Abstract
Galactites is a genus of flowering plants belonging to Asteraceae family. This genus is mainly represented by the Galactites elegans (All.) Nyman ex Soldano, the milky thistle, a plant of Mediterranean origin. Galactites elegans is consumed as a monofloral boar thistle honey. Chromatography separation of CHCl3 and n-BuOH extracts of aerial parts of G. elegans led to isolation of 18 pure compounds. Their structures were elucidated by 1D-and 2D-NMR spectroscopy and confirmed by mass spectrometry analysis. Sinapic aldehyde, abietin, chlorogenic acid, neochlorogenic acid, 8α-hydroxypinoresinol, 9α-hydroxypinoresinol, pinoresinol, 4-ketopinoresinol, nortrachelogenin, and erythro-guaiacylglycerol-β-O-4′-dihydroconiferyl alcohol were isolated from CHCl3 extract, while luteolin 4′-O-glucuronide, naringenin-7-O-neohesperidoside, kaempferol-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside, apigenin-7-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside, quercitrin, quercetin-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside, ciwujiatone, and nortrachelogenin-4,4′-di-O-β-D-glucopyranoside were obtained from n-BuOH extract. The majority of isolated compounds displayed a significant antioxidant potential in vitro test (DPPH). The ability of compounds to reduce the level of peroxides in control and BHP-treated Jurkat cells was studied. The lignan derivatives were also able to reduce at 50 μM the basal level of peroxides in Jurkat cells as well as counteract peroxide increase induced by BHP treatment. Particularly 8α-hydroxypinoresinol was the most active showing 70% of peroxide level inhibition.
1. Introduction
Galactites is a genus of flowering plants belonging to Asteraceae Compositae (commonly referred to as the aster, daisy, composite, or sunflower family) which is a very large and widespread family of flowering plants (Angiospermae). Many members belonging to this family are herbaceous, but a significant number are also shrubs, vines, or trees. The family has a worldwide distribution most commonly in the arid and semiarid regions of subtropical and lower temperate latitudes [1]. Asteraceae is an economically important family, providing products such as cooking oils, lettuce, sunflower seeds, artichokes, sweetening agents, coffee substitutes, and herbal teas. Plants in Asteraceae are medically important in areas that do not have access to Western medicine. They are also commonly featured in medical and phytochemical journals because the sesquiterpene lactone compounds contained within them are an important cause of allergic contact dermatitis [2].
This genus is mainly represented by the Galactites elegans (All.) Nyman ex Soldano, the milky thistle, a plant of Mediterranean origin (synonym: Galactites tomentosa Moench; common name: Scarlina). Galactites elegans is consumed as a monofloral boar thistle honey. This plant prefers sunny places and usually grows on the uncultivated or barren grounds, waste places, well-drained soils, pastures, and roadsides [3–5].
In our systematic search for polyphenolic constituents from Algerian plants, we have investigated the aerial parts of Galactites elegans and report herein isolation and structural elucidation of 18 compounds and their antioxidant activities.
2. Material and Methods
2.1. Chemicals and Reagents
Anhydrous sodium carbonate, Folin-Ciocalteu's phenol reagent, and methanol (analytical reagent and HPLC gradient grade) were purchased from Merck (Darmstadt, Germany). Ethylenediaminetetraacetic acid (EDTA), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 3-(2-pyridyl)-5,6-bis(4-phenyl-sulphonic acid)-1,2,4-triazine (ferrozine), iron (II) chloride (FeCl2), gallic acid, 2,6-di-tert-butyl-4-methylphenol (BHT), butylated hydroxyanisole (BHA), and dimethylsulphoxide (DMSO) were purchased from Sigma-Aldrich GmbH (Taufkirchen, Germany). All other chemicals were analytical grade and obtained from either Sigma or Merck. RPMI-1640 medium was from BioWhittaker Lonza (NJ, USA). Fetal bovine serum (FBS) was from GIBCO (Life Technologies, Grand Island, NY, USA). 2′,7′-Dichlorofluorescein diacetate (DCFH-DA), tert-butyl hydroperoxide (BHP), and all the other chemicals were from Sigma-Aldrich (St. Louis, MO, USA)
2.2. General Experimental Procedures
Briefly optical rotations were measured on a Perkin-Elmer 241 polarimeter equipped with a sodium lamp (589 nm) and a 1 dm microcell. UV spectra were recorded on a Perkin-Elmer-Lambda spectrophotometer. NMR experiments were performed on a Bruker DRX-600 spectrometer at 300 K. HRESIMS were acquired in positive ion mode on a Q-TOF premier spectrometer equipped with a nanoelectrospray ion source (Waters-Milford, MA, USA). Column chromatography was performed over Sephadex LH-20 (Amersham Biosciences; Uppsala, Sweden). Silica gel 60 (0.040–0.063 mm; Carlo Erba; Milan, Italy) was used as column material. HPLC separation was conducted on a Shimadzu LC-8A series pumping system equipped with a Shimadzu RID-10A refractive index detector and Shimadzu injector on a C18μ-Bondapak column (30 cm x 7.8 mm, 10 μm Waters, flow rate 2.0 mL min−1). TLC was performed on precoated Kiesel gel 60 F254 plates (Merck; Darmstadt, Germany); compounds were detected by Ce(SO4)2/H2SO4 (Sigma-Aldrich, Milan, Italy) solution; and reagent grade chemicals (Carlo Erba; Milan, Italy) were used throughout [6, 7].
