 as a critical regulator of breast and prostate cancer metastasis (Airoldi and others 2016; Sorrentino and others 2018). IL-30 is also expressed in triple-negative HER2+ breast cancer tumors. In addition, expression of IL-30 was correlated with disease stage and reoccurrence. Mice injected with triple-negative HER2+ breast cancer cells treated with IL-30 displayed increased proliferation and vascular dissemination of breast cancer cells. Silencing of STAT1/3 signaling, downstream targets of IL-30, prevented the expression of CXCL1, CSF1, IL-1β, IL-6, IL-8, PTGS2/COX2, and Myc, which are known to promote tumor growth and metastasis (Airoldi and others 2016). Expression of IL-30 has also been identified in prostate cancer cells and cancer infiltrating leukocytes (Pflanz and others 2002). IL-30 expression is correlated with grade and stage of disease in prostate cancer patients (Di Meo and others 2014). Sorrentino and others (2018) found that IL-30 secreted by human and murine prostate cancer stem-like cells (PCSLCs) had significant autocrine and paracrine affects, which supported PCSLC viability, self-renewal, and tumorgenicity. Therefore, they concluded that IL-30 could be a potential target for development of therapeutic monoclonal antibodies for cancer treatment.
Interleukin-31
IL-31 was reported in 2004 by Dillon and others (2004). It is a member of the IL-6 family of cytokines, a 4-helix bundle cytokine, which is produced by Th2 cells upon activation. It signals through a receptor composed of 2 chains, an IL-31 receptor A and oncostatin M receptor (OSMRbeta). The receptor is expressed constitutively in epithelial cells but is induced upon activation in monocytes. IL-31 seems to mediate pruritus, as this is an evident phenotype in IL-31 transgenic mice, which also develops alopecia and skin lesions. These observations strongly suggest that IL-31 is involved in dermatitis present in allergic diseases.
Sonkoly and others (2006) showed that IL-31 is overexpressed in pruritic atopic dermatitis skin. Highest levels were observed in prurigo nodularis, one of the most pruritic forms of chronic skin inflammation. In vivo, staphylococcal enterotoxin B (SEB) (a superantigen targeting Vβ8) induced IL-31 expression in atopic individuals, and in vitro SEB induced IL-31 production in leukocytes. IL-31 receptor A showed the most abundant expression in dorsal root ganglia, the site where the cell bodies of cutaneous sensory neurons reside. Cells expressing IL-31RA mRNA were detected in the dorsal root and trigeminal ganglia, and these cells also expressed OSMRbeta, but the expression of these receptor chains appears at different times during development (Bando and others 2006).
Expression of IL-31 correlates with the enhanced expression of IL-4 and IL-13 (2 other Th2 cytokines) in atopic dermatitis and atopic contact dermatitis (Neis and others 2006). However, IL-31 mRNA was not increased in psoriasis. These observations strongly suggested that IL-31 is associated with itching; however, the situation may be more complex since other studies have found IL-31 levels strongly increased in atopic dermatitis but they do not correlate with the intensity of the disease according to the SCORAD severity score (Ozceker and others 2018). However, support for the concept that IL-31 is a pivotal cytokine regulating pruritus comes from studies on contact dermatitis with Il31−/− mice, which exhibit reduced scratching frequency and duration during hapten-induced contact dermatitis (Takamori and others 2018). Eosinophils can also be potent sources of IL-31 (Rudrich and others 2018), and anti-IL-31 antibodies ameliorate scratching behavior in a mouse model of atopic dermatitis (Grimstad and others 2009).
Interleukin-32
Kim and others (2005) reported the identification of a novel cytokine produced by a cell line (human lung carcinoma A549) transfected with an IL-18 receptor β chain. This rendered the cell responsive to IL-18, and it produced several cytokines, including a novel one called IL-32. It is expressed by human peripheral blood mononuclear cells (PBMCs) upon activation, induced in human epithelial cells by IFN-γ, and in natural killer (NK) cells by IL-12 plus IL-18. In fact, it was originally called NK transcript 4. Its expression pattern mirrors other inflammatory cytokines, so it is not surprising that it is also capable of inducing the production of other cytokines such as TNF-α, IL-1β, IL-6, and various CXC chemokines in various cells. There are various isoforms of IL-32, but the gamma isoform has been reported to be the most active (Straub and Feuer 1989). IL-32 signals through p38 mitogen-activated protein kinase (MAPK) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways, and has been implicated in inflammatory disorders, inflammatory bowel disease (IBD), and influenza virus A infections. It has also been associated with several autoimmune diseases such as rheumatoid arthritis (RA), ulcerative colitis, and Crohn's disease (Felaco and others 2009). However, the specific role of this proinflammatory cytokine in these diseases is not yet known. It has also been implicated in the latent stage of tuberculosis (Montoya and others 2014).
IL-32 is likely to have both intracellular and extracellular functions (Heinhuis and others 2012). It could be involved in resistance against intracellular pathogens (Dos Santos and others 2018). Intracellular IL-32 may be involved in the regulation of cell activation and cell death. IL-32 also exhibits angiogenic properties (Nold-Petry and others 2014).
IL-32 has also been implicated in cancer progression. For example, IL-32 promotes the production of IL-2 by bone marrow (BM) cells, which may in turn promote the proliferation of multiple myeloma cells (Lin and others 2017). IL-32 expression has been associated with hypoxia in multiple myeloma cells, and high expression is associated with poor survival and bone loss (Zahoor and others 2017).
Given that there are various isoforms of IL-32, it represents a situation where each isoform may have different expression patterns, properties, and functions. We can expect more future reports where specific isoforms of IL-32 will be functionally characterized.
Interleukin-33
IL-33 is a member of the IL-1 superfamily. IL-33 induces Th2 responses in T cells, mast cells, basophils, and eosinophils. Previously known as nuclear factor in high endothelial venules (NF-HEV), it was discovered in high endothelial cells. Baekkevold and others (2003) discovered IL-33 by using molecular and bioinformatic approaches to study genes preferentially expressed by HEV cells, although it was found to be also expressed in human lymphoid organs [tonsils, lymph nodes, and Peyer's patches (PPs)] and in single cells of the T cell zones of lymph nodes. Nevertheless, its function was uncertain, and it was pointed out as a possible transcription factor in charge of the regulation of expression after cytokine signaling in HEV cells.
In 2005, researchers at Merck Research Labs showed that IL-33 signals through a ST2 receptor, a previously orphan IL-1 receptor family member, which is expressed in human and murine Th2 cells, mast cells, eosinophils, and basophils, and it is involved in Th2 responses (Coyle and others 1999; Schmitz and others 2005). Using a pull-down method with biotinylated IL-33 to capture the ST2 receptor and a GFP-NF-κB reporter-transfected cell line, Shmidtz and others (2005) proved that IL-33 was binding and signaling through ST2. Naturally, stimulation with IL-33 drives the expression of Th2 cytokines, IL-4, IL-5, and IL-13, and leads to an increase in the secretion of IgE, IgA and eosinophilia in vivo (Schmitz and others 2005; Yagami and others 2010). The connection of IL-33 with asthma and other Th2-related diseases was immediately drawn.
Using microarrays, the group that originally reported IL-33 described that it is constitutively expressed in lymphoid tissues, epithelial cells, fibroblasts, mucosal tissues, tumor cells, and vascular tissues where its expression is particularly high (Moussion and others 2008). The same group previously demonstrated that IL-33 has transcriptional regulation properties, and that it can be located in the nucleus of HEVs cells, drawing a parallel to IL-1α (Schmitz and others 2005). IL-1α is another member of the IL-1 family that shares structural and functional characteristics with IL-33. Both have 100 amino acid residues at the N-terminus and are present in high concentrations as precursors in the intracellular compartment of expressing cells, due to the lack of signal peptide (Kim and others 2013). The maturation and activity of IL-1 family members IL-1α and IL-1β start after the release from intracellular compartments mediated by mechanical or infection-related cell death, effectively making them representatives of molecules known as alarmins (Bianchi 2007). Alarmins are the endogenous equivalents of pathogen-associated molecular patterns (PAMPs) and are released during nonprogrammed cell death. Once released, alarmins can signal immune system cells such as macrophages and dendritic cells to initiate a cellular immune response to resolve inflammation (Bianchi 2007). Given that IL-33 is constitutively expressed in healthy barrier tissues, its similarities with other members of the IL-1 family of alarmins led the Girard group to conclude that IL-33 is an alarmin.
Asthma leads to chronic inflammation and persistent airway remodeling in the lungs (Busse and others 1999). IL-33 is upregulated in bronchoalveolar lavage fluid from asthma patients, and a single nucleotide polymorphism in ST2 is strongly associated with asthma in different human populations (Gudbjartsson and others 2009; Prefontaine and others 2010). Yagami and others (2010) showed that IL-33 acts directly in lung endothelial and epithelial cells but not in fibroblast or muscle cells by inducing the expression of CXCL8, having a direct effect in controlling neutrophil recruitment to the lungs, and thus regulating inflammation in the lungs. Furthermore, in vitro stimulation of human microvascular endothelial cells from lung blood vessels with IL-33 led to an increase of IL-6 and CCL2 secretion (Yagami and others 2010). From the analysis of these data, we can conclude that IL-33 plays a major role in lung inflammation, and that the IL-33/SP2 signaling axis is important in the pathogenesis of asthma and allergies.
Recently, IL-33 has also been implicated in the regulation of immune responses in the gut. It has been reported that IL-33 is upregulated in patients with IBD (Kobori and others 2010; Pastorelli and others 2010; Seidelin and others 2010). Colonic T regulatory cells (Tregs) and group 2 innate lymphoid cells (ILC2s) are known to respond to IL-33 in the gut (reviewed in Cayrol and Girard 2018). Furthermore, IL-33 has been shown to be a key cytokine in the maturation, proliferation, and function of colonic Tregs, with an antagonistic activity to IL-23, a key inflammatory molecule of the gut (Schiering and others 2014; Cayrol and Girard 2018). From the research done in the last 15 years, we can conclude that IL-33 has emerged as a key regulatory cytokine in barrier tissues, making it an important target for inhibition therapy in autoimmune diseases such as IBD, RA (Verri and others 2010), asthma, and allergies.
We should note that a recent study on the ancestral origins of the IL-1 family concluded that IL-33 and IL-18 have poor sequence similarity and no chromosomal evidence of common ancestry with the IL-1β cluster, and therefore should not be included in the IL-1 ligand ancestral family (Rivers-Auty and others 2018). This observation has important functional implications. IL-33 and IL-18 therefore represent genes derived from an ancestral gene that was also the ancestor of the IL-1β cluster. However, since this split occurred a long time (millions of years) ago, each of these genes (IL-1β cluster, IL-18, and IL-33) has had a long time to evolve independently ever since. For this reason, we can predict that each of these genes should exhibit very different functional properties. In contrast, other IL-1 family members derived directly from the IL-1β cluster in more recent evolutionary times should exhibit similar functions to other members of the IL-1β family cluster.
