Abstract
Helminth parasites are complex metazoans that belong to different taxonomic families but that collectively share the capacity to downregulate the host immune response directed toward themselves (parasite-specific immunoregulation). During long-standing chronic infection, these helminths appear able to suppress immune responses to bystander pathogens/antigens and atopic, autoimmune, and metabolic disorders. Helminth-induced immunoregulation occurs through the induction of regulatory T cells or Th2-type cells (or both). However, secreted or excreted parasite metabolites, proteins, or extracellular vesicles (or a combination of these) may also directly induce signaling pathways in host cells. Therefore, the focus of this review will be to highlight recent advances in understanding the immune responses to helminth infection, emphasizing the strategies/molecules and some of the mechanisms used by helminth parasites to modulate the immune response of their hosts.
Keywords: helminth, immune regulation, parasites, immune response, type-2 immunity, regulatory response.
Introduction
Helminth parasites belong to a diverse group of complex metazoans from different taxonomic families. Collectively, helminth infections are a major public health problem worldwide, and recent estimates suggest that 1.5 billion people have one or more of the common helminth infections ( Table 1), most of whom reside in low- and middle-income countries in the endemic areas of Asia, Latin America, the Caribbean, and sub-Saharan Africa 1.
Table 1. Human helminth infections of public health importance.
| Helminth species | Disease or condition in
humans |
Estimate prevalence
worldwide |
Habitat of adult worm
in humans |
|---|---|---|---|
| Nematodes | |||
| Ascaris lumbricoides | Ascariasis | 804 million | Small intestine |
| Ascaris suum | |||
| Trichuris trichiura | Trichuriasis | 477 million | Large intestine |
| Enterobius vermicularis | Enterobiasis (Oxyuriasis) | >200 million | |
| Toxocara canis | Visceral or ocular larva
migrans |
Unknown | N/A |
| Necator americanus | Necatoriasis | 472 million | Small intestine |
| Ancylostoma duodenale | Ancylostomiasis | ||
| Ancylostoma ceylanicum | |||
| Strongyloides stercoralis | Strongyloidiasis | 30–100 million | |
| Wuchereria bancrofti | Lymphatic filariasis | 44 million | Lymphatic vessels |
| Brugia malayi or Brugia timori | |||
| Onchocerca volvulus | Onchocerciasis (river
blindness) |
17 million | Subcutaneous tissue |
| Trichinella spiralis | Trichinellosis | 0.066 million | Small intestine |
| Trematodes | |||
| Schistosoma mansoni | Intestinal schistosomiasis | 206 million | Mesenteric veins |
| Schistosoma haematobium | Urogenital schistosomiasis | Venous plexus of urinary
bladder |
|
| Schistosoma japonicum | Intestinal schistosomiasis | Mesenteric veins | |
| Fasciola hepatica | Fascioliasis | 80 million | Bile ducts |
| Clonorchis sinensis | Clonorchiasis | Bile ducts and gall
bladder |
|
| Opisthorchis spp. | Opisthorchiasis | ||
| Paragonimus spp. | Paragonimiasis | Lungs | |
| Cestodes | |||
| Echinococcus granulosus | Hydatid disease | 0.8 million | N/A |
| Echinococcus multilocularis | Alveolar echinococcosis | 0.019 million | N/A |
|
Cysticercus cellulosae
( Taenia solium larva) |
Cysticercosis and
Neurocysticercosis |
1 million | N/A |
| Taenia solium | Intestinal taeniasis | 0.38 million | Small intestine |
N/A, not applicable. There is no development of adult worms in humans.
These many helminths each have significant differences in their biological life cycles along with marked variation in tissue tropism. These differences are reflected in the differences in clinical outcomes seen among the helminth parasites. Pathologic consequences of most helminth infection have been associated with both the parasite intensity (or burden) and the relative acuteness or chronicity of the infection.
Despite these helminth species-specific differences, helminths as a group have been shown to modulate/regulate the host response to themselves (parasite-specific immunoregulation) 2– 4. However, with long-standing chronic infection, these parasites can alter the immune response to bystander pathogens/antigens 5, 6, including vaccines 7, 8, and allergens 9, 10. In addition, they have been associated with modulation of the severity of inflammatory bowel disease (IBD) 11, diabetes 12, and arthritis 13.
Because of the helminths’ capacity to regulate the host immune response, a regulation that can be partially reversed by anthelmintic therapy, there has been widespread interest in understanding the mechanisms underlying helminth-induced immune regulation along with those parasite-encoded molecules that may be driving such regulation. In particular, the so-called excretory/secretory (ES) products from helminth parasites have gained the most attention, as they may be targets for anthelmintic vaccines, diagnostics, and drugs or they could be useful as potential therapeutics for inflammatory and autoimmune disorders. Therefore, the focus of this review will be to highlight recent advances in understanding the immune responses to helminth infection, emphasizing the strategies/molecules used by helminth parasites to modulate the immune response of their hosts.