2.3. Plant Material
The aerial parts of Galactites elegans, voucher specimen (Gae alg0312-2012), were collected in the end of March 2013 (flowering stage) in Hamma Bouziane, Constantine, Algeria. Fresh aerial parts were dried to constant weight at room temperature.
2.4. Extraction and Isolation
Dried and powdered aerial parts of G. elegans (966 g) were macerated with MeOH-H2O (8:2) at room temperature. The operation repeated 3 times. The hydromethanolic extract was concentrated to dryness (under low pressure). The residue was suspended in H2O and successively partitioned with petroleum ether for 1 time then CHCl3, EtOAc, and n-BuOH (3 mL ×300 mL, each), respectively, affording a CHCl3 soluble fraction (2 g), an EtOAc-soluble fraction (5.5 g), and a n-BuOH soluble fraction (19 g).
A part of butanolic extract (2.79 g) was submitted to chromatographic separation on a Sephadex LH-20 column, using MeOH as mobile phase; fractions were collected, analyzed by TLC on silica 60 F254 gel-coated glass sheets using CHCl3:MeOH:H2O (80:18:2, v/v/v) and n-BuOH–AcOH–H2O (60:15:25, v/v/v) as eluent, and grouped to obtain 26 fractions.
The compound luteolin 4′-O-glucuronide [8] (7.3 mg) was obtained directly from the fraction 22. Fraction 6 was chromatographed using RP18 HPLC with MeOH/H2O (42:58, v/v) as mobile phase (flow rate 2.0 mL min−1) to yield pure compound nortrachelogenin 4,4′-di-O-ß-D-glucopyranoside (2) [9] (1.1 mg, tR 36 min). Fraction 8 was isolated using RP18 HPLC with MeOH/H2O (37:63, v/v) as mobile phase (flow rate 2.0 mL min−1) to yield pure compounds chlorogenic acid [10] (8.1 mg, tR 8 min), neochlorogenic acid [11] (3.0 mg, tR 9 min), naringenin-7-O-neohesperidoside [12] (19.2 mg, tR 27 min), quercetin-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside [13] (3.0 mg, tR 52 min), apigenin-7-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside [14] (1.4 mg, tR 62 min), and Kaempferol-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside [13] (1.9 mg, tR 82 min). Fraction 9 was separated using RP18 HPLC with MeOH/H2O (35:65, v/v) as mobile phase (flow rate 2.0 mL min−1) to yield pure compound quercitrin [15] (24.7 mg, tR 23 min). Fraction 14 was chromatographed using RP18 HPLC with MeOH/H2O (2:3, v/v) as mobile phase (flow rate 2.0 mL min−1) to yield pure compound quercitrin [15] (5.7 mg, tR 34 min).
