Abstract
Schinus molle L. is used to treat various diseases; however, the literature lacks information regarding its possible immunotoxic effects. The aim of the study was to investigate the immunotoxic effects of essential oil from leaves of Schinus molle L. in cultures of human lymphocytes and macrophages. The cultures were treated with essential oil (EO) of Schinus molle L. and subsequently subjected to genotoxic analysis (comet assay), mutagenic analysis (micronucleus frequency and chromosomal aberration), and cytotoxic (cell viability) and functional parameters (interleukins secretions). Our analyses have determined that the essential oil from leaves of Schinus molle L. presents several compounds with α-pinene being the major compound; in addition, the compound verbenene was firstly identified; genotoxic effects were detected only in macrophages and only at the two highest concentrations tested. An important finding is that Schinus molle L. oil causes an activation of the immune system. This action has its mechanism centered by the cascade nitric oxide-interleukin-10-tumor necrosis factor alpha.
1. Introduction
Natural products have been used by humanity since time immemorial [1], and the knowledge of medicinal plants is, in most cases, the only therapeutic resource in many communities and various ethnic groups [2]. Thus, these products can be found as much in the poorest regions of Brazil and in large cities in the country, where they are sold in street markets, popular markets, and are even grown in residential yards [2].
Given this situation, Schinus molle L. has been used as a treatment option for the Brazilian population, which relates its use to various activities, such as antiviral and antibacterial usages, treatment of respiratory and urinary infections, and rheumatism alleviation and as an antidepressant [3]. Despite its widespread use in folk medicine, there are no studies in the literature reporting the possible toxic effects of its essential oil (EO).
Reduced resistance to infectious disease was a well-documented consequence of primary and acquired immunodeficiencies, but a novel finding following xenobiotic exposure. The awareness of the consequences of altered immune function is the most likely outcome of inadvertent exposure. The human health implications of studies in which chemical exposure reduced resistance to infection drove an early focus on immunosuppression within the toxicology community [4].
Thus, the importance of this work, which aimed to investigate the possible immunotoxic effects of the plant in question, is evident to corroborate the literature, which lacks such information. To the best of our knowledge, this is the first work with this species to address aspects of cellular, genetic, and functional damage in these cell types.
2. Materials and Methods
2.1. Botanical Identification of Schinus molle L.
The material was attained from a collection of specimen. A voucher specimen was sent to the UFSM Herbarium (Federal University of Santa Maria), identified by the botanical herbarium, and cataloged under the number SMDB-13507.
2.2. Essential Oil Extraction of Schinus molle L.
The plant material of choice was fresh leaves collected inside the city of Uruguaiana in Rio Grande do Sul state in June 2015. For the extraction, sheets of 30 g were used, which were triturated and subjected to hydrodistillation in a Clevenger apparatus for eight hours. The yield was calculated considering the weight/weight of oil.
2.3. Determination of Concentrations
Due to a lack of studies on the plant, doses were chosen to allow a broad-spectrum evaluation, which enabled the determination of a median lethal dose (LD50) [5]. Therefore, concentrations of 100 μg/mL, 10 μg/mL, 1 μg/mL, 0.1 μg/mL, and 0.01 μg/mL were initially tested in cultures of lymphocytes and macrophages, and after analysis of cell proliferation, the LD50 was determined for the various test cells. The LD50 was determined by the statistical method of nonlinear regression for the different cultures grown.
2.4. Photochemical Composition
The essential oil was subjected to separation by gas chromatography with detection by mass spectrometry (CG-MS). The analysis was performed on a model 6890N chromatograph coupled with a model 5975B mass detector, both from Agilent Technologies. Chromatographic conditions were as follows: initial oven temperature was 50°C for one minute, followed by heating at 50°C/min to 300°C, keeping this temperature for 9 minutes, for a total of 60 minutes. Separation was achieved on a DB-5MS column (30 m x 320 μm x 0.25 μm) at a constant rate of 1.5 mL/min of helium; detection was performed in quadrupole equipment using ionizing electrons [6]. Identification of components was done based on the retention index (RI), determined by the homologous series of n-alkanes, C7-C30 under similar experimental conditions, compared with the search mass spectra libraries (NIST and WILEY) and mass spectra data in the literature [7].
2.5. Lymphocyte Culture
The lymphocyte cultures were prepared using 0.5 ml of venous blood (collected by voluntary venipuncture) and immediately transferred to a culture medium containing 10 ml of RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 1% streptomycin-penicillin, as described in previous work by our group [5, 8, 9] and approved by the Research Ethics Committee of the Federal University of Pampa (n°. 27045614.0.0000.5323). The cells were placed in a greenhouse at 37°C in a 5% CO2 environment for 72 hours.