Interleukin-34
IL-34 is a cytokine that also binds the macrophage colony stimulating factor-1 receptor (M-CSF-1). Its discovery was a “tour de force.” Lin and others (2008) performed a functional screening of the extracellular proteome. This was represented by a comprehensive set of recombinant secreted proteins and the extracellular domains of transmembrane proteins. Each protein was tested in several functional assays. One of the secreted proteins, designated IL-34, stimulated monocyte viability. They also reported that IL-34 binds CSF-1. However, it quickly became apparent that IL-34 and M-CSF did not exhibit identical biological activities. They differ in their capacity to induce the production of chemokines such as CCL2 and CCL24 in primary macrophages. Interestingly, IL-34 and M-CSF do not share sequence homology, but some antibodies against CSF-1 can block the binding of 1 ligand but not another. This suggests that these ligands bind different parts of CSF-1, suggesting that they can mediate different bioactivities and signal transduction pathways.
Both M-CSF and IL-34 mediate osteoclastogenesis (Chen and others 2011) in combination with RANKL. This suggests their potential therapeutic role in osteoporosis.
IL-34 is produced by keratinocytes in the skin and neurons in the brain. IL34−/− mice displayed a marked reduction of Langerhans cells and microglia, whereas monocytes, dermal and lymphoid tissue macrophages were unaffected. Langerhans cell survival is dependent on IL-34, whereas microglia and their yolk sac precursors develop independently of IL-34 but rely on it for their maintenance in the adult brain (Greter and others 2012).
There have been other intriguing studies that have implicated IL-34. For example, Bezie and others (2015) reported that IL-34 is specifically produced by T regulatory cells and mediates transplant tolerance. In a rat cardiac allograft model, treatment of rats with IL-34 promoted allograft tolerance that was mediated by induction of Tregs. Treatment of human macrophages with IL-34 greatly expanded CD8+ and CD4+ FoxP3+ Tregs, cells that exhibited greater suppressive potential than non-IL-34 expanded Tregs.
In addition, IL-34 is known to influence the phenotype of myeloid lineage cells that express CSF-1R. For example, it has been reported to inhibit acute rejection of rat liver transplantation by inducing the polarization of Kupffer cells to the M2 phenotype (Zhao and others 2018). Similarly, it has been reported to be expressed at the fetal–maternal interface and to induce immunoregulatory macrophages of a decidual phenotype (Lindau and others 2018).
IL-34 levels in serum have been reported in various diseases, including systemic lupus erythematosus (SLE) (Xie and others 2018), periodontal disease (Guruprasad and Pradeep 2018), patients experiencing acute rejection after liver transplantation (San Segundo and others 2016), development of liver fibrosis in patients with Hepatitis B (Wang and others 2018) and RA (Zhou and others 2016). The potential role of IL-34 in these diseases remains to be clarified.
Finally, IL-34 may play a role in both cancer and fibrosis. High coexpression of IL-34 and M-CSF correlates with tumor progression and poor survival in lung cancer (Baghdadi and others 2018). It also has been implicated in colon cancer (Franze and others 2018), and it may play a role in the functional polarization of tumor-associated macrophages (Jeannin and others 2018). It has been reported to promote fibrocyte proliferation, suggesting that it is a profibrotic factor (Galligan and Fish 2017).
We conclude that IL-34 has strong potential to be a target for development of therapeutic antibodies.
Interleukin-35
IL-35 was named a little over a decade ago in 2017 independently by 2 laboratories, Niedbala and others (2007) and Collision and others (2007). However, it was first described in 1997 but not named until 2017 (Devergne and others 1997; Collison and others 2007). IL-35 has gained much interest for its role in strongly inhibiting immune cell function, with prospects in development of therapeutics for autoimmune disease. As part of the IL-12 family, IL-35 consists of a heterodimeric glycoprotein formed by disulfide-linked IL-12alpha chain (p35) and IL-27 b chain EBV-induced gene 3 (Ebi3) (Tedder and Leonard 2014). Collision and others (2007) originally reported that IL-35 was produced by populations of thymus-derived natural regulatory T cells (nTregs) and was required for their maximal suppressive activity. Regulatory B cells (Bregs) have also been reported to produce anti-inflammatory cytokines, including IL-10 and IL-35 (Pusic and others 2014; Shen and others 2014). Like other IL-12 cytokine family members, IL-35R binds to activate STAT proteins (Collison and others 2012). Binding of IL-35 to IL-35R activates STAT1 and STAT4 in T cells (Pusic and others 2014). However, in B cells IL-35 signaling mediates STAT1 and STAT3 (Shen and others 2014).
IL-35 has gained much interest for its suppressive role. Ebi3 is a downstream target of FoxP3, restricting its expression to the Treg lineage. IL-35 has been shown to prevent Th1 and Th17 proliferation by arresting T cells in the G1 phase of cell division (Wirtz and others 2011). Although IL-35 is able to block T cell proliferation, it does not promote the conversion of Th1 cells to Tregs as IFN-γ is a strong negative regulator of Ebi3 and P35 transcription (Vignali and Kuchroo 2012). In Th2 cells, IL-35 blocks Th2 differentiation by repressing GATA3 and IL-4 expression. In addition, IL-35, like transforming growth factor-beta (TGF-β) and IL-10, can induce regulatory T cells (iTregs). Recent studies have focused on the role of IL-35 in autoimmunity. Numerous studies have demonstrated that IL-35 plays a protective role in mouse models of human autoimmune disease such as experimental autoimmune encephalomyelitis, experimental autoimmune uveitis, encephalitis, allergic airway model, diabetes, and colitis (Collison and others 2007; Canda-Sanchez and others 2009; Huang and others 2011; Bettini and others 2012; Wang and others 2014).
Recently, Bregs have also been identified as potent producers of IL-35 (Yanaba and others 2008). Bregs are believed to mediate their immunosuppressive capabilities through the secretion of IL-10 upon stimulation of TLR agonists CD40 L and IL-21 (Yoshizaki and others 2012). Recent reports have suggested that IL-35, in addition to IL-10, contributes to Breg suppressive function. Bregs have been shown to be capable of producing IL-35 (Shen and others 2014; Wang and others 2014). Addition of rIL-35 results in selective proliferation of CD19+CD5+B220lo, which display enhanced secretion of IL-10 and IL-35. Similarly, rIL-35 inhibits B220hi B cell proliferation (Wang and others 2014). IL-35 produced by Bregs acts in an autocrine manner to stimulate the IL-35R on Bregs and also in a paracrine fashion to promote the generation of induced Tregs (Wilson and others 2010).
Alternatively, recent studies have suggested that IL-35 plays a protective role in tumor immune escape. Ebi3 overexpression has been reported in Hodgkin's lymphoma (Niedobitek and others 2002) and lung cancer cells (Nishino and others 2011). In addition, tumors expressing IL-35 have an increase in myeloid-derived suppressor cells, which are known to be immune suppressive and inhibit cytotoxic T cell responses (Wang and others 2013).
While IL-35 may be an intriguing cytokine to treat autoimmune diseases, recombinant IL-35 is difficult to obtain, thus limiting immunology research and its potential production as a therapeutic. IL-35 is not secreted as a disulfide-linked heterodimer, therefore only ∼4% of secreted Ebi3 is coprecipitated with IL-12p35 (Devergne and others 1997). Although approaches to generate rIL-35 in mouse models and attempts to covalently link Ebi3 and IL-12p35 have been made, production remains inefficient (Collison and others 2007; Aparicio-Siegmund and others 2014; Shen and others 2014).
While the immune inhibitory role of IL-35 has been well characterized in mouse models, the immunosuppressive effects of IL-35 in humans are modest (Bardel and others 2008). However, Seyeri and others (2010) reported that human Tregs produced IL-35 under strong stimulation. Thus, the role of IL-35 in human Tregs still warrants future investigation.
Interleukin-36
IL-36 is a very interesting group of cytokines, derived from a member of the IL-1 family. In humans, it exists as 4 distinct secreted proteins, previously orphan IL-1 family ligands; IL-36α, IL-36β, IL-36γ, and the receptor antagonist IL-36ra. All of them require post-transcriptional processing to be functional and bind the IL-36 receptor (IL-36R) (Towne and others 2011). Due to the proinflammatory nature of all the IL-36 members and the similarity of the responses they elicit signaling through the IL-36R, they were designated under the IL-36 name in 2010 (Dinarello and others 2010).
The first member of the IL-36 cytokine group termed IL-1HY1 (now IL-36ra) was discovered in 1999 by Mulero and collaborators, using a cDNA screening derived from human liver and spleen. The cDNA caught the authors' attention due to the relatively high homology (65%) to IL-1ra (Mulero and others 1999). The next year, 4 different groups would publish the discovery of new members of the IL-1 family, (Barton and others 2000; Busfield and others 2000; Kumar and others 2000; Smith and others 2000). The function of these cytokines would not become apparent until their receptor was identified, IL-36R, formerly known as IL-1 receptor-related protein 2 (IL-1rp2). Debets and collaborators reported that IL-36α elicited NF-κB signaling through IL-1Rrp2 and was strongly inhibited by IL-36ra (Debets and others 2001). Interestingly, they also found high expression of IL-36α, IL-36ra, and IL-36R in skin and a significant upregulation in lesional psoriasis. It was later found that all the proposed members of the IL-36 cytokine subfamily signal through IL-36R and activate the NF-κB, MAPKs, JNK, and ERK1/2 kinase cascade, which leads to CXCL8, TNF-α, IL-17A, IL-23, and IL-6 secretion. Interestingly, IL-36ra functions as a direct antagonist of the other IL-36 members (Towne and others 2004; Blumberg and others 2010; Towne and others 2011).
IL-36R is mainly expressed in T cells and is especially predominant in naïve CD4 T cells (Vigne and others 2012; Walsh and Fallon 2018). Stimulation with IL-36 induced naïve T cell proliferation and enhanced IL-2 secretion. Furthermore, IL-36β along with IL-12 promotes Th1 polarization and plays an important role against Mycobacterium infections (Vigne and others 2012). IL-36 cytokines are mainly expressed in skin keratinocytes, myeloid cells, Langerhans cells, and mucosal epithelium (Gresnigt and van de Veerdonk 2013; Gabay and Towne 2015; Walsh and Fallon 2018). This expression pattern and the responding cells indicate that the IL-36 cytokines are likely very important in skin homeostasis and defense. As such, studies with an IL36α−/− mouse showed that IL-36A (but not IL-36β or IL-36γ) is required to induce and maintain psoriasis-like disease in a mouse model of psoriasis (Milora and others 2015). Interestingly, the same group found that IL-36α acts in a self-amplifying loop with IL-1α, which is also required to maintain skin inflammation during psoriatic lesion flares (Milora and others 2015).
Ongoing clinical studies are evaluating the potential efficacy of IL-36R blockage using a therapeutic monoclonal antibody in psoriasis. A hint of the potential use of an IL-36R blocking monoclonal antibody came from an unlikely source, a rare human disease caused by a loss of function mutation on the IL36RA gene, termed deficiency of IL-36 receptor antagonist (DITRA) syndrome (Marrakchi and others 2011). Patients with DITRA syndrome develop generalized pustular psoriasis, and some have been successfully treated with anakinra, a recombinant human IL-1ra originally developed for the treatment of RA (Rossi-Semerano and others 2013; Ganesan and others 2017; Molho-Pessach and others 2017). There is no certainty that the administration of IL-36R blocking antibody will help resolve skin inflammation in autoimmunity, but the concept is supported by a wide body of research that suggests that it will be effective in these indications. In fact, there are currently at least 3 active clinical trials testing the efficacy of a humanized anti-IL-36R antibody (Tsai and Tsai 2017).