A part of CHCl3 extract (1.87 g) was fractionated by column chromatography (CC) of Silica gel eluted with CHCl3 followed by increasing concentrations of MeOH in CHCl3 (between 1% and 100%), fractions were collected and monitored by TLC to obtain 20 fractions. Fraction 4 was chromatographed using RP18 HPLC with MeOH/H2O (2:3, v/v) as mobile phase (flow rate 2.0 mL min−1) to yield pure compounds ciwujiatone [16] (0.7 mg, tR 10 min), 4-ketopinoresinol [17] (3) (1.9 mg, tR 40 min), pinoresinol [18] (1.2 mg, tR 48 min), and nortrachelogenin [19] (1) (2.1 mg, tR 57 min). Fraction 10 was chromatographed using RP18 HPLC with MeOH/H2O (35:65, v/v) as mobile phase (flow rate 2.0 mL min−1) to yield pure compound 8α-hydroxypinoresinol [20] (4) (1.8 mg, tR 10 min). The Fraction 11 was separated using RP18 HPLC with MeOH/H2O (2:3, v/v) as mobile phase (flow rate 2.0 mL min−1) to yield pure compound 9α-hydroxypinoresinol [21] G4 (2.2 mg, tR 38 min). Fraction 12 was chromatographed using RP18 HPLC with MeOH/H2O (35:65, v/v) as mobile phase (flow rate 2.0 mL min−1) to yield pure compounds sinapic aldehyde [22] (0.7 mg, tR 16 min) and abietin [23] (1.1 mg, tR 17 min). Fraction 15 was chromatographed using RP18 HPLC with MeOH/H2O (7:18, v/v) as mobile phase (flow rate 2.0 mL min−1) to yield pure compound erythro-guaiacylglycerol-β-O-4′-dihydroconiferyl alcohol [24] (1.3 mg, tR 64 min).
The structure of each compound was determined by NMR (see Figures S1-S11 in the Supplementary Material for the 1H NMR spectra of the tested lignans and glycosides).
2.5. Antioxidant Activity
2.5.1. Determination of Total Phenolic Contents
Total phenolic contents of the samples were analyzed using the Folin-Ciocalteu reagent according to the method of Milella [25] using gallic acid as standard, with some modifications [26]. The fraction solutions were mixed with 0.2 mL of 50% Folin-Ciocalteu reagent and allowed to react for 3 min and 1 mL aqueous solution of 2% Na2CO3 was added. At the end of incubation for 45 min at room temperature, absorbance of each mixture was measured at 760 nm. The same procedure was also applied to the standard solutions of gallic acid. Total phenolic contents were expressed as μg gallic acid equivalents per mg of the fractions.
2.6. DPPH Radical Scavenging Assay
Radical scavenging activity was determined by a spectrophotometric method based on the reduction of a methanol solution of DPPH using the method of Blois [27]. The sample solutions were added to 0.004% methanol solution of DPPH. The mixture was shaken vigorously and left to stand at room temperature for 30 min in the dark. The absorbance was measured at 517 nm against a blank by a spectrophotometer (Rayleigh, UV-2601). Scavenging of DPPH radical was calculated according to formula:
| (1) |
where Acontrol is the absorbance of the control reaction (containing all reagents except the test compound) and Asample is the absorbance of the test compound. DPPH scavenging activity was expressed as IC50 values (μg/mL) for comparison. The IC50 value of each sample was defined as the concentration of sample required for a 50% decrease in absorbance of the blank. BHT and BHA were used as positive controls.
2.7. Metal Chelating Activity on Ferrous Ions (Fe2+)
Metal chelating activity was determined according to the method of Decker and Welch [28], with some modifications [29, 30]. Briefly, 0.5 mL of the samples was mixed with 0.05 mL 2 mM FeCl2 and 0.1 mL 5 mM ferrozine. The mixture was diluted with methanol (2 mL) and left standing at room temperature for 10 minutes. The absorbance of the solution was measured spectrophotometrically at 562 nm. EDTA was used as a positive control.
2.8. Cell Cultures
Jurkat cells (a T-cell leukemia cell line obtained from Cell Bank in GMP-IST, Genova, Italy) were maintained in RPMI 1640 medium supplemented with 10% (v/v) FBS, 2 mM L-glutamine, and antibiotics at 37°C in humidified atmosphere with 5% CO2. To ensure logarithmic growth, cells were subcultured every three days. Stock solutions (50 mM) of polyphenolic compounds in DMSO were stored at -20°C and appropriately diluted in the same solvent or directly in the medium just before use (DMSO never exceeding 0,5%).
2.9. Peroxide Depletion Activity of Test Compounds by Cytofluorometry
The evaluation of intracellular peroxides concentration was performed according to Rothe [30] with some modifications. In detail, Jurkat cells were collected by centrifugation and suspended in RPMI containing 5% FBS at a density of 5 x 105 cells/mL. FBS concentration was lowered to 2% to increase the uptake rate of flavonoids and reagents in the short-incubation time (1 hr) chosen for the assay. Cell suspensions were incubated with increasing concentrations of each chemical or vehicle only at 37°C. For each sample duplicate test tubes were prepared. After 30 min of incubation, BHP (550 μM final concentration) or an equal volume of vehicle was added. This allowed us to monitor the effect of flavonoids on BHP-induced peroxide elevation or the basal levels of peroxides, respectively. In the last 15 min of incubation cells were loaded with DCFH-DA (8 μM final concentration). Test tubes were gentle mixed several times along the incubation period (1 hr). Cells suspension were then washed and resuspended in an equal volume of medium and 10,000 events were analyzed for DCF-fluorescence by cytofluorometry (BD FACSCalibur™ instrument, Becton Dickinson, San Jose, CA, USA). DCF green fluorescence was analyzed in the FL1 channel (λexc 488 nm; (λem 535 nm). Before the cytofluorometric analysis an aliquot of each sample was withdrawn to evaluate cell viability by Trypan-blue exclusion test.