2.6. Macrophage Culture
The isolation of leukocytes from whole blood was carried out by centrifugation, and the density difference between the leukocyte cells was determined using centrifugation methods and tack plastic/glass monocytes, as described in previous studies [10–12]. Cells of interest were obtained, and the activation of monocytes occurred using lipopolysaccharide (LPS) 1 μg/mL in RPMI 1640 supplemented with 1% penicillin-streptomycin and 25% FBS at 37 ± 1°C and atmosphere containing 5% CO2 and 95% humidity for 2 hours. This exposure resulted in the formation of dendritic cells derived from monocytes to be used in the experiment [13, 14].
2.7. Treatments of Cultures
All cultures received the addition of the test compound at a final volume of 100 µL. The groups to be tested were the negative control (NC) phosphate buffer pH 7.4 and the genotoxic positive control with bleomycin 3 μg/ml. Schinus molle L. EO at five different concentrations, ranging from their respective LD50 to LD50/10000, was used for each of the tested cells. For all protocols of the experiment, cultures were analyzed in triplicate (n = 3).
2.8. Cytotoxicity of Schinus molle L. Oil
The analyzed parameter for evaluation of cytotoxicity was cell viability through the loss of membrane integrity using the trypan blue method [15]. This requires a sample homogenate rate in contact with the trypan blue dye, which stains the viable cells. The analysis was performed using a microscope at 400x. One hundred cells were counted.
2.9. Genotoxicity of Schinus molle L. Oil
The genotoxicity was evaluated using a comet assay as described by Singh [16]. For this, previously prepared slides covered with high-melting-point agarose with a sample outlet were homogenized with low-melting-point agarose and after being dried were disposed in a vat containing lysing solution for one week. After this process, the slides were subjected to 20 min, 300 mA, 25 V electrophoresis in 300 mM NaOH buffer and pH>13. Held in electrophoresis, the slides were neutralized and dried at room temperature. After drying, the slides were fixed, dried again, rehydrated, stained with silver nitrate solution, and dried at room temperature. The DNA damage was calculated from the cells with different classifications of damage; the damage index ranges from 0 (100 cells x 0, when there was no damage) to 400 (100 cells x 4, when the maximum damage occurred).
2.10. Mutagenicity of Schinus molle L. Oil
The micronucleus test was the parameter used to evaluate mutagenicity. For this, the cultures were used, and the method described above was performed according to the technique described by Schmid [17] and presented as an index as described by Fenech [18]. Besides this, a chromosomal instability test was also carried out. The chromosomal instability was evaluated by the cytogenetics for Band G, a technique described by Yunis [19], which requires the addition of 10 μL/mL colchicine to each properly treated cell culture. The addition was followed by incubation for 1 hour at 37°C. After incubation, the samples were centrifuged for 10 minutes at 1800 rpm. After centrifugation, the cell pellet was suspended in hypotonic KCl solution and incubated at 37°C for 16 minutes. Another centrifugation was conducted, and the pellet was suspended in acetic acid/methanol (1:3) and again subjected to successive centrifugations. Finally, the pellets obtained were placed onto precleaned slides with 70% ethanol and drying was performed at room temperature. Staining was done with giemsa dye, and the slides were analyzed under a microscope at 1000x magnification.
2.11. Interleukins Secretions
For the determination of tumor necrosis factor-α (TNF-α), interleukine-10 (IL-10), interleukine-6 (IL-6), and nitric oxide production, measurements were made using ELISA kits according to the manufacturer's instructions. All tests were performed in triplicate. The results of these tests were expressed in percentage of production in relation to the negative control.
2.12. Statistical Analysis
All analyses were performed using specific statistical software. The analyses were assessed by analysis of variance (ANOVA) followed by post hoc Bonferroni test. The results were considered significant at p<0.05.
3. Results and Discussion
The yield achieved from EO extraction of Schinus molle L. leaves in June/2015 was 0.364%. After determining the yield, work began assessing the phytochemical composition thereof by GC-MS, which resulted in the detection of thirty compounds (Table 1); of these, the highest concentrations found were of α-Pinene, Sabinene, Bicyclogermacrene, and Limonene, with percentage of 23.49, 11.36, 10.13, and 9.02, respectively. These four compounds together represent more than 50% of the compounds present and identified in the oil. Other studies have also identified α-pinene as a major compound present in the EO of leaves of Schinus terebinthifolius R. and Schinus lentiscifolius M. [20, 21].