In recent years, it has been observed that IL-36 family members, especially IL-36α, are upregulated during IBD pathogenesis (Russell and others 2016). Walsh and collaborators found that the expression of IL-36α is upregulated in human patients with ulcerative colitis and mouse models of sterile inflammation. The inflammation was significantly reduced in a dextran sulfate colitis model using IL-36r−/− mice. Furthermore, other studies indicate that IL-36 cytokines are also necessary for effective tissue repair in the gut (Medina-Contreras and others 2016; Scheibe and others 2017). The studies on the role of IL-36 family in IBD further improve the case for the development of IL-36R blocking therapies, and highlight the need for further research on the role of IL-36 cytokines in the homeostasis of the gut.
Interleukin-37
Another member of the IL-1 cytokine family, originally known as IL-1 F7, shares structural similarities with IL-18 and can bind to the IL-18 receptor (IL-18Rα) although at much lower affinity (Dunn and others 2001; Nold and others 2010). It has 5 isoforms (IL-37a, b, c, d, and e), and each isoform expression is tissue specific (Boraschi and others 2011; Jia and others 2018). IL-37 was first described as an IL-1 family member (which was identified using the same approach that led to the identification of other IL-1 family members) through bioinformatics analyses of the human genome for uncharacterized genes that share similarity and/or share chromosomal location with other known IL-1 family members. In fact, IL-37 was described simultaneously with the previously discussed IL-36 cytokines by 3 different groups using similar approaches (Busfield and others 2000; Smith and others 2000; Dunn and others 2001). Dunn and collaborators reported that IL-37 could bind to IL-18R suggesting a function similar or parallel to IL-18 (Dunn and others 2001). IL-37 is expressed in various tissues, but its expression is particularly high in thymus, testis, and uterus as well as macrophages, dendritic cells (DCs), tonsillar B cells, and plasma cells (Pan and others 2001; Cavalli and Dinarello 2018). IL-37 is also expressed in gut and skin epithelial cancers (Cavalli and Dinarello 2018).
Remarkably, IL-37 is an anti-inflammatory cytokine. It was until 2010, almost 10 years after the first report by Nold and collaborators who found that the expression of IL-37 by epithelium and macrophages suppressed almost completely the expression of several proinflammatory cytokines (mainly IL-1β, IL-6, and TNF-α) and chemokines (CCL2, CXCL2, and CXCL8) in vitro, and protected mice against sepsis induced by LPS in vivo (Nold and others 2010). They also found that IL-37 forms a complex with SMAD3, an important transcription factor in the TGF-β pathway (Takimoto and others 2010), and is necessary for IL-37-driven responses. Taken together, these observations indicated that IL-37 was likely to be a novel anti-inflammatory cytokine.
Most of the effects of IL-37 in metabolic, autoimmune, and infectious diseases have been tested in several metabolic, autoimmune, and infectious models using a transgenic mouse that overexpresses human IL-37 (IL-37-tg) (Nold and others 2010). In these experiments, IL-37 has proven to have a protective effect on tissue damage and mediates a reduction in the secretion of proinflammatory cytokines in several models, including colitis, RA, allergy, and fungal pulmonary infections (McNamee and others 2011; Moretti and others 2014; Lunding and others 2015; Ye and others 2015; Cavalli and Dinarello 2018). In addition, IL-37 appears to play a role in the regulation of fat metabolism, aortic disease, and aging (Siffring and others 1989; Ballak and others 2014; Henry and others 2015). Examination of the research done with IL-37 in mouse models of metabolic, autoimmune, and infectious diseases indicates that IL-37 is important in homeostasis and as an immune regulator. While involvement of IL-37 has also been reported in human disease (Imaeda and others 2013; Hojen and others 2015; Xia and others 2015; Yin and others 2017), there are currently no human clinical trials evaluating the effects of exogenous administration on humans. IL-37 seems as a potential candidate for supplementation therapy in conjunction with blocking therapy (the most likely candidates would be monoclonal antibodies).
Interleukin-38
IL-38 was another IL-1 family protein discovered in the early 2000s along with IL-36 and IL-37. The IL-38 gene was identified and termed IL-1HY2 by Lin and collaborators in 2001. They reported that IL-1HY2 shares sequence 52% and structural similarity to IL-1ra, and also significant similarity to IL-36ra (Lin and others 2001; van de Veerdonk and others 2018). The IL38 gene is located in chromosome 2, along with other members of the IL-1 family (Bensen and others 2001), indicating that it is part of recent evolutionary gene duplication. IL-38 is expressed in skin, fetal liver, placenta, brain, thymus, and tonsils (Bensen and others 2001; Lin and others 2001; van de Veerdonk and others 2018). Remarkably, years later van de Veerdonk and collaborators reported that IL-38 is an accessory IL-36 receptor antagonist that binds to the IL-36 receptor to decrease secretion of IL-22 and IL-17 after stimulation with heat-killed Candida albicans in memory T cells. It also reduced the production of CXCL8 by PBMCs induced by LPS, similar to the canonical IL-36ra (van de Veerdonk and others 2012). The confirmation of IL-38 binding to IL-36R came by using immobilized extracellular domains of the IL-1 family receptors to screen receptor binding and IL-38 and IL-36ra bound to the extracellular domain of IL-36 (van de Veerdonk and others 2012). In addition, PBMCs treated with IL-38 siRNA produced up to 28-fold more proinflammatory cytokines (including IL-36, CCL2, and APRIL), suggesting that IL-38 exhibits an antagonist function (Rudloff and others 2015).
Several genome-wide association study (GWAS) studies have associated IL-38 with human diseases where other IL-1 family cluster alleles have been implicated, including ankylosing spondylitis, RA, and cardiovascular disease (Chou and others 2006; Rahman and others 2006; Jung and others 2010; Lopez-Mejias and others 2016). IL-38 has been reported to be increased in patients with SLE, and its levels directly correlate with an increased risk of renal lupus and central nervous system lupus (Rudloff and others 2015). It has also been found that IL-38 is increased in patients with hidradenitis suppurativa, Hepatitis B, gestational diabetes mellitus, asthma, lung fibrosis, and lung adenocarcinoma (Chu and others 2016; Wang and others 2016a; Takada and others 2017; Tominaga and others 2017; Yu and others 2017; Hessam and others 2018).
Interestingly, in Hepatitis B, IL-38 expression appears to be a marker of good prognosis (patients with high levels of IL-38 were more likely to respond to therapy). In the case of asthma and cancer, it appears to be the opposite; in asthma, it correlated with lower numbers of circulating Tregs, and in cancer it was associated with higher expression of PD-L1 by the tumors (Chu and others 2016; Wang and others 2016a; Takada and others 2017). The function and role of IL-38 have not been extensively explored, and it is one of the IL-1 family members for which less information is available. However, the data available strongly suggest that it is an attractive candidate to be a potential target for human diseases where the IL-36/IL-36R axis has been implicated.
Interleukin-39
In 2016, Wang and others (2016c) described the most recently discovered member of the IL-12 cytokine family, IL-39. IL-39 is a heterodimer composed of the IL-23p19 alpha subunit and Ebi3 beta subunit. IL-39 was found to be secreted as a heterodimer by activated B cells, similar to IL-35. After LPS stimulation, IL-39 was significantly upregulated in B cells. In addition, p19 and Ebi3 mRNA was found to be expressed by DCs and macrophages (Wang and others 2016c). Similarly to IL-35, IL-39 has been shown to mediate inflammatory responses through activation of STAT1/STAT3 (Floss and others 2017).
SLE is an autoimmune disease in which B cells increase the production of autoantibodies (Oku and Atsumi 2018). Wang and others found that activated B cells in lupus-prone mice secreted IL-39. In addition, administration of IL-39 in vitro and in vivo induced the differentiation and/or expansion of neutrophils, which contribute to disease (Wang and others 2016b, 2016c). Knockdown of p19 or Ebi3 reduced lupus severity in mice. Therefore, IL-39 may be used as a potential target for treating SLE.
Interleukin-40
IL-40 is the last cytokine to be discovered. We reported it in October 2017 (Catalan-Dibene and others 2017). The discovery of IL-40 followed a different approach than other cytokines in this review. The gene encoding IL-40 is annotated in the human genome as C17orf99, and it is mainly expressed by fetal liver, BM, and activated B cells, and encodes a small secreted protein (27 kDa) of 265 amino acids, including a 20-amino-acid signal peptide. We became interested in this gene while screening the body index of gene expression (BIGE) database (Roth and others 2006) for uncharacterized genes related to the immune system. C17orf99 was identified as a gene of interest because of its expression pattern (obtained from the BIGE database) and the presence of a signal peptide. Remarkably, it is not structurally related to any other cytokine family, indicating that it likely has unique evolutionary history. An estimated 10% of the human genome encodes secreted proteins, and C17orf99 had been identified as a potential gene of interest on a wide screen of uncharacterized secreted genes (Clark and others 2003). Furthermore, bioinformatics analyses revealed that gene orthologs are only present in mammals, leading us to hypothesize that the function of C17orf99 should be related to a mammalian-specific immune function. That hypothesis proved correct when we measured immunoglobulin (Ig) levels in the milk of lactating Il40−/− mice. There was a significant decrease in the concentration of IgA in the milk of Il40−/− mice, and we also found that IL-40 is normally expressed in the mammary gland before the onset of IgA production. In fact, we found a general IgA deficiency in Il40−/− mice (not only in the mammary gland). Interestingly, the gut of these mice is affected by the lack of IL-40, since we found a lower number (and smaller size) of PPs, which could be explained by a significant decrease in the number of IgA+ B cells in the PPs as well as a dysregulated microbiota.
The IgA deficiency indicated that the function of IL-40 is important for normal B cell function in the periphery. Thus, we decided to explore a possible function of IL-40 in the BM, where B cells originate. We hypothesized that B cell development in the BM is impaired in Il40−/− mice. We first observed that mice have reduced numbers of mature B cells as well as Pre-B cells in the BM. Furthermore, the cells producing IL-40 in the BM are stromal cells, probably a subtype involved in lymphopoiesis. Following these results, we wanted to know whether B cells in the periphery were also affected in the Il40−/− mice. In fact, all B cell populations (transitional, follicular, and marginal zone) in the spleen were significantly reduced when compared with WT mice, confirming that IL-40 plays a role in B cell maturation in the BM and in the periphery.
We also analyzed the expression of IL-40 by activated B cells. We found that IL-40 can be produced by spleen B cells upon activation with anti-IgM, anti-CD-40, and IL-4, but that their ability to produce IL-40 increases significantly if the B cells are polarized in vitro with TGF-β. Therefore, TGF-β is necessary to have increased expression of IL-40 by activated B cells, and is also necessary for IgA class switching in naïve B cells (Sonoda and others 2009). These observations suggest that IL-40 is likely present during IgA responses, although we were unable to demonstrate the role of IL-40 in class switching. In addition, we observed that human B cell lymphoma cell lines (OCI-Ly1) constitutively express IL-40, suggesting a potential role of IL-40 in human B cell-associated diseases.