2.10. Statistical Analysis
All experiments were performed in triplicate and the results were expressed as mean ± SD. Statistical analyses were performed using the SPSS 11.5 (SPSS, Chicago, IL). For DPPH activity, differences among means were done by analysis of variance (ANOVA), and averages were compared using the Duncan test. For other tests, differences between treatment groups were analyzed by the student test. Differences were considered significant when P <0.05.
3. Results and Discussion
3.1. Antioxidant Activities of Extracts and Components from Galactites elegans
3.1.1. Total Phenolic Content
Phenolic compounds are characterized by having at least one aromatic ring with one or more hydroxyl groups attached which directly contribute to the antioxidant properties [31]. Therefore, it is important to evaluate the total phenolic in the extracts from Galactites elegans. The contents of total phenolic compounds in the extracts, expressed as μg gallic acid equivalents per milligram of dry extract, are shown in Table 1.
Table 1.
Antioxidant activities of the extracts from Galactites elegans1.
| Material | DPPH | Total phenolic content (µgGAE/mg of material) | Metal chelating activity |
|---|---|---|---|
| IC50 (µg/mL) | (%) | ||
| Ex. CHCl3 | 41.2 ± 1.3d | 116.5 ± 0.7b | NA2 |
| Ex. n-Butanol | 52.1 ± 1.1c | 94.4 ± 0.6a | 38.5 ± 1.4b |
| BHT | 22.3 ± 0.8b | NS3 | NS3 |
| BHA | 19.1 ± 0.4a | NS3 | NS3 |
| EDTA | NS3 | NS3 | 93.7 ± 0.3a |
1Values represent averages ± standard deviations for triplicate experiments. Values in the same column with different superscripts are significantly (p < 0.05) different. 2Not active. 3Not studied.
3.1.2. DPPH Radical Scavenging Activity
DPPH radical scavenging activities of the extracts and the reference synthetic agents are given in Table 1. According to these IC50 values, the DPPH radical scavenging abilities among the different extracts were in the order of CHCl3> n-BuOH (P < 0.05). Furthermore, pure compounds radical scavenging abilities is reported in Table 2. Lower IC50 value indicates higher free radical scavenging activity.
Table 2.
IC50 values of polyphenols tested against the DPPH radical.
| Compound | IC50 (μM)1 |
|---|---|
| BHT (positive control) | 98.8 ± 4.5 |
| BHA (positive control) | 105.4 ± 5.3 |
| 4-ketopinoresinol (3) | 143.3 ± 13.1 |
| 8α-hydroxypinoresinol (4) | 71.5± 5.9 |
| 9α-hydroxypinoresinol | 84.0± 2.9 |
| Abietin | >500 |
| apigenin-7-O-a-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside (isorhoifolin) | >500 |
| chlorogenic acid | 59.8 ± 4.9 |
| Ciwujiatone | 64.7 ± 5.3 |
| erythro-guaiacylglycerol-β-O-4′-dihydroconiferyl alcohol | >500 |
| kaempferol-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside (biorobin) | 39.4 ± 2.8 |
| luteolin 4′-O-glucuronide | 38.9 ± 2.5 |
| naringenin-7-O-neohesperidoside | 116.0 ± 9.7 |
| neochlorogenic acid | 65.8 ± 4.8 |
| nortrachelogenin (1) | 38.6 ± 2.7 |
| nortrachelogenin-4,4′-di-O-β-D-glucopyranoside (2) | 34.4 ± 2.3 |
| pinoresinol | 50.8 ± 3.1 |
| quercetin-3-O-α-l-rhamnopyranosyl-(1→6)-β-D-glucopyranoside (rutin) | 63.0 ± 4.6 |
| quercitrin | 12.2 ± 1.0 |
| sinapic aldehyde | 53.4 ± 3.8 |
1Values represent averages ± standard deviations for triplicate experiments.