Table 1.
Composition of essential oil of S. molle L.
| Compounds | RIa | RIb | Percentage |
|---|---|---|---|
| α-Pinene | 941 | 939 | 23.49 ± 0.2 |
| Sabinene | 978 | 976 | 11.36 ± 0.14 |
| Bicyclogermacrene | 1495 | 1494 | 10.13 ± 0.08 |
| Limonene | 1030 | 1031 | 9.02 ± 0.09 |
| Spathulenol | 1576 | 1576 | 6.59 ± 0.17 |
| β-pinene | 980 | 980 | 6.09 ± 0.54 |
| β-caryophyllene | 1417 | 1418 | 5.28 ± 0.36 |
| Germacrene-D | 1480 | 1480 | 4.21 ± 0.47 |
| Eugenol | 1356 | 1356 | 2.96 ± 0.08 |
| Myrcene | 991 | 991 | 2.83 ± 0.09 |
| α-Bisabolene | 1504 | 1504 | 2.57 ± 0.07 |
| 1.8 cineol | 1035 | 1033 | 2.04 ± 0.02 |
| Terpinen-4-ol | 1177 | 1177 | 1.74 ± 0.03 |
| α-Humulene | 1451 | 1454 | 1.73 ± 0.01 |
| α-Terpinene | 1019 | 1018 | 1.46 ± 0.06 |
| Hexadecane | 1600 | 1601 | 1.07 ± 0.9 |
| Citronellal | 1153 | 1153 | 1.05 ± 0.08 |
| Germacrene-B | 1557 | 1556 | 1.02 ± 0.01 |
| Citronellyl acetate | 1354 | 1354 | 0.83 ± 0.02 |
| Verbenene | 969 | 967 | 0.74 ± 0.3 |
| Aromadendrene | 1442 | 1439 | 0.65 ± 0.2 |
| δ-terpinene | 1063 | 1062 | 0.38 ± 0.1 |
| α-Cadinene | 1538 | 1538 | 0.35 ± 0.06 |
| Camphene | 953 | 953 | 0.27 ± 0.08 |
| p-Cymene | 1023 | 1026 | 0.25 ± 0.09 |
| Caryophyllene oxide | 1580 | 1581 | 0.25 ± 0.05 |
| (E)-β-cymene | 1052 | 1050 | 0.16 ± 0.01 |
| γ-Cadinene | 1523 | 1521 | 0.09 ± 0.02 |
| α-Thujene | 930 | 931 | 0.08 ± 0.01 |
| α-Terpineol | 1191 | 1189 | 0.07 ± 0.03 |
|
| |||
| Total identified (%) | 98.76 ± 0.08 | ||
Relative proportions of the essential oil constituents were expressed as percentages ± SD (n=3).
aExperimental retention indices (based on homologous series of n-alkane C7-C30).
bRetention indices from the literature (Adams, 1995).
α-pinene (2,6,6-trimethylbicyclo [3.1.1] hept-2-ene) is a monoterpene [22] that can be found in different EOs and has various biological uses, such as larvicidals, detergents, and insecticides, among others [23], and numerous studies have sought to elucidate its toxicological profile. Additionally, among the compounds identified, over 65% had already been described in the literature, as compounds present in the leaves of Schinus molle L. [3, 20, 24, 25]; however, the verbenene compound was previously identified only in the EO of Schinus molle L. fruit and not in its leaves [26], and other compounds, such as citronella, citronella acetate, eugenol, and hexadecane, have not been phytochemically identified for this species before, according to the literature.
The determination of LD50 was carried out per the results found in the analysis of cell proliferation, which was evaluated by the statistical method of nonlinear regression for both types of cell studied. For this, first we made a curve with five EO concentrations in cell cultures, which were 100, 10, 1, 0.1, and 0.01 μg/mL, obtaining at the end of the analysis LD50 30.07 μg/mL for human lymphocytes (Figure 1(a)) and 42.07 μg/mL for human macrophages (Figure 1(b)).
Figure 1.
Assessment of cell proliferation for determining the LD50 of the essential oil of Schinus molle L. culture of (a) human lymphocytes and (b) human macrophages by linear regression. Data expressed as mean ± standard deviation and it was performed at each test triplicate. In each graph the different letters represent a statistically significant difference at p <0.05.