In recent years, the importance of B cells in autoimmunity has become evident. Rituximab (an antibody against CD20, a marker of human B cells) has been approved for treatment of several autoimmune diseases (Musette and Bouaziz 2018). Recently, another anti-CD20 antibody has been approved for multiple sclerosis (Greenfield and Hauser 2018). These developments point to a pivotal role of B cells in autoimmunity. Given that the effectiveness of these treatments depends on their ability to eliminate B cells and not plasma cells (since the latter do not express CD20), their mechanism of action does not depend on the elimination of autoimmune antibodies, but rather on the elimination of effector functions directly mediated by B cells. The most likely effector function therefore would be cytokine production. From this perspective, the identification of a novel B cell-associated cytokine is a very interesting development (Luu and others 2014). We are confident that future studies will reveal very interesting biology associated with IL-40 in human disease.
Conclusion
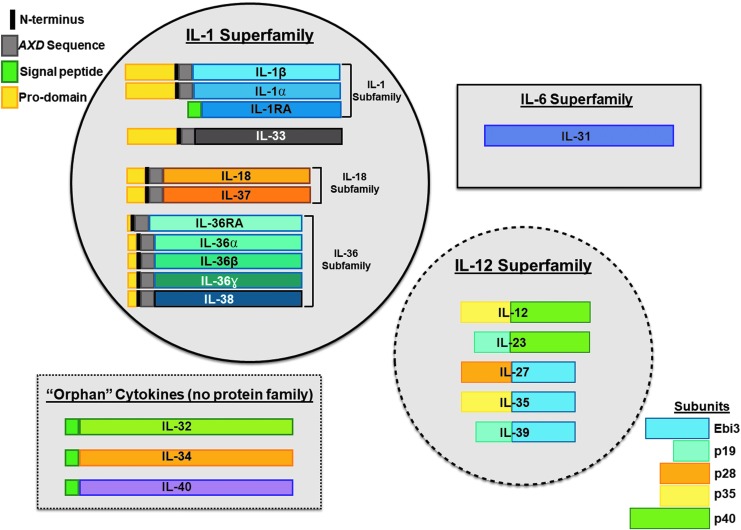
We have reviewed the current state of research of 10 of the latest cytokines described to date (summarized in Table 1 and Fig. 1). It is apparent from the information already available on these proteins that their potential functional role(s) in both innate and acquired immune responses and their potential role in human disease are vast. Still, it is apparent that the research focusing on these novel cytokines is still in early stages. As an example, a literature search on publications mentioning IL-10 in the US National Library of Medicine of the National Institutes of Health (pubmed.gov) yields 56,241 publications, while a similar search for IL-35, one of the most “popular” new cytokines, yields only 409 results. This suggests that there are many novel fields of research to be defined based on the physiology of these novel cytokines.
Table 1.
Summarized Characteristics of IL-30 to IL-40
| Cytokine | Other names | Gene superfamily | Chromosome location | Receptor | Functions | Main human expression tissuesa | Producing cells | Immune cells responding | Potential role in disease | In-depth reviews |
|---|---|---|---|---|---|---|---|---|---|---|
| IL-30 | p28, IL-27p28b | IL-12 | 16p12.1 | IL-27Rα | Suppression of IL-17A production, anti-inflammatory | Placenta, BM, liver, stomach, skin, testis | Monocytes, macrophages, DCs | T cells | Prostate cancer, breast cancer | Di Carlo (2014) |
| IL-31 | None | IL-6 | 12q24.31 | IL-31RA/OSMRβ | Pruritus, proinflammatory | BM, tonsil, spleen, skin | Eosinophils, CD4+ T cells | Epithelial cells, monocytes | Pruritic atopic dermatitis, airway hypersensitivity, IBD | Zhang and others (2008) |
| IL-32 | NK4 | None | 16p13.3 | Unknown | Angiogenesis, IL-2 production in BM, proinflammatory | Appendix, lung, duodenum, small intestine | Monocytes, T cells, NK cells, epithelial cells | Monocytes, macrophages, BM stroma | Cancer, tuberculosis, influenza virus A infection, RA, ulcerative colitis, Crohn's disease, chronic obstructive pulmonary disease | Khawar and others (2016); Sloot and others (2018) |
| IL-33 | NF-HEV, IL-1 F11 | IL-1 | 9p24.1 | ST2 | Alarmin, proinflammatory | Skin, stomach, cervix, endometrium, nasopharynx, bronchus | Endothelial cells, epithelial cells, fibroblast, mucosal epithelium | T cells, mast cells, eosinophils, basophils, ILC2s | Asthma, IBD | Liew and others (2016) |
| IL-34 | C16orf77 | None | 16q22.1 | CSF-1R, PTP-ζ, CD138 | Myeloid cell proliferation, anti-inflammatory | Spleen,c skinc | Keratinocytes, neurons, Tregs, monocytes, macrophages, DCs | Macrophages, monocytes, Langerhans cells, microglia, kupffer cells, DCs | Osteoporosis, lupus, periodontal disease, Hepatitis B, RA, transplant rejection, lung cancer, fibrosis | Guillonneau and others (2017) |
| IL-35 | None | IL-12 | 3q25.33 (IL12A); 19p13.3 (EBI3) | IL-12Rβ2/IL-12Rβ2; gp130/gp130; IL-12Rβ2/gp130 | Prevents Th1 and Th17 cell proliferation, induces Treg/Breg polarization, anti-inflammatory | Placenta, spleen,c | Tregs, Bregs, | Macrophages, T cells | Multiple sclerosis, diabetes, colitis, Hodgkin's lymphoma, lung cancer | Choi and others (2015) |
| IL-36α IL-36β IL-36γ IL-36RA | FIL1ɛ/IL-1 F6, FIL1η/IL-1 F8, IL-1 F9, FIL1δ/IL-1 F5 | IL-1 | 2q14.1 | IL-36R | Th1 responses, proinflammatory | Skin, esophagus, tonsil | Keratinocytes, mucosal epithelial cells, monocytes, macrophages, Langerhans cells, CD4+ T cells | Epithelial cells, macrophages, DCs, T cells, B cells, plasma cells | DITRA,d lesional psoriasis, tuberculosis, IBD | Gabay and Towne (2015) |
| IL-37 | FIL1ζ, IL-1 F7 | IL-1 | 2q14.1 | IL-18Rα | Anti-inflammatory | Skin | Epithelial cells, keratinocytes, monocytes, NK cells, B cells | Epithelial cells, fibroblasts, monocytes, macrophages, DCs, T cells | SLE, RA, IBD, ankylosing spondylitis, Graves' disease, HIV infection, coronary artery disease | Jia and others (2018) |
| IL-38 | IL-1 F10, FIL1θ | IL-1 | 2q14.1 | IL-1R; IL-36R; IL-1RAPL1 | Blocking of IL-36, anti-inflammatory | Skin | Epithelial cells, B cells | Epithelial cells, macrophages, DCs, B cells, plasma cells | RA, atherosclerosis, cardiovascular disease, SLE, hidradenitis suppurativa, Hepatitis B, diabetes, asthma, fibrosis, lung cancer | Garraud and others (2018); van de Veerdonk and others (2018) |
| IL-39 | None | IL-12 | 12q13.3 (P19); 19p13.3 (EBI3) | Unknown | Proinflammatory | Spleen | Macrophages, DCs, B cells | Neutrophils | Lupus | None |
| IL-40 | C17orf99 | None | 17q25.3 | Unknown | Involved in IgA production, B cell homeostasis and development | BM, fetal liver | B cells, BM stroma | B cells | B cell lymphoma | None |
Data from protein atlas of human expression (www.proteinatlas.org; Uhlen and others 2015).
IL-30 is a subunit of IL-27 but exhibits activity by itself.
RNA expression.
Deficiency of IL-36 receptor antagonist is a disease caused by the lack of functional IL-36ra.
CSF, colony stimulating factor; DCs, dendritic cells; DITRA, deficiency of IL-36 receptor antagonist; IBD, inflammatory bowel disease; IL, interleukin; NF-HEV, nuclear factor in high endothelial venules; NK, natural killer; OSMRβ, oncostatin M receptor beta; RA, rheumatoid arthritis.
FIG. 1.
Graphical representation of IL-30 to IL-40 and which cytokine superfamilies they belong to (or not). The relationships of the IL-1 superfamily cytokines are based on their evolutionary analysis as described by Rivers-Auty and others (2018). IL, interleukin.
One of the biggest challenges after the identification of a novel cytokine is to start defining its biology. Usually one of the first steps is the production of a knockout mouse. However, identifying a phenotype of a new knockout mouse is not easy either. These considerations make this type of research (searching for novel cytokines) a very challenging project. Importantly, evolutionary considerations can suggest functional roles. Even chromosomal location can provide important clues about the function of a particular gene (Zlotnik and Yoshie 2012).
The discovery of cytokines in the late 20th and early 21st centuries was supported by advances in molecular biology techniques and the development of gene databases and bioinformatics. Many of the cytokines, including several in this review, were found because they belong to cytokine superfamilies and share similarities to previously known cytokines. In our experience, it has been very difficult to uncover the physiology of IL-40, because it is encoded by a previously uncharacterized gene for which no information was available. This led us to rely on the characterization of an Il-40-/- mouse, which indicates a role for IL-40 in IgA production and B cell development. A key question is whether there remain many other cytokines to be identified. Ten percent of the human genome is estimated to encode secreted proteins, suggesting that there may be more. Given the popularity of databases and gene arrays, and novel techniques such as RNAseq and single-cell sequencing, we predict that cytokines produced by relatively rare cell subsets are yet to be identified. If so, we may also predict that their genes will not be related to any of the known cytokine families.
Some of these novel cytokines exhibit very important functions. Examples include cytokines such as IL-31 and its association with pruritus, IL-35, and its ability to polarize T cells and B cells into potent immune-suppressive cells or IL-36 and its role in skin homeostasis. We can therefore predict that the biology of these novel cytokines will open several very interesting fields of research in the future.
Acknowledgments
The study was supported by Grant R21AI128465 from NIAID/NIH. JCD was supported by a grant from UCMEXUS/CONACYT.
Author Disclosure Statement
No competing financial interests exist.