3.1.3. Metal Chelating Activity
Iron ions catalyse the conversion of less reactive species such as H2O2 or lipid peroxides into more reactive ones such as hydroxyl or peroxyl/alkoxyl radicals. Therefore, extracts with iron chelating ability can act as powerful antioxidants [32]. The metal chelating ability of the extracts was investigated by ferrozine assay. The chelating potential of n-BuOH extract was determined as 38.5 ± 1.4 % that was significantly lower (p< 0.01) than synthetic chelating agent EDTA (93.7 ± 0.3 %) at the concentration of 2 mg/mL. On the other hand, chloroformic extract was not effective at the tested concentration.
3.2. Antioxidant Potential of Test Compounds by Cytofluorometry
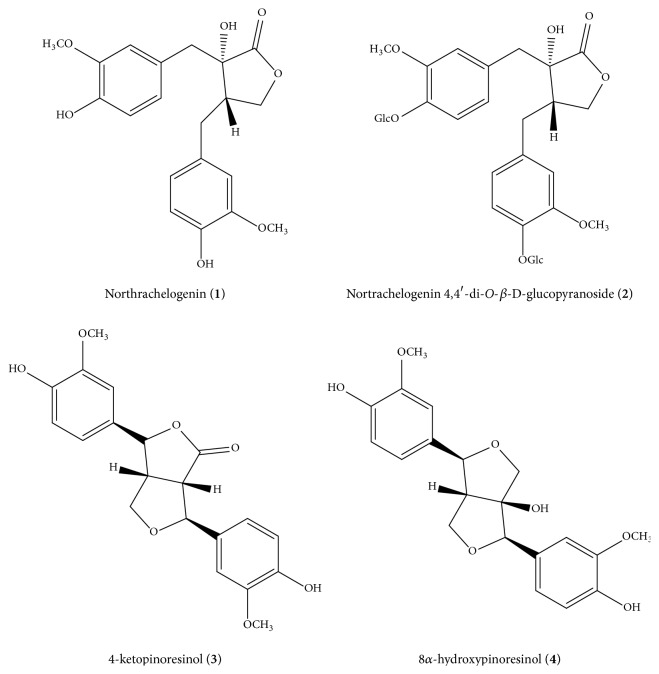
This study was carried out in order to discover natural compounds which could be used as active ingredients to improve the health and/or physical appearance of the skin or as preservatives or stabilizers for other active ingredients or vehicles in topical formulations. As reported in a recent work of Korte [33], natural products which show this kind of bioactivity are lignans and lignan esters. For these reasons, among all the tested molecules, pinoresinol and the northrachelogenin derivatives have been selected (Figure 1; see Figures S1-S4 in the Supplementary Material for the 1H NMR spectra of compounds).
Figure 1.
Structures of the tested lignans.
Particularly, the in cell antioxidant potential of nortrachelogenin (1), nortrachelogenin-4,4′-di-O-β-D-glucopyranoside (2), and 8α-hydroxypinoresinol (4) have been investigated.
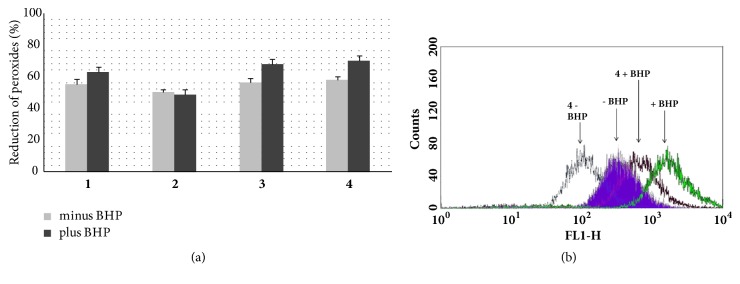
4-ketopinoresinol (3) was used as positive control as reported in Chen [17], however pinoresinol was not tested because of its high cytotoxicity, instead 9 hydropinoresinol was not active [21]. The ability of test compounds to reduce the level of peroxides in control and BHP-treated Jurkat cells was measured by cytofluorometry. Each chemical was tested at 25 μM and 50 μM concentrations, being the latter the maximum dose not cytotoxic. They were able to reduce the basal level of peroxides in Jurkat cells as well as counteract peroxide increase induced by BHP treatment. The slight lower antioxidant potential of nortrachelogenin 4,4′-di-O-ß-D-glucopyranoside (2) could be ascribed to the presence of a carbohydrate moiety which, if on one hand could slight contribute to the antioxidant activity and on the other hand might reduce nortrachelogenin 4,4′-di-O-ß-D-glucopyranoside availability for Jurkat cells. In addition, the sugar component seems to be responsible for the higher cytotoxic potential of nortrachelogenin 4,4′-di-O-ß-D-glucopyranoside, possibly due to perturbation of plasma membrane (Figure 2).