Work performed by Ruffa et al. [27], which investigated the cytotoxic effects of the methanol extract of Schinus molle L. in concentrations ranging from 15 to 100 μg/mL for hepatic carcinoma cell cultures, demonstrated a dose-dependent cytotoxic effect and that the LD50 of the studied cells was 50 ± 7 μg/mL. Thus, for the development of other tests, the concentrations to be tested were determined, based on LD50 found for lymphocytes and macrophages, and these were LD50, LD50/10, LD50/100, LD50/1000, and LD50/10000.
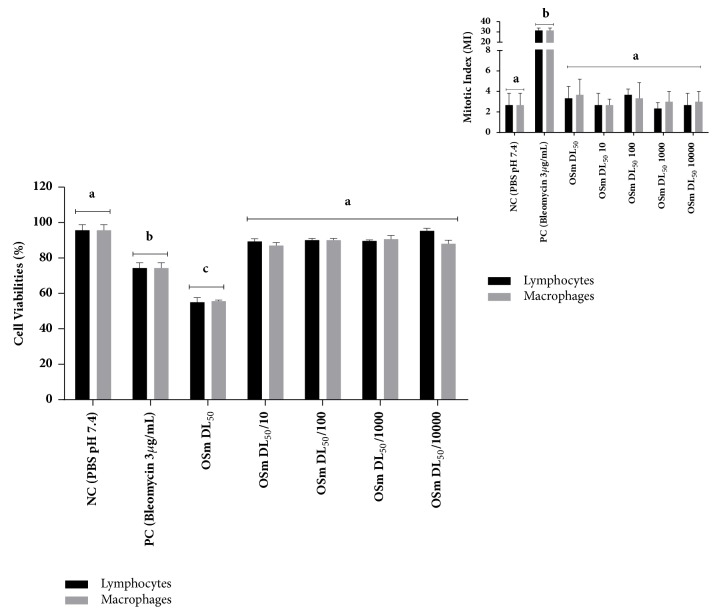
In Figure 2, we can observe the results of cell viability for the cells studied.
Figure 2.
Evaluation of cell viability in cultures of lymphocytes and human macrophages exposed to different concentrations of the essential oil of Schinus molle L. Data expressed as mean ± standard deviation and it was performed at each test triplicate (n=3). In each graph the different letters represent a statistically significant difference at p <0.05.
The cytotoxicity observed may be related to the presence of certain compounds in the EO, which may promote these cytotoxic effects together and/or in isolation, as described in the study by Sobral-Souza et al. [23], which investigated the cytotoxic effect of the compound α-pinene in cultures of fibroblasts at concentrations of 50 and 100 μg/mL. This study found a low cytotoxicity at a concentration of 50 µg/mL in fibroblast cultures. Thus, cytotoxicity could be related to the presence of certain compounds, such as, for example, α-pinene, which is the major compound of the EO studied; however, we cannot rule out the possibility that other compounds are contributing to this effect.
Regarding the mitotic index, none of the doses showed effects on the cells tested at these concentrations. Our results for S. molle L. are opposed to those found in the study conducted by Pawlowski et al. [20] which investigated the effects of EO extracted from the leaves of S. terebinthifolius and S. molle in root meristem onion and lettuce and found that the EO metaphase index was reduced. In addition, they showed that the groups treated with S. terebinthifolius EO compared to the NC promoted a reduction in the mitotic index of onions and lettuce, higher than 80% and 70%, respectively. The groups treated with S. molle L. had a reduced mitotic index for both onions and lettuce, obtaining around 20% and 30%, respectively.
Figure 3 summarizes the genotoxic effects of S. molle L. essential oil: in (a) the results of the micronuclei (expressed as nuclear division index), in (b) the numerical chromosomal instability, and in (c) the index of DNA damage.
Figure 3.
Genotoxic effects of S. molle L. essential oil. In (a) the results of the micronuclei (expressed as nuclear division index), in (b) the numerical chromosomal instability, and in (c) the index of DNA damage. Data expressed as mean ± standard deviation and it was performed at each test triplicate (n=3). In each graph the different letters represent a statistically significant difference at p <0.05.