References
- Airoldi I, Cocco C, Sorrentino C, Angelucci D, Di Meo S, Manzoli L, Esposito S, Ribatti D, Bertolotto M, Iezzi L, Natoli C, Di Carlo E. 2016. Interleukin-30 promotes breast cancer growth and progression. Cancer Res 76(21):6218–6229 [DOI] [PubMed] [Google Scholar]
- Aparicio-Siegmund S, Moll JM, Lokau J, Grusdat M, Schroder J, Plohn S, Rose-John S, Grotzinger J, Lang PA, Scheller J, Garbers C. 2014. Recombinant p35 from bacteria can form Interleukin (IL-)12, but Not IL-35. PLoS One 9(9):e107990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkevold ES, Roussigne M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard JP. 2003. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol 163(1):69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghdadi M, Endo H, Takano A, Ishikawa K, Kameda Y, Wada H, Miyagi Y, Yokose T, Ito H, Nakayama H, Daigo Y, Suzuki N, Seino KI. 2018. High co-expression of IL-34 and M-CSF correlates with tumor progression and poor survival in lung cancers. Sci Rep 8(1):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballak DB, van Diepen JA, Moschen AR, Jansen HJ, Hijmans A, Groenhof GJ, Leenders F, Bufler P, Boekschoten MV, Muller M, Kersten S, Li S, Kim S, Eini H, Lewis EC, Joosten LA, Tilg H, Netea MG, Tack CJ, Dinarello CA, Stienstra R. 2014. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat Commun 5:4711. [DOI] [PubMed] [Google Scholar]
- Bando T, Morikawa Y, Komori T, Senba E. 2006. Complete overlap of interleukin-31 receptor A and oncostatin M receptor beta in the adult dorsal root ganglia with distinct developmental expression patterns. Neuroscience 142(4):1263–1271 [DOI] [PubMed] [Google Scholar]
- Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L'Hermine A, Devergne O. 2008. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol 181(10):6898–6905 [DOI] [PubMed] [Google Scholar]
- Barton JL, Herbst R, Bosisio D, Higgins L, Nicklin MJ. 2000. A tissue specific IL-1 receptor antagonist homolog from the IL-1 cluster lacks IL-1, IL-1ra, IL-18 and IL-18 antagonist activities. Eur J Immunol 30(11):3299–3308 [DOI] [PubMed] [Google Scholar]
- Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. 2006. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol 7(9):929–936 [DOI] [PubMed] [Google Scholar]
- Bensen JT, Dawson PA, Mychaleckyj JC, Bowden DW. 2001. Identification of a novel human cytokine gene in the interleukin gene cluster on chromosome 2q12–14. J Interferon Cytokine Res 21(11):899–904 [DOI] [PubMed] [Google Scholar]
- Bettini M, Castellaw AH, Lennon GP, Burton AR, Vignali DA. 2012. Prevention of autoimmune diabetes by ectopic pancreatic beta-cell expression of interleukin-35. Diabetes 61(6):1519–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezie S, Picarda E, Ossart J, Tesson L, Usal C, Renaudin K, Anegon I, Guillonneau C. 2015. IL-34 is a Treg-specific cytokine and mediates transplant tolerance. J Clin Invest 125(10):3952–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi ME. 2007. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81(1):1–5 [DOI] [PubMed] [Google Scholar]
- Blumberg H, Dinh H, Dean C, Jr., Trueblood ES, Bailey K, Shows D, Bhagavathula N, Aslam MN, Varani J, Towne JE, Sims JE. 2010. IL-1RL2 and its ligands contribute to the cytokine network in psoriasis. J Immunol 185(7):4354–4362 [DOI] [PubMed] [Google Scholar]
- Boraschi D, Lucchesi D, Hainzl S, Leitner M, Maier E, Mangelberger D, Oostingh GJ, Pfaller T, Pixner C, Posselt G, Italiani P, Nold MF, Nold-Petry CA, Bufler P, Dinarello CA. 2011. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw 22(3):127–147 [DOI] [PubMed] [Google Scholar]
- Busfield SJ, Comrack CA, Yu G, Chickering TW, Smutko JS, Zhou H, Leiby KR, Holmgren LM, Gearing DP, Pan Y. 2000. Identification and gene organization of three novel members of the IL-1 family on human chromosome 2. Genomics 66(2):213–216 [DOI] [PubMed] [Google Scholar]
- Busse W, Elias J, Sheppard D, Banks-Schlegel S. 1999. Airway remodeling and repair. Am J Respir Crit Care Med 160(3):1035–1042 [DOI] [PubMed] [Google Scholar]
- Canda-Sanchez A, Salgado FJ, Perez-Diaz A, Varela-Gonzalez C, Arias P, Nogueira M. 2009. Differential distribution of both IL-12Rbeta chains in the plasma membrane of human T cells. J Membr Biol 227(1):1–12 [DOI] [PubMed] [Google Scholar]
- Catalan-Dibene J, Vazquez MI, Luu VP, Nuccio SP, Karimzadeh A, Kastenschmidt JM, Villalta SA, Ushach I, Pone EJ, Casali P, Raffatellu M, Burkhardt AM, Hernandez-Ruiz M, Heller G, Hevezi PA, Zlotnik A. 2017. Identification of IL-40, a novel B cell-associated cytokine. J Immunol 199(9):3326–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli G, Dinarello CA. 2018. Suppression of inflammation and acquired immunity by IL-37. Immunol Rev 281(1):179–190 [DOI] [PubMed] [Google Scholar]
- Cayrol C, Girard JP. 2018. Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol Rev 281(1):154–168 [DOI] [PubMed] [Google Scholar]
- Chen Z, Buki K, Vaaraniemi J, Gu G, Vaananen HK. 2011. The critical role of IL-34 in osteoclastogenesis. PLoS One 6(4):e18689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Leung PS, Bowlus C, Gershwin ME. 2015. IL-35 and autoimmunity: a comprehensive perspective. Clin Rev Allergy Immunol 49(3):327–332 [DOI] [PubMed] [Google Scholar]
- Chou CT, Timms AE, Wei JC, Tsai WC, Wordsworth BP, Brown MA. 2006. Replication of association of IL1 gene complex members with ankylosing spondylitis in Taiwanese Chinese. Ann Rheum Dis 65(8):1106–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M, Chu IM, Yung EC, Lam CW, Leung TF, Wong GW, Wong CK. 2016. Aberrant expression of novel cytokine IL-38 and regulatory T lymphocytes in childhood asthma. Molecules 21(7):E933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HF, Gurney AL, Abaya E, Baker K, Baldwin D, Brush J, Chen J, Chow B, Chui C, Crowley C, Currell B, Deuel B, Dowd P, Eaton D, Foster J, Grimaldi C, Gu Q, Hass PE, Heldens S, Huang A, Kim HS, Klimowski L, Jin Y, Johnson S, Lee J, Lewis L, Liao D, Mark M, Robbie E, Sanchez C, Schoenfeld J, Seshagiri S, Simmons L, Singh J, Smith V, Stinson J, Vagts A, Vandlen R, Watanabe C, Wieand D, Woods K, Xie MH, Yansura D, Yi S, Yu G, Yuan J, Zhang M, Zhang Z, Goddard A, Wood WI, Godowski P, Gray A. 2003. The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: a bioinformatics assessment. Genome Res 13(10):2265–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, Satoskar AR, Garcia KC, Hunter CA, Drake CG, Murray PJ, Vignali DA. 2012. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol 13(3):290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. 2007. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450(7169):566–569 [DOI] [PubMed] [Google Scholar]
- Cox JH, Kljavin NM, Ramamoorthi N, Diehl L, Batten M, Ghilardi N. 2011. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med 208(1):115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, Ottoson P, Persson P, Delaney T, Lehar S, Lin S, Poisson L, Meisel C, Kamradt T, Bjerke T, Levinson D, Gutierrez-Ramos JC. 1999. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med 190(7):895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabe S, Guay-Giroux A, Tormo AJ, Duluc D, Lissilaa R, Guilhot F, Mavoungou-Bigouagou U, Lefouili F, Cognet I, Ferlin W, Elson G, Jeannin P, Gauchat JF. 2009. The IL-27 p28 subunit binds cytokine-like factor 1 to form a cytokine regulating NK and T cell activities requiring IL-6R for signaling. J Immunol 183(12):7692–7702 [DOI] [PubMed] [Google Scholar]
- Debets R, Timans JC, Homey B, Zurawski S, Sana TR, Lo S, Wagner J, Edwards G, Clifford T, Menon S, Bazan JF, Kastelein RA. 2001. Two novel IL-1 family members, IL-1 delta and IL-1 epsilon, function as an antagonist and agonist of NF-kappa B activation through the orphan IL-1 receptor-related protein 2. J Immunol 167(3):1440–1446 [DOI] [PubMed] [Google Scholar]
- Devergne O, Birkenbach M, Kieff E. 1997. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci U S A 94(22):12041–12046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo E. 2014. Interleukin-30: a novel microenvironmental hallmark of prostate cancer progression. Oncoimmunology 3(1):e27618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, Haugen HS, Maurer M, Harder B, Johnston J, Bort S, Mudri S, Kuijper JL, Bukowski T, Shea P, Dong DL, Dasovich M, Grant FJ, Lockwood L, Levin SD, LeCiel C, Waggie K, Day H, Topouzis S, Kramer J, Kuestner R, Chen Z, Foster D, Parrish-Novak J, Gross JA. 2004. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol 5(7):752–760 [DOI] [PubMed] [Google Scholar]
- Di Meo S, Airoldi I, Sorrentino C, Zorzoli A, Esposito S, Di Carlo E. 2014. Interleukin-30 expression in prostate cancer and its draining lymph nodes correlates with advanced grade and stage. Clin Cancer Res 20(3):585–594 [DOI] [PubMed] [Google Scholar]
- Dinarello C, Arend W, Sims J, Smith D, Blumberg H, O'Neill L, Goldbach-Mansky R, Pizarro T, Hoffman H, Bufler P, Nold M, Ghezzi P, Mantovani A, Garlanda C, Boraschi D, Rubartelli A, Netea M, van der Meer J, Joosten L, Mandrup-Poulsen T, Donath M, Lewis E, Pfeilschifter J, Martin M, Kracht M, Muehl H, Novick D, Lukic M, Conti B, Solinger A, Kelk P, van de Veerdonk F, Gabel C. 2010. IL-1 family nomenclature. Nat Immunol 11(11):973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos JC, Damen M, Joosten LAB, Ribeiro-Dias F. 2018. Interleukin-32: an endogenous danger signal or master regulator of intracellular pathogen infections-Focus on leishmaniases. Semin Immunol. In press. [Epub ahead of print]; DOI: 10.1016/j.smim.2018.02.010 [DOI] [PubMed] [Google Scholar]
- Dunn E, Sims JE, Nicklin MJ, O'Neill LA. 2001. Annotating genes with potential roles in the immune system: six new members of the IL-1 family. Trends Immunol 22(10):533–536 [DOI] [PubMed] [Google Scholar]
- Felaco P, Castellani ML, De Lutiis MA, Felaco M, Pandolfi F, Salini V, De Amicis D, Vecchiet J, Tete S, Ciampoli C, Conti F, Cerulli G, Caraffa A, Antinolfi P, Cuccurullo C, Perrella A, Theoharides TC, Conti P, Toniato E, Kempuraj D, Shaik YB. 2009. IL-32: a newly-discovered proinflammatory cytokine. J Biol Regul Homeost Agents 23(3):141–147 [PubMed] [Google Scholar]
- Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. 2007. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol 8(12):1372–1379 [DOI] [PubMed] [Google Scholar]