Figure 2.
In-cells antioxidant potential of pinoresinol and the northrachelogenin derivatives. (a) Unstimulated (white bars) and BHP-stimulated (black bars) Jurkat cells were incubated with each tested compounds (50 μM) or vehicle only. Cellular concentrations of peroxides (DCF fluorescence) were measured by cytofluorometry. Data shown were obtained using the mean fluorescence values and are the mean values ± SD of at least three experiments performed in duplicate. P values were always <0.01. (b) Representative histograms obtained with 8α-hydroxypinoresinol (4).
4. Conclusion
All the isolates (except chlorogenic acid) were first reported from the genus Galactites and the majority of isolated phenolic components displayed a significant antioxidant potential in vitro assay (DPPH). The lignan derivatives were also able to reduce at 50 μM the basal level of peroxides in Jurkat cells as well as counteract peroxide increase induced by BHP treatment.
Particularly, 8α-hydroxypinoresinol (4) was the most active compound showing 70% of peroxide level inhibition. The slight lower antioxidant potential of nortrachelogenin 4,4′-di-O-β-D-glucopyranoside (2) (45% of peroxide level inhibition) could be ascribed to the presence of a carbohydrate moiety.
This result suggests that active fractions could be used as a source of antioxidant agent for pharmaceutical and cosmetic preparations.
Acknowledgments
This work was supported financially by University of Basilicata (Local Interest Funds 2015), Potenza, Italy.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
All authors declare that there are no conflicts of interest regarding the publication of this article.
Authors' Contributions
Omar Tebboub and Roberta Cotugno are equal contributing authors.
Supplementary Materials
Figure S1: 1H NMR spectrum of compound 1 (CD3OD, 600 MHz). Figure S2: 1H NMR spectrum of compound 2 (CD3OD, 600 MHz). Figure S3: 1H NMR spectrum of compound 3 (CD3OD, 600 MHz). Figure S4: 1H NMR spectrum of compound 4 (CD3OD, 600 MHz). Figure S5: 1H NMR spectrum of abietin (CD3OD, 600 MHz). Figure S6: 1H NMR spectrum of luteolin 4′-O-glucuronide (CD3OD, 600 MHz). Figure S7: 1H NMR spectrum of naringenin-7-O-neohesperidoside (CD3OD, 600 MHz). Figure S8: 1H NMR spectrum of kaempferol-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside (CD3OD, 600 MHz). Figure S9: 1H NMR spectrum of apigenin-7-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside (CD3OD, 600 MHz). Figure S10: 1H NMR spectrum of quercitrin (CD3OD, 600 MHz). Figure S11: 1H NMR spectrum of quercetin-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside (CD3OD, 600 MHz).
References
- 1.Barkley T., Brouillet L., Strother J. Flora of North America, Asteraceae, part 1. New York, Ny, USA: Oxford University Press; 2006. [Google Scholar]
- 2.Heinrich M., Robles M., West J. E., Ortiz De Montellano B. R., Rodriguez E. Ethnopharmacology of Mexican asteraceae (compositae) Annual Review of Pharmacology and Toxicology. 1998;38:539–565. doi: 10.1146/annurev.pharmtox.38.1.539. [DOI] [PubMed] [Google Scholar]
- 3.Moench C. Methodus plantas horti botanici et agri Marburgensis . Marburgi Cattorum: in officina nova libraria academiae; 1794. [DOI] [Google Scholar]
- 4.Pignatti S. Note critiche sulla flora d'Italia. VII. Supplemento. Giornale Botanico Italiano. 2009;116(1-2):93–95. doi: 10.1080/11263508209428041. [DOI] [Google Scholar]
- 5.Jerković I., Roje M., Tuberoso C. I. G., et al. Bioorganic research of galactites tomentosa moench. Honey extracts: Enantiomeric purity of chiral marker 3-phenyllactic acid. Chirality. 2014;26(8):405–410. doi: 10.1002/chir.22340. [DOI] [PubMed] [Google Scholar]
- 6.Milella L., Milazzo S., De Leo M., et al. α-Glucosidase and α-Amylase Inhibitors from Arcytophyllum thymifolium. Journal of Natural Products. 2016;79(8):2104–2112. doi: 10.1021/acs.jnatprod.6b00484. [DOI] [PubMed] [Google Scholar]