In the evaluation of DNA damage, only LD50 and LD50/10 showed significant damage to the macrophages compared to the NC group, representing more than 20% DNA damage each. By contrast, the EO caused no significant DNA damage at any of the concentrations tested in the lymphocytes culture; in the same way it did not change the frequency of micronuclei, certifying that the EO concentrations tested showed no mutagenic effects in the same cells. As for the macrophages, it presented significant, concentration-dependent changes, with a higher frequency of micronuclei compared to the NC for LD50, LD50/10, and LD50/100. These results may be related to the presence of α-pinene, which at concentrations of 25, 30, and 35 μM is able to promote significant changes in the frequency of micronuclei and concentration-dependent genotoxicity in hamster cell cultures (V79-Cl3), as described in research by Catanzaro et al. [28]. Türkez and Aydin [29] evaluated the effect of exposure of human lymphocytes to α-pinene (10–200 μg/mL) using mutagen parameters (micronucleus test and chromosomal aberration) and concluded that the cells did not change significantly compared to the control. As for the analysis of chromosomal instability developed in this study, the tested concentrations did not promote changes in any of the studied cells, so that they also did not cause changes in the mitotic index for either HLs or HMs compared to the NC.
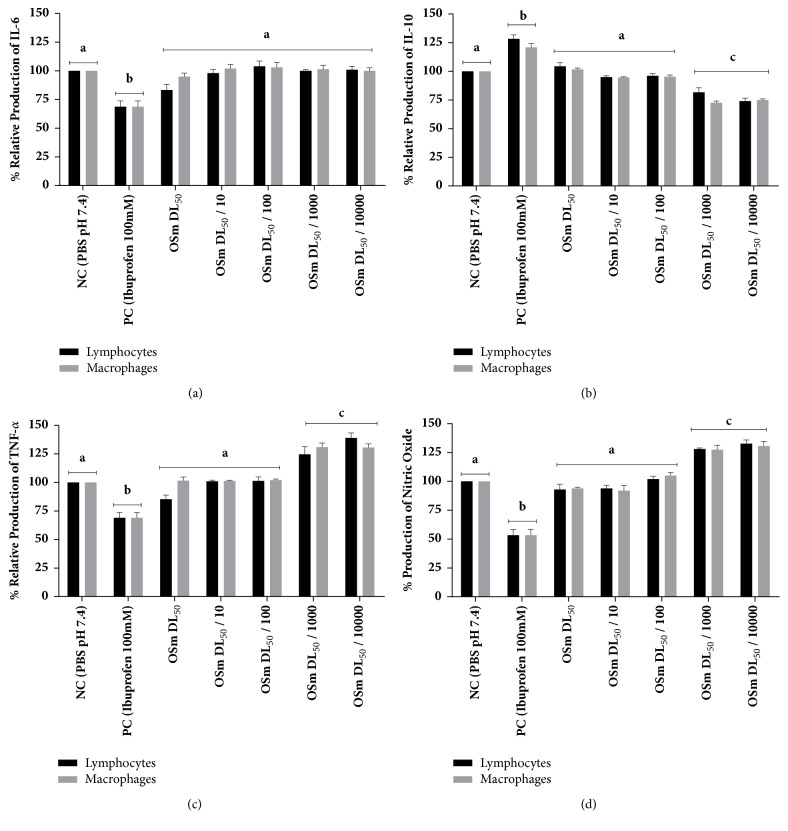
Figure 4 shows functional evaluation parameters of the immune system cells. In (a) we have the results of interleukin-6 secretion, in (b) the secretion of interleukin-10, in (c) the production of tumor necrosis factor alpha, and in (d) the production of nitric oxide (NO).
Figure 4.
Functional evaluation of the immune system cells. In (a) we have the results of interleukin-6 secretion, in (b) the secretion of interleukin-10, in (c) the production of tumor necrosis factor alpha, and in (d) the production of nitric oxide (NO). Data expressed as mean ± standard deviation and it was performed at each test triplicate (n=3). In each graph the different letters represent a statistically significant difference at p <0.05.
Cytokines are proteins, which are engaged in the communication between cells of the immune system. IL-6 is a 184-amino acid glycosylated protein, which can be synthesized and secreted by many cell types including monocytes, T-cells, fibroblasts, and endothelial cells. IL-6 binds to a specific receptor (IL-6R), an 80 kDa. During inflammation or infection, IL-6 is secreted by neutrophils, monocytes, macrophages, fibroblasts, endothelial cells, smooth muscle cells, and T-cells type I transmembrane protein [30]. In our study, none of the concentrations tested caused alterations in the relative production of IL-6, both in lymphocytes and in macrophages. Interleukin-10 (IL-10) is a potent anti-inflammatory cytokine that was originally labeled cytokine synthesis inhibitory factor due to its ability to inhibit production of TNF-α. These properties prompted early and extensive efforts to utilize IL-10 to modulate immune response in humans. Almost all leukocytes, including T and B cells, dendritic cells, NK cells, mast cells, neutrophils, eosinophils, and keratinocytes produce IL-10 [31]. Tumor necrosis factor (TNF-α) is a central proinflammatory cytokine involved in various inflammatory conditions. TNF is a trimeric protein, expressed from a wide variety of cells, and exists in both soluble and membrane-associated forms [32]. Our results showed that there was a reduction in IL-10 production against low oil concentrations and this is reflected in an increase in the production of TNF-α, which are directly related. Nitric oxide (NO) is a molecule utilized throughout the animal kingdom as a signaling or toxic agent between cells. Generated by many cell types in a variety of tissues, in mammals it acts as a vascular relaxing agent, a neurotransmitter, and an inhibitor of platelet aggregation. NO plays several roles in immunity—as a toxic agent towards infectious organisms, an inducer or suppressor of apoptosis, or an immunoregulator [33]. Few works report the relationship between the Schinus genus and immune system mediators.