- Floss DM, Schonberg M, Franke M, Horstmeier FC, Engelowski E, Schneider A, Rosenfeldt EM, Scheller J. 2017. IL-6/IL-12 cytokine receptor shuffling of extra- and intracellular domains reveals canonical STAT activation via synthetic IL-35 and IL-39 signaling. Sci Rep 7(1):15172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franze E, Dinallo V, Rizzo A, Di Giovangiulio M, Bevivino G, Stolfi C, Caprioli F, Colantoni A, Ortenzi A, Grazia AD, Sica G, Sileri PP, Rossi P, Monteleone G. 2018. Interleukin-34 sustains pro-tumorigenic signals in colon cancer tissue. Oncotarget 9(3):3432–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Towne JE. 2015. Regulation and function of interleukin-36 cytokines in homeostasis and pathological conditions. J Leukoc Biol 97(4):645–652 [DOI] [PubMed] [Google Scholar]
- Galligan CL, Fish EN. 2017. Interleukin-34 promotes fibrocyte proliferation. J Interferon Cytokine Res 37(10):440–448 [DOI] [PubMed] [Google Scholar]
- Ganesan R, Raymond EL, Mennerich D, Woska JR, Jr, Caviness G, Grimaldi C, Ahlberg J, Perez R, Roberts S, Yang D, Jerath K, Truncali K, Frego L, Sepulveda E, Gupta P, Brown SE, Howell MD, Canada KA, Kroe-Barrett R, Fine JS, Singh S, Mbow ML. 2017. Generation and functional characterization of anti-human and anti-mouse IL-36R antagonist monoclonal antibodies. MAbs 9(7):1143–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers C, Spudy B, Aparicio-Siegmund S, Waetzig GH, Sommer J, Holscher C, Rose-John S, Grotzinger J, Lorenzen I, Scheller J. 2013. An interleukin-6 receptor-dependent molecular switch mediates signal transduction of the IL-27 cytokine subunit p28 (IL-30) via a gp130 protein receptor homodimer. J Biol Chem 288(6):4346–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraud T, Harel M, Boutet MA, Le Goff B, Blanchard F. 2018. The enigmatic role of IL-38 in inflammatory diseases. Cytokine Growth Factor Rev 39:26–35 [DOI] [PubMed] [Google Scholar]
- Greenfield AL, Hauser SL. 2018. B-cell therapy for multiple sclerosis: entering an era. Ann Neurol 83(1):13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresnigt MS, van de Veerdonk FL. 2013. Biology of IL-36 cytokines and their role in disease. Semin Immunol 25(6):458–465 [DOI] [PubMed] [Google Scholar]
- Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kundig TM, Frei K, Ginhoux F, Merad M, Becher B. 2012. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 37(6):1050–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimstad O, Sawanobori Y, Vestergaard C, Bilsborough J, Olsen UB, Gronhoj-Larsen C, Matsushima K. 2009. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp Dermatol 18(1):35–43 [DOI] [PubMed] [Google Scholar]
- Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, Thorleifsson G, Helgadottir H, Steinthorsdottir V, Stefansson H, Williams C, Hui J, Beilby J, Warrington NM, James A, Palmer LJ, Koppelman GH, Heinzmann A, Krueger M, Boezen HM, Wheatley A, Altmuller J, Shin HD, Uh ST, Cheong HS, Jonsdottir B, Gislason D, Park CS, Rasmussen LM, Porsbjerg C, Hansen JW, Backer V, Werge T, Janson C, Jonsson UB, Ng MC, Chan J, So WY, Ma R, Shah SH, Granger CB, Quyyumi AA, Levey AI, Vaccarino V, Reilly MP, Rader DJ, Williams MJ, van Rij AM, Jones GT, Trabetti E, Malerba G, Pignatti PF, Boner A, Pescollderungg L, Girelli D, Olivieri O, Martinelli N, Ludviksson BR, Ludviksdottir D, Eyjolfsson GI, Arnar D, Thorgeirsson G, Deichmann K, Thompson PJ, Wjst M, Hall IP, Postma DS, Gislason T, Gulcher J, Kong A, Jonsdottir I, Thorsteinsdottir U, Stefansson K. 2009. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet 41(3):342–347 [DOI] [PubMed] [Google Scholar]
- Guillonneau C, Bezie S, Anegon I. 2017. Immunoregulatory properties of the cytokine IL-34. Cell Mol Life Sci 74(14):2569–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruprasad CN, Pradeep AR. 2018. Interleukin-34 levels in gingival crevicular fluid and plasma in periodontal health and disease with and without type-2 diabetes mellitus. J Investig Clin Dent 9(2):e12317. [DOI] [PubMed] [Google Scholar]
- Guzzo C, Che Mat NF, Gee K. 2010. Interleukin-27 induces a STAT1/3- and NF-kappaB-dependent proinflammatory cytokine profile in human monocytes. J Biol Chem 285(32):24404–24411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinhuis B, Netea MG, van den Berg WB, Dinarello CA, Joosten LA. 2012. Interleukin-32: a predominantly intracellular proinflammatory mediator that controls cell activation and cell death. Cytokine 60(2):321–327 [DOI] [PubMed] [Google Scholar]
- Henry CJ, Casas-Selves M, Kim J, Zaberezhnyy V, Aghili L, Daniel AE, Jimenez L, Azam T, McNamee EN, Clambey ET, Klawitter J, Serkova NJ, Tan AC, Dinarello CA, DeGregori J. 2015. Aging-associated inflammation promotes selection for adaptive oncogenic events in B cell progenitors. J Clin Invest 125(12):4666–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessam S, Sand M, Gambichler T, Skrygan M, Ruddel I, Bechara FG. 2018. Interleukin-36 in hidradenitis suppurativa: evidence for a distinctive proinflammatory role and a key factor in the development of an inflammatory loop. Br J Dermatol 178(3):761–767 [DOI] [PubMed] [Google Scholar]
- Hojen JF, Rasmussen TA, Andersen KL, Winckelmann AA, Laursen RR, Gunst JD, Moller HJ, Fujita M, Ostergaard L, Sogaard OS, Dinarello CA, Tolstrup M. 2015. Interleukin-37 expression is increased in chronic HIV-1-infected individuals and is associated with inflammation and the size of the total viral reservoir. Mol Med 21:337–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CH, Loo EX, Kuo IC, Soh GH, Goh DL, Lee BW, Chua KY. 2011. Airway inflammation and IgE production induced by dust mite allergen-specific memory/effector Th2 cell line can be effectively attenuated by IL-35. J Immunol 187(1):462–471 [DOI] [PubMed] [Google Scholar]
- Imaeda H, Takahashi K, Fujimoto T, Kasumi E, Ban H, Bamba S, Sonoda H, Shimizu T, Fujiyama Y, Andoh A. 2013. Epithelial expression of interleukin-37b in inflammatory bowel disease. Clin Exp Immunol 172(3):410–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannin P, Paolini L, Adam C, Delneste Y. 2018. The roles of CSFs on the functional polarization of tumor-associated macrophages. FEBS J 285(4):680–699 [DOI] [PubMed] [Google Scholar]
- Jia H, Liu J, Han B. 2018. Reviews of interleukin-37: functions, receptors, and roles in diseases. Biomed Res Int 2018:3058640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MY, Kang SW, Kim SK, Kim HJ, Yun DH, Yim SV, Hong SJ, Chung JH. 2010. The interleukin-1 family gene polymorphisms in Korean patients with rheumatoid arthritis. Scand J Rheumatol 39(3):190–196 [DOI] [PubMed] [Google Scholar]
- Khawar MB, Abbasi MH, Sheikh N. 2016. IL-32: a novel pluripotent inflammatory interleukin, towards gastric inflammation, gastric cancer, and chronic rhino sinusitis. Mediators Inflamm 2016:8413768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Lee Y, Kim E, Kwak A, Ryoo S, Bae SH, Azam T, Kim S, Dinarello CA. 2013. The interleukin-1alpha precursor is biologically active and is likely a key alarmin in the IL-1 family of cytokines. Front Immunol 4:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. 2005. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity 22(1):131–142 [DOI] [PubMed] [Google Scholar]
- Kobori A, Yagi Y, Imaeda H, Ban H, Bamba S, Tsujikawa T, Saito Y, Fujiyama Y, Andoh A. 2010. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol 45(10):999–1007 [DOI] [PubMed] [Google Scholar]
- Kumar S, McDonnell PC, Lehr R, Tierney L, Tzimas MN, Griswold DE, Capper EA, Tal-Singer R, Wells GI, Doyle ML, Young PR. 2000. Identification and initial characterization of four novel members of the interleukin-1 family. J Biol Chem 275(14):10308–10314 [DOI] [PubMed] [Google Scholar]
- Liew FY, Girard JP, Turnquist HR. 2016. Interleukin-33 in health and disease. Nat Rev Immunol 16(11):676–689 [DOI] [PubMed] [Google Scholar]
- Lin H, Ho AS, Haley-Vicente D, Zhang J, Bernal-Fussell J, Pace AM, Hansen D, Schweighofer K, Mize NK, Ford JE. 2001. Cloning and characterization of IL-1HY2, a novel interleukin-1 family member. J Biol Chem 276(23):20597–20602 [DOI] [PubMed] [Google Scholar]
- Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein SK, Williams LT. 2008. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 320(5877):807–811 [DOI] [PubMed] [Google Scholar]
- Lin X, Yang L, Wang G, Zi F, Yan H, Guo X, Chen J, Chen Q, Huang X, Li Y, Zhang E, Wu W, Yang Y, He D, He J, Cai Z. 2017. Interleukin-32alpha promotes the proliferation of multiple myeloma cells by inducing production of IL-6 in bone marrow stromal cells. Oncotarget 8(54):92841–92854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau R, Mehta RB, Lash GE, Papapavlou G, Boij R, Berg G, Jenmalm MC, Ernerudh J, Svensson-Arvelund J. 2018. Interleukin-34 is present at the fetal-maternal interface and induces immunoregulatory macrophages of a decidual phenotype in vitro. Hum Reprod 33(4):588–599 [DOI] [PubMed] [Google Scholar]
- Lopez-Mejias R, Genre F, Remuzgo-Martinez S, Gonzalez-Juanatey C, Robustillo-Villarino M, Llorca J, Corrales A, Vicente E, Miranda-Filloy JA, Magro C, Tejera-Segura B, Ramirez Huaranga MA, Pina T, Blanco R, Alegre-Sancho JJ, Raya E, Mijares V, Ubilla B, Minguez Sanchez MD, Gomez-Vaquero C, Balsa A, Pascual-Salcedo D, Lopez-Longo FJ, Carreira P, Gonzalez-Alvaro I, Rodriguez-Rodriguez L, Fernandez-Gutierrez B, Ferraz-Amaro I, Castaneda S, Martin J, Gonzalez-Gay MA. 2016. Influence of elevated-CRP level-related polymorphisms in non-rheumatic Caucasians on the risk of subclinical atherosclerosis and cardiovascular disease in rheumatoid arthritis. Sci Rep 6:31979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunding L, Webering S, Vock C, Schroder A, Raedler D, Schaub B, Fehrenbach H, Wegmann M. 2015. IL-37 requires IL-18Ralpha and SIGIRR/IL-1R8 to diminish allergic airway inflammation in mice. Allergy 70(4):366–373 [DOI] [PubMed] [Google Scholar]
- Luu VP, Vazquez MI, Zlotnik A. 2014. B cells participate in tolerance and autoimmunity through cytokine production. Autoimmunity 47(1):1–12 [DOI] [PubMed] [Google Scholar]
- Marrakchi S, Guigue P, Renshaw BR, Puel A, Pei XY, Fraitag S, Zribi J, Bal E, Cluzeau C, Chrabieh M, Towne JE, Douangpanya J, Pons C, Mansour S, Serre V, Makni H, Mahfoudh N, Fakhfakh F, Bodemer C, Feingold J, Hadj-Rabia S, Favre M, Genin E, Sahbatou M, Munnich A, Casanova JL, Sims JE, Turki H, Bachelez H, Smahi A. 2011. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med 365(7):620–628 [DOI] [PubMed] [Google Scholar]