- 7.Gualtieri M. J., Malafronte N., Vassallo A., et al. Bioactive limonoids from the leaves of Azaridachta indica (Neem) Journal of Natural Products. 2014;77(3):596–602. doi: 10.1021/np400863d. [DOI] [PubMed] [Google Scholar]
- 8.Sayed H. M., Mohamed M. H., Farag S. F., Mohamed G. A., Omobuwajo O. R. M., Proksch P. Fructose-amino acid conjugate and other constituents from Cyperus rotundus L. Natural Product Research (Formerly Natural Product Letters) 2008;22(17):1487–1497. doi: 10.1080/14786410802038556. [DOI] [PubMed] [Google Scholar]
- 9.Liu X.-T., Wang Z.-X., Yang Y., et al. Active components with inhibitory activities on IFN-γ/STAT1 and IL-6/STAT3 signaling pathways from Caulis Trachelospermi. Molecules. 2014;19(8):11560–11571. doi: 10.3390/molecules190811560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Q., Zhang Y.-T., Yang C.-R., Xu M. Phenolic antioxidants from green tea produced from camellia crassicolumna var. multiplex. Journal of Agricultural and Food Chemistry. 2009;57(2):586–590. doi: 10.1021/jf802974m. [DOI] [PubMed] [Google Scholar]
- 11.Aversano R., Contaldi F., Adelfi M. G., et al. Comparative metabolite and genome analysis of tuber-bearing potato species. Phytochemistry. 2017;137:42–51. doi: 10.1016/j.phytochem.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Brito A., Ramirez J. E., Areche C., Sepúlveda B., Simirgiotis M. J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules. 2014;19(11):17400–17421. doi: 10.3390/molecules191117400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han J.-T., Bang M.-H., Chun O.-K., Kim D.-O., Lee C.-Y., Baek N.-I. Flavonol glycosides from the aerial parts of Aceriphyllum rossii and their antioxidant activities. Archives of Pharmacal Research. 2004;27(4):390–395. doi: 10.1007/BF02980079. [DOI] [PubMed] [Google Scholar]
- 14.Tao L., Huang J., Zhao Y., Li C. Chemical constituents in Buddleja albiflora. Zhongguo Zhongyao Zazhi. 2009;34(23):3043–3046. [PubMed] [Google Scholar]
- 15.Dai X., Ding Y., Zhang Z., Cai X., Li Y. Quercetin and quercitrin protect against cytokine-induced injuries in RINm5F β-cells via the mitochondrial pathway and NF-κB signaling. International Journal of Molecular Medicine. 2013;31(1):265–271. doi: 10.3892/ijmm.2012.1177. [DOI] [PubMed] [Google Scholar]
- 16.Deng Y., Chin Y.-W., Chai H., Keller W. J., Kinghorn A. D. Anthraquinones with quinone reductase-inducing activity and benzophenones from Morinda citrifolia (Noni) roots. Journal of Natural Products. 2007;70(12):2049–2052. doi: 10.1021/np070501z. [DOI] [PubMed] [Google Scholar]
- 17.Chen H.-H., Chen Y.-T., Huang Y.-W., Tsai H.-J., Kuo C.-C. 4-Ketopinoresinol, a novel naturally occurring ARE activator, induces the Nrf2/HO-1 axis and protects against oxidative stress-induced cell injury via activation of PI3K/AKT signaling. Free Radical Biology & Medicine. 2012;52(6):1054–1066. doi: 10.1016/j.freeradbiomed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y., Wang W., Tang L., et al. Lignan and flavonoid glycosides from Urtica laetevirens Maxim. Journal of Natural Medicines. 2009;63(1):100–101. doi: 10.1007/s11418-008-0274-8. [DOI] [PubMed] [Google Scholar]
- 19.Eklund P. C., Långvik O. K., Wärnå J. P., Salmi T. O., Willför S. M., Sjöholm R. E. Chemical studies on antioxidant mechanisms and free radical scavenging properties of lignans. Organic & Biomolecular Chemistry. 2005;3(18):3336–3347. doi: 10.1039/b506739a. [DOI] [PubMed] [Google Scholar]
- 20.Wangteeraprasert R., Lipipun V., Gunaratnam M., Neidle S., Gibbons S., Likhitwitayawuid K. Bioactive compounds from Carissa spinarum. Phytotherapy Research. 2012;26(10):1496–1499. doi: 10.1002/ptr.4607. [DOI] [PubMed] [Google Scholar]