Our work is the first to evaluate these markers in these cells. We can infer that the results obtained are a cascade of effects. Oil components such as α-pinene, which are known to have this action (REF), inflame the production of nitric oxide, which in turn inhibits the production of interleukin-10 and results in increased production of tumor necrosis factor alpha. By adding these actions, we have an immune response activation by the components of the oil. Our study needs to determine whether this immune activation would be beneficial (in the case of a foreign agent) or harmful to the body (in a mechanism like an autoimmune disease) [34–36].
4. Conclusion
The essential oil from leaves of Schinus molle L. presents various compounds, α-pinene being the major compound; in addition, the compound verbenene was first identified in the essential oil of the leaves of Schinus molle L. The EO promoted cytotoxicity in both types of cell tested, but genotoxic effects were detected only in macrophages and only at the two highest concentrations tested; as compared to the mutagenic effects, the EO did not cause chromosomal alterations or alterations in the index of cell division but did lead to an increase in the frequency of concentration-dependent micronuclei of macrophages. An important finding is that Schinus molle L. oil causes an activation of the immune system. This action has its mechanism centered by the cascade nitric oxide-interleukin-10-tumor necrosis factor alpha. However, it is worth noting the importance of knowledge of the EO constituents of Schinus molle L. to obtain further clarification concerning its toxicity, as this plant is widely used in folk medicine to treat various infirmities.
Acknowledgments
The authors would like to thank the professors from NAPO (Center for Analysis and Organic Research at UFSM) for providing the GC/MS chromatograms and spectra, A.F. Morel (Department of Chemistry at UFSM) for the assessment of the n-alkane series, and Renato Zacchia (Botanical Department of the Federal University of Santa Maria) for providing the identification of S. molle L. Also, they are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Financiadora de Estudos e Projetos (FINEP) for financial support to conduct this study.
Data Availability
Any and all data files are available for evaluation as per request to the corresponding author's e-mail.
Conflicts of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Authors' Contributions
Jonathaline Apollo Duarte (jonathalineapollo@yahoo.com.br), Léa Augusta de Bairros Zambrano (bize_zambrano@hotmail.com), Luciane Dias Quintana (lucianequintana@hotmail.com), Mariana Balhego Rocha (marianabalhego@hotmail.com), and Elizandra Gomes Schmitt (danda_schmitt2@hotmail.com) conducted the experiments on the cell cultures. Aline Augusti Boligon (alineboligon@hotmail.com) and Marli Matiko Anraku de Campos (marlimatiko@yahoo.com) were responsible for conducting phytochemical analyses, and Luís Flávio Souza de Oliveira (luisoliveira@unipampa.edu.br) and Michel Mansur Machado (michelmachado@unipampa.edu.br) were responsible for the experimental design, data analysis, and final writing of the manuscript.