- McNamee EN, Masterson JC, Jedlicka P, McManus M, Grenz A, Collins CB, Nold MF, Nold-Petry C, Bufler P, Dinarello CA, Rivera-Nieves J. 2011. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A 108(40):16711–16716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Contreras O, Harusato A, Nishio H, Flannigan KL, Ngo V, Leoni G, Neumann PA, Geem D, Lili LN, Ramadas RA, Chassaing B, Gewirtz AT, Kohlmeier JE, Parkos CA, Towne JE, Nusrat A, Denning TL. 2016. Cutting edge: IL-36 receptor promotes resolution of intestinal damage. J Immunol 196(1):34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milora KA, Fu H, Dubaz O, Jensen LE. 2015. Unprocessed interleukin-36alpha regulates psoriasis-like skin inflammation in cooperation with interleukin-1. J Invest Dermatol 135(12):2992–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molho-Pessach V, Alyan R, Gordon D, Jaradat H, Zlotogorski A. 2017. Secukinumab for the treatment of deficiency of interleukin 36 receptor antagonist in an adolescent. JAMA Dermatol 153(5):473–475 [DOI] [PubMed] [Google Scholar]
- Montoya D, Inkeles MS, Liu PT, Realegeno S, Teles RM, Vaidya P, Munoz MA, Schenk M, Swindell WR, Chun R, Zavala K, Hewison M, Adams JS, Horvath S, Pellegrini M, Bloom BR, Modlin RL. 2014. IL-32 is a molecular marker of a host defense network in human tuberculosis. Sci Transl Med 6(250):250ra114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti S, Bozza S, Oikonomou V, Renga G, Casagrande A, Iannitti RG, Puccetti M, Garlanda C, Kim S, Li S, van de Veerdonk FL, Dinarello CA, Romani L. 2014. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog 10(11):e1004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussion C, Ortega N, Girard JP. 2008. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel “alarmin”? PLoS One 3(10):e3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulero JJ, Pace AM, Nelken ST, Loeb DB, Correa TR, Drmanac R, Ford JE. 1999. IL1HY1: a novel interleukin-1 receptor antagonist gene. Biochem Biophys Res Commun 263(3):702–706 [DOI] [PubMed] [Google Scholar]
- Musette P, Bouaziz JD. 2018. B cell modulation strategies in autoimmune diseases: new concepts. Front Immunol 9:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neis MM, Peters B, Dreuw A, Wenzel J, Bieber T, Mauch C, Krieg T, Stanzel S, Heinrich PC, Merk HF, Bosio A, Baron JM, Hermanns HM. 2006. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol 118(4):930–937 [DOI] [PubMed] [Google Scholar]
- Niedbala W, Cai B, Wei X, Patakas A, Leung BP, McInnes IB, Liew FY. 2008. Interleukin 27 attenuates collagen-induced arthritis. Ann Rheum Dis 67(10):1474–1479 [DOI] [PubMed] [Google Scholar]
- Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. 2007. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol 37(11):3021–3029 [DOI] [PubMed] [Google Scholar]
- Niedobitek G, Pazolt D, Teichmann M, Devergne O. 2002. Frequent expression of the Epstein-Barr virus (EBV)-induced gene, EBI3, an IL-12 p40-related cytokine, in Hodgkin and Reed-Sternberg cells. J Pathol 198(3):310–316 [DOI] [PubMed] [Google Scholar]
- Nishino R, Takano A, Oshita H, Ishikawa N, Akiyama H, Ito H, Nakayama H, Miyagi Y, Tsuchiya E, Kohno N, Nakamura Y, Daigo Y. 2011. Identification of Epstein-Barr virus-induced gene 3 as a novel serum and tissue biomarker and a therapeutic target for lung cancer. Clin Cancer Res 17(19):6272–6286 [DOI] [PubMed] [Google Scholar]
- Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. 2010. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol 11(11):1014–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nold-Petry CA, Rudloff I, Baumer Y, Ruvo M, Marasco D, Botti P, Farkas L, Cho SX, Zepp JA, Azam T, Dinkel H, Palmer BE, Boisvert WA, Cool CD, Taraseviciene-Stewart L, Heinhuis B, Joosten LA, Dinarello CA, Voelkel NF, Nold MF. 2014. IL-32 promotes angiogenesis. J Immunol 192(2):589–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku K, Atsumi T. 2018. Systemic lupus erythematosus: nothing stale her infinite variety. Mod Rheumatol 28(5):758–765 [DOI] [PubMed] [Google Scholar]
- Owaki T, Asakawa M, Kamiya S, Takeda K, Fukai F, Mizuguchi J, Yoshimoto T. 2006. IL-27 suppresses CD28-mediated [correction of medicated] IL-2 production through suppressor of cytokine signaling 3. J Immunol 176(5):2773–2780 [DOI] [PubMed] [Google Scholar]
- Ozceker D, Bulut M, Ozbay AC, Dilek F, Koser M, Tamay Z, Guler N. 2018. Assessment of IL-31 levels and disease severity in children with atopic dermatitis. Allergol Immunopathol (Madr) 46(4):322–325 [DOI] [PubMed] [Google Scholar]
- Pan G, Risser P, Mao W, Baldwin DT, Zhong AW, Filvaroff E, Yansura D, Lewis L, Eigenbrot C, Henzel WJ, Vandlen R. 2001. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine 13(1):1–7 [DOI] [PubMed] [Google Scholar]
- Pastorelli L, Garg RR, Hoang SB, Spina L, Mattioli B, Scarpa M, Fiocchi C, Vecchi M, Pizarro TT. 2010. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci U S A 107(17):8017–8022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. 2004. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol 172(4):2225–2231 [DOI] [PubMed] [Google Scholar]
- Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. 2002. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 16(6):779–790 [DOI] [PubMed] [Google Scholar]
- Pot C, Apetoh L, Awasthi A, Kuchroo VK. 2011. Induction of regulatory Tr1 cells and inhibition of T(H)17 cells by IL-27. Semin Immunol 23(6):438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, Martin JG, Hamid Q. 2010. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol 125(3):752–754 [DOI] [PubMed] [Google Scholar]
- Pusic KM, Pusic AD, Kemme J, Kraig RP. 2014. Spreading depression requires microglia and is decreased by their M2a polarization from environmental enrichment. Glia 62(7):1176–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman P, Sun S, Peddle L, Snelgrove T, Melay W, Greenwood C, Gladman D. 2006. Association between the interleukin-1 family gene cluster and psoriatic arthritis. Arthritis Rheum 54(7):2321–2325 [DOI] [PubMed] [Google Scholar]
- Rivers-Auty J, Daniels MJD, Colliver I, Robertson DL, Brough D. 2018. Redefining the ancestral origins of the interleukin-1 superfamily. Nat Commun 9(1):1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-Semerano L, Piram M, Chiaverini C, De Ricaud D, Smahi A, Kone-Paut I. 2013. First clinical description of an infant with interleukin-36-receptor antagonist deficiency successfully treated with anakinra. Pediatrics 132(4):e1043–e1047 [DOI] [PubMed] [Google Scholar]
- Roth RB, Hevezi P, Lee J, Willhite D, Lechner SM, Foster AC, Zlotnik A. 2006. Gene expression analyses reveal molecular relationships among 20 regions of the human CNS. Neurogenetics 7(2):67–80 [DOI] [PubMed] [Google Scholar]
- Rudloff I, Godsell J, Nold-Petry CA, Harris J, Hoi A, Morand EF, Nold MF. 2015. Brief report: interleukin-38 exerts antiinflammatory functions and is associated with disease activity in systemic lupus erythematosus. Arthritis Rheumatol 67(12):3219–3225 [DOI] [PubMed] [Google Scholar]
- Rudrich U, Gehring M, Papakonstantinou E, Illerhaus A, Engmann J, Kapp A, Hartmann K, Meyer NH, Gibbs BF, Raap U. 2018. Eosinophils are a major source of interleukin-31 in bullous pemphigoid. Acta Derm Venereol 98(8):766–771 [DOI] [PubMed] [Google Scholar]
- Russell SE, Horan RM, Stefanska AM, Carey A, Leon G, Aguilera M, Statovci D, Moran T, Fallon PG, Shanahan F, Brint EK, Melgar S, Hussey S, Walsh PT. 2016. IL-36alpha expression is elevated in ulcerative colitis and promotes colonic inflammation. Mucosal Immunol 9(5):1193–1204 [DOI] [PubMed] [Google Scholar]
- San Segundo D, Ruiz P, Irure J, Arias-Loste MT, Cuadrado A, Puente A, Casafont F, Lopez-Hoyos M, Crespo J, Fabrega E. 2016. Serum levels of interleukin-34 during acute rejection in liver transplantation. Transplant Proc 48(9):2977–2979 [DOI] [PubMed] [Google Scholar]
- Scheibe K, Backert I, Wirtz S, Hueber A, Schett G, Vieth M, Probst HC, Bopp T, Neurath MF, Neufert C. 2017. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut 66(5):823–838 [DOI] [PubMed] [Google Scholar]
- Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, Harrison OJ, Owens BMJ, Lohning M, Belkaid Y, Fallon PG, Powrie F. 2014. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513(7519):564–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. 2005. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23(5):479–490 [DOI] [PubMed] [Google Scholar]
- Seidelin JB, Bjerrum JT, Coskun M, Widjaya B, Vainer B, Nielsen OH. 2010. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol Lett 128(1):80–85 [DOI] [PubMed] [Google Scholar]
- Seyerl M, Kirchberger S, Majdic O, Seipelt J, Jindra C, Schrauf C, Stockl J. 2010. Human rhinoviruses induce IL-35-producing Treg via induction of B7-H1 (CD274) and sialoadhesin (CD169) on DC. Eur J Immunol 40(2):321–329 [DOI] [PubMed] [Google Scholar]
- Shen P, Roch T, Lampropoulou V, O'Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C, Li R, Jouneau L, Boudinot P, Wilantri S, Sakwa I, Miyazaki Y, Leech MD, McPherson RC, Wirtz S, Neurath M, Hoehlig K, Meinl E, Grutzkau A, Grun JR, Horn K, Kuhl AA, Dorner T, Bar-Or A, Kaufmann SHE, Anderton SM, Fillatreau S. 2014. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507(7492):366–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimozato O, Sato A, Kawamura K, Chiyo M, Ma G, Li Q, Tagawa M. 2009. The secreted form of p28 subunit of interleukin (IL)-27 inhibits biological functions of IL-27 and suppresses anti-allogeneic immune responses. Immunology 128(1 Suppl):e816–e825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siffring PA, Gupta NC, Mohiuddin SM, Esterbrooks DJ, Hilleman DE, Cheng SC, Sketch MH, Sr., Frick MP. 1989. Myocardial uptake and clearance of T1–T201 in healthy subjects: comparison of adenosine-induced hyperemia and exercise stress. Radiology 173(3):769–774 [DOI] [PubMed] [Google Scholar]
- Sloot YJE, Smit JW, Joosten LAB, Netea-Maier RT. 2018. Insights into the role of IL-32 in cancer. Semin Immunol. In press. [Epub ahead of print]; DOI: 10.1016/j.smim.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Smith DE, Renshaw BR, Ketchem RR, Kubin M, Garka KE, Sims JE. 2000. Four new members expand the interleukin-1 superfamily. J Biol Chem 275(2):1169–1175 [DOI] [PubMed] [Google Scholar]
- Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, Alenius H, Dieu-Nosjean MC, Meller S, Rieker J, Steinhoff M, Hoffmann TK, Ruzicka T, Zlotnik A, Homey B. 2006. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol 117(2):411–417 [DOI] [PubMed] [Google Scholar]
- Sonoda E, Matsumoto R, Hitoshi Y, Ishii T, Sugimoto M, Araki S, Tominaga A, Yamaguchi N, Takatsu K. 1989. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med 170(4):1415–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino C, Ciummo SL, Cipollone G, Caputo S, Bellone M, Di Carlo E. 2018. Interleukin-30/IL27p28 shapes prostate cancer stem-like cell behavior and is critical for tumor onset and metastasization. Cancer Res 78(10):2654–2668 [DOI] [PubMed] [Google Scholar]
- Straub FB, Feuer G. 1989. Adenosinetriphosphate. The functional group of actin. 1950. Biochim Biophys Acta 1000:180–195 [PubMed] [Google Scholar]
- Stumhofer JS, Hunter CA. 2008. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett 117(2):123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O'Shea JJ, Hennighausen L, Ernst M, Hunter CA. 2006. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 7(9):937–945 [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. 2007. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol 8(12):1363–1371 [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Tait ED, Quinn WJ, 3rd, Hosken N, Spudy B, Goenka R, Fielding CA, O'Hara AC, Chen Y, Jones ML, Saris CJ, Rose-John S, Cua DJ, Jones SA, Elloso MM, Grotzinger J, Cancro MP, Levin SD, Hunter CA. 2010. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol 11(12):1119–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K, Okamoto T, Tominaga M, Teraishi K, Akamine T, Takamori S, Katsura M, Toyokawa G, Shoji F, Okamoto M, Oda Y, Hoshino T, Maehara Y. 2017. Clinical implications of the novel cytokine IL-38 expressed in lung adenocarcinoma: possible association with PD-L1 expression. PLoS One 12(7):e0181598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori A, Nambu A, Sato K, Yamaguchi S, Matsuda K, Numata T, Sugawara T, Yoshizaki T, Arae K, Morita H, Matsumoto K, Sudo K, Okumura K, Kitaura J, Matsuda H, Nakae S. 2018. IL-31 is crucial for induction of pruritus, but not inflammation, in contact hypersensitivity. Sci Rep 8(1):6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto T, Wakabayashi Y, Sekiya T, Inoue N, Morita R, Ichiyama K, Takahashi R, Asakawa M, Muto G, Mori T, Hasegawa E, Saika S, Hara T, Nomura M, Yoshimura A. 2010. Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development. J Immunol 185(2):842–855 [DOI] [PubMed] [Google Scholar]
- Tedder TF, Leonard WJ. 2014. Autoimmunity: regulatory B cells—IL-35 and IL-21 regulate the regulators. Nat Rev Rheumatol 10(8):452–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Okamoto M, Kawayama T, Matsuoka M, Kaieda S, Sakazaki Y, Kinoshita T, Mori D, Inoue A, Hoshino T. 2017. Overexpression of IL-38 protein in anticancer drug-induced lung injury and acute exacerbation of idiopathic pulmonary fibrosis. Respir Investig 55(5):293–299 [DOI] [PubMed] [Google Scholar]
- Towne JE, Garka KE, Renshaw BR, Virca GD, Sims JE. 2004. Interleukin (IL)-1 F6, IL-1 F8, and IL-1 F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-kappaB and MAPKs. J Biol Chem 279(14):13677–13688 [DOI] [PubMed] [Google Scholar]
- Towne JE, Renshaw BR, Douangpanya J, Lipsky BP, Shen M, Gabel CA, Sims JE. 2011. Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36alpha, IL-36beta, and IL-36gamma) or antagonist (IL-36Ra) activity. J Biol Chem 286(49):42594–42602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YC, Tsai TF. 2017. Anti-interleukin and interleukin therapies for psoriasis: current evidence and clinical usefulness. Ther Adv Musculoskelet Dis 9(11):277–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F. 2015. Proteomics. Tissue-based map of the human proteome. Science 347(6220):1260419. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk FL, de Graaf DM, Joosten LA, Dinarello CA. 2018. Biology of IL-38 and its role in disease. Immunol Rev 281(1):191–196 [DOI] [PubMed] [Google Scholar]
- van de Veerdonk FL, Stoeckman AK, Wu G, Boeckermann AN, Azam T, Netea MG, Joosten LA, van der Meer JW, Hao R, Kalabokis V, Dinarello CA. 2012. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc Natl Acad Sci U S A 109(8):3001–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verri WA, Jr, Souto FO, Vieira SM, Almeida SC, Fukada SY, Xu D, Alves-Filho JC, Cunha TM, Guerrero AT, Mattos-Guimaraes RB, Oliveira FR, Teixeira MM, Silva JS, McInnes IB, Ferreira SH, Louzada-Junior P, Liew FY, Cunha FQ. 2010. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann Rheum Dis 69(9):1697–1703 [DOI] [PubMed] [Google Scholar]
- Vignali DA, Kuchroo VK. 2012. IL-12 family cytokines: immunological playmakers. Nat Immunol 13(8):722–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne S, Palmer G, Martin P, Lamacchia C, Strebel D, Rodriguez E, Olleros ML, Vesin D, Garcia I, Ronchi F, Sallusto F, Sims JE, Gabay C. 2012. IL-36 signaling amplifies Th1 responses by enhancing proliferation and Th1 polarization of naive CD4+ T cells. Blood 120(17):3478–3487 [DOI] [PubMed] [Google Scholar]
- Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. 2003. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity 19(5):645–655 [DOI] [PubMed] [Google Scholar]
- Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. 2006. IL-27 limits IL-2 production during Th1 differentiation. J Immunol 176(1):237–247 [DOI] [PubMed] [Google Scholar]
- Walsh PT, Fallon PG. 2018. The emergence of the IL-36 cytokine family as novel targets for inflammatory diseases. Ann N Y Acad Sci 1417(1):23–34 [DOI] [PubMed] [Google Scholar]
- Wang HJ, Jiang YF, Wang XR, Zhang ML, Gao PJ. 2016a. Elevated serum interleukin-38 level at baseline predicts virological response in telbivudine-treated patients with chronic hepatitis B. World J Gastroenterol 22(18):4529–4537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, Egwuagu CE. 2014. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med 20(6):633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu X, Zhang Y, Wang Z, Zhu G, Han G, Chen G, Hou C, Wang T, Ma N, Shen B, Li Y, Xiao H, Wang R. 2016b. Interleukin (IL)-39 [IL-23p19/Epstein-Barr virus-induced 3 (Ebi3)] induces differentiation/expansion of neutrophils in lupus-prone mice. Clin Exp Immunol 186(2):144–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wei Y, Xiao H, Liu X, Zhang Y, Han G, Chen G, Hou C, Ma N, Shen B, Li Y, Egwuagu CE, Wang R. 2016c. A novel IL-23p19/Ebi3 (IL-39) cytokine mediates inflammation in Lupus-like mice. Eur J Immunol 46(6):1343–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YQ, Cao WJ, Gao YF, Ye J, Zou GZ. 2018. Serum interleukin-34 level can be an indicator of liver fibrosis in patients with chronic hepatitis B virus infection. World J Gastroenterol 24(12):1312–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu JQ, Liu Z, Shen R, Zhang G, Xu J, Basu S, Feng Y, Bai XF. 2013. Tumor-derived IL-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis. J Immunol 190(5):2415–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MS, Taylor MD, O'Gorman MT, Balic A, Barr TA, Filbey K, Anderton SM, Maizels RM. 2010. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol 40(6):1682–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz S, Billmeier U, McHedlidze T, Blumberg RS, Neurath MF. 2011. Interleukin-35 mediates mucosal immune responses that protect against T-cell-dependent colitis. Gastroenterology 141(5):1875–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojno ED, Hunter CA. 2012. New directions in the basic and translational biology of interleukin-27. Trends Immunol 33(2):91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Shen H, Lu J. 2015. Elevated serum and synovial fluid levels of interleukin-37 in patients with rheumatoid arthritis: attenuated the production of inflammatory cytokines. Cytokine 76(2):553–557 [DOI] [PubMed] [Google Scholar]
- Xie HH, Shen H, Zhang L, Cui MY, Xia LP, Lu J. 2018. Elevated serum interleukin-34 level in patients with systemic lupus erythematosus is associated with disease activity. Sci Rep 8(1):3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, Saito H, Matsuda A. 2010. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol 185(10):5743–5750 [DOI] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. 2008. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28(5):639–650 [DOI] [PubMed] [Google Scholar]
- Ye L, Jiang B, Deng J, Du J, Xiong W, Guan Y, Wen Z, Huang K, Huang Z. 2015. IL-37 alleviates rheumatoid arthritis by suppressing IL-17 and IL-17-triggering cytokine production and limiting Th17 cell proliferation. J Immunol 194(11):5110–5119 [DOI] [PubMed] [Google Scholar]
- Yin D, Naji DH, Xia Y, Li S, Bai Y, Jiang G, Zhao Y, Wang X, Huang Y, Chen S, Fa J, Tan C, Zhou M, Zhou Y, Wang L, Liu Y, Chen F, Liu J, Chen Q, Tu X, Xu C, Wang QK. 2017. Genomic variant in IL-37 confers a significant risk of coronary artery disease. Sci Rep 7:42175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, Spolski R, Poe JC, Leonard WJ, Tedder TF. 2012. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 491(7423):264–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Liu J, Zhang R, Huang X, Sun T, Wu Y, Hambly BD, Bao S. 2017. IL-37 and 38 signalling in gestational diabetes. J Reprod Immunol 124:8–14 [DOI] [PubMed] [Google Scholar]
- Zahoor M, Westhrin M, Aass KR, Moen SH, Misund K, Psonka-Antonczyk KM, Giliberto M, Buene G, Sundan A, Waage A, Sponaas AM, Standal T. 2017. Hypoxia promotes IL-32 expression in myeloma cells, and high expression is associated with poor survival and bone loss. Blood Adv 1(27):2656–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Putheti P, Zhou Q, Liu Q, Gao W. 2008. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev 19(5–6):347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Pan G, Tang C, Li Z, Zheng D, Wei X, Wu Z. 2018. IL-34 inhibits acute rejection of rat liver transplantation by inducing kupffer cell M2 polarization. Transplantation 102(6):e265–e274 [DOI] [PubMed] [Google Scholar]
- Zhou RP, Wu XS, Xie YY, Dai BB, Hu W, Ge JF, Chen FH. 2016. Functions of interleukin-34 and its emerging association with rheumatoid arthritis. Immunology 149(4):362–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. 2012. The chemokine superfamily revisited. Immunity 36(5):705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]