- 21.Steffan B., Wätjen W., Michels G., et al. Polyphenols from plants used in traditional Indonesian medicine (Jamu): Uptake and antioxidative effects in rat H4IIE hepatoma cells. Journal of Pharmacy and Pharmacology. 2005;57(2):233–240. doi: 10.1211/0022357055317. [DOI] [PubMed] [Google Scholar]
- 22.Potapovich M. V., Kurchenko V. P., Metelitza D. I., Shadyro O. I. Antioxidant activity of oxygen-containing aromatic compounds. Applied Biochemistry and Microbiology. 2011;47(4):346–355. doi: 10.1134/S0003683811040144. [DOI] [PubMed] [Google Scholar]
- 23.Gao D.-F., Zhang Y.-J., Yang C.-R., Chen K.-K., Jiang H.-J. Phenolic antioxidants from green tea produced from Camellia taliensis. Journal of Agricultural and Food Chemistry. 2008;56(16):7517–7521. doi: 10.1021/jf800878m. [DOI] [PubMed] [Google Scholar]
- 24.Li L., Seeram N. P. Maple syrup phytochemicals include lignans, coumarins, a stilbene, and other previously unreported antioxidant phenolic compounds. Journal of Agricultural and Food Chemistry. 2010;58(22):11673–11679. doi: 10.1021/jf1033398. [DOI] [PubMed] [Google Scholar]
- 25.Milella L., Bader A., De Tommasi N., Russo D., Braca A. Antioxidant and free radical-scavenging activity of constituents from two Scorzonera species. Food Chemistry. 2014;160:298–304. doi: 10.1016/j.foodchem.2014.03.097. [DOI] [PubMed] [Google Scholar]
- 26.Yegenoglu H., Aslim B., Oke F. Comparison of antioxidant capacities of ganoderma lucidum (curtis) P. Karst and funalia trogii (Berk.) bondartsev & singer by using different in vitro methods. Journal of Medicinal Food. 2011;14(5):512–516. doi: 10.1089/jmf.2010.0144. [DOI] [PubMed] [Google Scholar]
- 27.Blois M. S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 28.Decker E. A., Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. Journal of Agricultural and Food Chemistry. 1990;38(3):674–677. doi: 10.1021/jf00093a019. [DOI] [Google Scholar]
- 29.Oke F., Aslim B. Biological potentials and cytotoxicity of various extracts from endemic Origanum minutiflorum O. Schwarz & PH Davis. Food and Chemical Toxicology. 2010;48(6):1728–1733. doi: 10.1016/j.fct.2010.03.053. [DOI] [PubMed] [Google Scholar]
- 30.Rothe G., Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2',7'-dichlorofluorescin. Journal of Leukocyte Biology. 1990;47(5):440–448. doi: 10.1002/jlb.47.5.440. [DOI] [PubMed] [Google Scholar]
- 31.Cartea M. E., Francisco M., Soengas P., Velasco P. Phenolic compounds in Brassica vegetables. Molecules. 2011;16(1):251–280. doi: 10.3390/molecules16010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halliwell B. The wanderings of a free radical. Free Radical Biology & Medicine. 2009;46(5):531–542. doi: 10.1016/j.freeradbiomed.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Korte H., Lehtola V.-M., Unkila M., Hiilovaara-Teijo M., Ahotupa M. Lignan formulations. Google Patents; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: 1H NMR spectrum of compound 1 (CD3OD, 600 MHz). Figure S2: 1H NMR spectrum of compound 2 (CD3OD, 600 MHz). Figure S3: 1H NMR spectrum of compound 3 (CD3OD, 600 MHz). Figure S4: 1H NMR spectrum of compound 4 (CD3OD, 600 MHz). Figure S5: 1H NMR spectrum of abietin (CD3OD, 600 MHz). Figure S6: 1H NMR spectrum of luteolin 4′-O-glucuronide (CD3OD, 600 MHz). Figure S7: 1H NMR spectrum of naringenin-7-O-neohesperidoside (CD3OD, 600 MHz). Figure S8: 1H NMR spectrum of kaempferol-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside (CD3OD, 600 MHz). Figure S9: 1H NMR spectrum of apigenin-7-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside (CD3OD, 600 MHz). Figure S10: 1H NMR spectrum of quercitrin (CD3OD, 600 MHz). Figure S11: 1H NMR spectrum of quercetin-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside (CD3OD, 600 MHz).
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.