References
- 1.Viegas Jr C., Bolzani V. S., Barreiro E. J. Os produtos naturais e a química medicinal moderna. Química Nova. 2006;29(2):326–337. doi: 10.1590/s0100-40422006000200025. [DOI] [Google Scholar]
- 2.Maciel M. A. M., Pinto A. C., Veiga V. F., Jr. Medicinal plants: the need for multidisciplinary scientific studies. Química Nova. 2002;25(3):429–438. doi: 10.1590/s0100-40422002000300016. [DOI] [Google Scholar]
- 3.Martins M. D. R., Arantes S., Candeias F., Tinoco M. T., Cruz-Morais J. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. Journal of Ethnopharmacology. 2014;151(1):485–492. doi: 10.1016/j.jep.2013.10.063. [DOI] [PubMed] [Google Scholar]
- 4.Germolec D., Luebke R., Rooney A., Shipkowski K., Vandebriel R., van Loveren H. Immunotoxicology: A brief history, current status and strategies for future immunotoxicity assessment. Current Opinion in Toxicology. 2017;5:55–59. doi: 10.1016/j.cotox.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Güez C. M., de Souza R. O., Fischer P., et al. Evaluation of basil extract (Ocimum basilicum L.) on oxidative, anti-genotoxic and anti-inflammatory effects in human leukocytes cell cultures exposed to challenging agents. Brazilian Journal of Pharmaceutical Sciences. 2017;53(1) [Google Scholar]
- 6.Da Cunha F. A. B., Wallau G. L., Pinho A. I., et al. Eugenia uniflora leaves essential oil induces toxicity in Drosophila melanogaster: involvement of oxidative stress mechanisms. Toxicology Research. 2015;4(3):634–644. doi: 10.1039/C4TX00162A. [DOI] [Google Scholar]
- 7.Adams R. P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 5. Texensis Publishing; 2017. [Google Scholar]
- 8.dos Santos Montagner G. F. F., Sagrillo M., Machado M. M., et al. Toxicological effects of ultraviolet radiation on lymphocyte cells with different manganese superoxide dismutase Ala16Val polymorphism genotypes. Toxicology in Vitro. 2010;24(5):1410–1416. doi: 10.1016/j.tiv.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Zuravski L., Coelho R. P., Duarte J. A., et al. Protective Role of Golden Flaxseed (Linum usitatissimum L.) Against Oxidative Damage in Lipids and Proteins of Healthy Volunteers. Journal of Biosciences and Medicines. 2015;3(10):45–53. doi: 10.4236/jbm.2015.310006. [DOI] [Google Scholar]
- 10.Davis G. E. The Mac-1 and p150,95 β2 integrins bind denatured proteins to mediate leukocyte cell-substrate adhesion. Experimental Cell Research. 1992;200(2):242–252. doi: 10.1016/0014-4827(92)90170-D. [DOI] [PubMed] [Google Scholar]
- 11.Delirezh N., Shojaeefar E. Phenotypic and functional comparison between flask adherent and magnetic activated cell sorted monocytes derived dendritic cells. Iranian Journal of Immunology. 2012;9(2):98–108. [PubMed] [Google Scholar]
- 12.Rosa E. D., Amaral Q. D. F. D., Duarte J. A., et al. Antigenotoxic, antimutagenic and cytoprotective potential of Salvia hispanica L. seed extract on human leukocytes exposed to oxidative damage. Journal of Functional Foods. 2017;38:505–509. doi: 10.1016/j.jff.2017.09.052. [DOI] [Google Scholar]
- 13.Vogel D. Y. S., Glim J. E., Stavenuiter A. W. D., et al. Human macrophage polarization in vitro: maturation and activation methods compared. Immunobiology. 2014;219(9):695–703. doi: 10.1016/j.imbio.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Arnold C. E., Gordon P., Barker R. N., Wilson H. M. The activation status of human macrophages presenting antigen determines the efficiency of Th17 responses. Immunobiology. 2015;220(1):10–19. doi: 10.1016/j.imbio.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Burow M. E., Weldon C. B., Tang Y., et al. Differences in susceptibility to tumor necrosis factor α-induced apoptosis among MCF-7 breast cancer cell variants. Cancer Research. 1998;58(21):4940–4946. [PubMed] [Google Scholar]
- 16.Singh N. P., McCoy M. T., Tice R. R., Schneider E. L. A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental Cell Research. 1988;175(1):184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 17.Schmid W. The micronucleus test. Mutation Research/Environmental Mutagenesis and Related Subjects. 1975;31(1):9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 18.Fenech M. The in vitro micronucleus technique. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2000;455(1-2):81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 19.Yunis J. J. High resolution of human chromosomes. Science. 1976;191(4233):1268–1270. doi: 10.1126/science.1257746. [DOI] [PubMed] [Google Scholar]
- 20.Pawlowski Â., Kaltchuk-Santos E., Zini C. A., Caramão E. B., Soares G. L. G. Essential oils of Schinus terebinthifolius and S. molle (Anacardiaceae): mitodepressive and aneugenic inducers in onion and lettuce root meristems. South African Journal of Botany. 2012;80:96–103. doi: 10.1016/j.sajb.2012.03.003. [DOI] [Google Scholar]
- 21.Pawlowski A., Kaltchuk-Santos E., Brasil M. C., Caramão E. B., Zini C. A., Soares G. L. G. Chemical composition of Schinus lentiscifolius March. essential oil and its phytotoxic and cytotoxic effects on lettuce and onion. South African Journal of Botany. 2013;88:198–203. doi: 10.1016/j.sajb.2013.07.026. [DOI] [Google Scholar]
- 22.Aydin E., Türkez H., Geyikoğlu F. Antioxidative, anticancer and genotoxic properties of α-pinene on N2a neuroblastoma cells. Biologia (Poland) 2013;68(5):1004–1009. [Google Scholar]
- 23.Sobral-Souza C. E., Leite N. F., Brito D. I., et al. Avaliação da atividade citotóxica e potencial antiparasitário in vitro do a-pineno e carvacrol. Acta Toxicológica Argentina. 2014;22(2):76–81. [Google Scholar]
- 24.dos Santos A. C. A., Rossato M., Agostini F., et al. Caracterização química de populações de Schinus molle L. do Rio Grande do Sul. Revista Brasileira de Farmacognosia. 2008;5(S2):1014–1016. [Google Scholar]
- 25.Gomes V., Agostini G., Agostini F., Atti dos Santos A. C., Rossato M. Variation in the essential oils composition in Brazilian populations of Schinus molle L. (Anacardiaceae) Biochemical Systematics and Ecology. 2013;48:222–227. doi: 10.1016/j.bse.2013.01.003. [DOI] [Google Scholar]
- 26.Bendaoud H., Romdhane M., Souchard J. P., Cazaux S., Bouajila J. Chemical composition and anticancer and antioxidant activities of Schinus molle L. and Schinus terebinthifolius Raddi berries essential oils. Journal of Food Science. 2010;75(6):C466–C472. doi: 10.1111/j.1750-3841.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- 27.Ruffa M. J., Ferraro G., Wagner M. L., Calcagno M. L., Campos R. H., Cavallaro L. Cytotoxic effect of Argentine medicinal plant extracts on human hepatocellular carcinoma cell line. Journal of Ethnopharmacology. 2002;79(3):335–339. doi: 10.1016/S0378-8741(01)00400-7. [DOI] [PubMed] [Google Scholar]
- 28.Catanzaro I., Caradonna F., Barbata G., Saverini M., Mauro M., Sciandrello G. Genomic instability induced by a-pinene in Chinese hamster cell line. Mutagenesis. 2012;27(4):463–469. doi: 10.1093/mutage/ges005. [DOI] [PubMed] [Google Scholar]
- 29.Türkez H., Aydın E. In vitro assessment of cytogenetic and oxidative effects of α-pinene. Toxicology & Industrial Health. 2016;32(1):168–176. doi: 10.1177/0748233713498456. [DOI] [PubMed] [Google Scholar]
- 30.Schaper F., Rose-John S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine & Growth Factor Reviews. 2015;26(5):475–487. doi: 10.1016/j.cytogfr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Saxena A., Khosraviani S., Noel S., Mohan D., Donner T., Hamad A. R. A. Interleukin-10 paradox: A potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine. 2015;74(1):27–34. doi: 10.1016/j.cyto.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebrec H., Ponce R., Preston B. D., Iles J., Born T. L., Hooper M. Tumor necrosis factor, tumor necrosis factor inhibition, and cancer risk. Current Medical Research and Opinion. 2015;31(3):557–574. doi: 10.1185/03007995.2015.1011778. [DOI] [PubMed] [Google Scholar]
- 33.Coleman J. W. Nitric oxide in immunity and inflammation. International Immunopharmacology. 2001;1(8):1397–1406. doi: 10.1016/S1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues K. A. D. F., Amorim L. V., Dias C. N., Moraes D. F. C., Carneiro S. M. P., Carvalho F. A. D. A. Syzygium cumini (L.) Skeels essential oil and its major constituent α-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. Journal of Ethnopharmacology. 2015;160:32–40. doi: 10.1016/j.jep.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 35.Rindelaub J. D., McAvey K. M., Shepson P. B. The photochemical production of organic nitrates from α-pinene and loss via acid-dependent particle phase hydrolysis. Atmospheric Environment. 2015;100:193–201. doi: 10.1016/j.atmosenv.2014.11.010. [DOI] [Google Scholar]
- 36.Bison J. V., Cardoso-Gustavson P., de Moraes R. M., et al. Volatile organic compounds and nitric oxide as responses of a Brazilian tropical species to ozone: the emission profile of young and mature leaves. Environmental Science and Pollution Research. 2017:1–9. doi: 10.1007/s11356-017-0744-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any and all data files are available for evaluation as per request to the corresponding author's e-mail.