Acuteness and chronicity of infection drive distinct immune profiles
The complexity of the life cycles of helminth parasites that have multiple developmental stages of the parasite each with a distinct antigenic repertoire and often distinct tropisms for particular organ systems (for example, intestinal and airway mucosa in larval Ascaris lumbricoides and hookworm infections; skin/subcutaneous tissue and draining lymph nodes in Onchocerca volvulus infection; the hepatic portal system for Schistosoma mansoni; and the muscle and the brain for Taenia solium cysticerci) makes it difficult to generalize about helminths as a single group 2. Normally, however, infection occurs through the ingestion of eggs or exposure to infective larvae. Once in contact with their mammalian hosts, the parasite progressively develops during the migration of the larval stages through the host’s systems/organs that culminate in their maturation into adult worms within a specific habitat that reflects each helminth’s tropism for a particular anatomical niche. As these developmental transitions and migration occur over a period of time (from weeks to years, depending on the parasite and its particular mammalian host), immune responses are often regulated differently on the basis of the resident tissue or perhaps by the life span of the parasite.
One example of the complex developmental and migratory processes that occur following helminth infection is that caused by the roundworms A. lumbricoides, a parasite that, by current estimates, is harbored by more than 800 million people worldwide 14. Human infection occurs following the ingestion of parasite eggs containing the third-stage infective larvae (L3) that hatch in the small intestine. After penetrating the intestine at the level of the caecum or proximal colon, these L3 migrate through the portal vessels to the liver and subsequently to the lungs. There they migrate through the lung parenchyma and penetrate into the alveolar spaces, causing a range of symptoms, including wheezing, dyspnea, cough, and substernal pain 15, 16. This early/acute phase of infection has been called larval ascariasis 17. These migrating Ascaris larvae induce a local inflammatory response in the lungs of humans (causing a Löffler’s-like syndrome 18) and of experimentally infected mice. In mice, the inflammation has been characterized as a type 2 response (dominated by IL-4 and IL-13 and some IL-5). Tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) levels are also seen in the lung induced by larval migration. At the peak of Ascaris larval migration (~8 days post-infection), there is a marked production of IL-6, thought to be related to the prominent neutrophil infiltration 19. When the larvae start to leave the lung tissue to migrate back to the small intestine to complete their life cycle, the neutrophil infiltrate in the lung is replaced by both alternatively activated (or M2) macrophages (AAM) (Fizz1+, Arginase-I+) and eosinophils that play a key role in tissue remodeling and prevention of re-infection 20. Once back in the small intestine, the larvae mature into adult worms, establishing a long-term chronic infection characterized by a profoundly diminished helminth-specific response 21, 22.
Over the last 20 years, several experimental studies using intestinal nematodes of rodents such as Heligmosomoides polygyrus or Nippostrongylus brasiliensis have provided a detailed description of a “protective” immune response associated with worm expulsion 23– 26. Although the mechanisms of larval killing are less well-studied, it is known that early in infection, prior to adult worm development and establishment, mucosal epithelial sensor cells secrete a group of alarmins—for example, IL-25, thymic stromal lymphopoietin (TSLP), and IL-33—that promote the activation and differentiation of innate and adaptive type 2 cells, leading to the secretion of a myriad of cytokines, including IL-4, IL-5, IL-9, and IL-13 26, 27. These type 2-associated cytokines result in goblet cell hyperplasia, mucus hyper-secretion, and smooth muscle contraction and other immunological changes such as eosinophilia and the differentiation of AAM macrophages 26, 28.
Recently, a novel subset of epithelial cells, termed tuft cells, was identified in the small intestine. These tuft cells constitutively express IL-25. Von Moltke et al. 29 and Gerbe et al. 30 showed that after infection by the rodent hookworm N. brasiliensis, tuft cells produce IL-25 that in turn activates type 2 innate lymphoid cells (ILC2s) to produce IL-13 that subsequently acts on epithelial crypt progenitors to promote differentiation and increased frequency of both tuft and goblet cells. As reviewed by Grencis and Worthington 31, this tuft cell–ILC2 circuit loop orchestrates a rapid and effective anti-helminth immune effector response that leads to worm expulsion.
For helminth infection in humans, the immune response during the early/acute phase of infection involves the induction of type 2-associated cytokines (IL-4, IL-5, IL-9, and IL-13) first by innate lymphocytes (ILC2) and later by effector antigen-specific polyfunctional CD4 T cells 32. This relatively early phase also induces high antigen-specific IgG4 and IgE levels as well as peripheral and tissue eosinophilia and expanded populations of AAM 33, 34.
In peripheral blood, this polarized type 2 response occurs at the time of patency when egg laying (for example, S. mansoni) 35 or microfilarial release (for example, Wuchereria bancrofti) from adult females occurs 36, resulting in a significant modulation of Th1 responses (IL-2 and interferon-gamma [IFN-γ]). However, this persistent dominant Th2 response over the course of the helminth infection also induces expansion of natural 37– 39 and helminth-induced 40, 41 regulatory T (Treg) cells and immunoregulatory monocytes 42– 44; this same response drives B-cell class-switching to IgG4 45. This new regulatory environment, characterized by low parasite antigen-specific lymphocyte proliferation, higher antigen-specific IgG4/IgE ratios, and increased levels of the regulatory cytokines IL-10 and transforming growth factor-beta (TGF-β), is the hallmark of an asymptomatic, chronic infection 46– 49.
In chronic filarial infections, microfilaremia is observed in clinically asymptomatic patients. Interestingly, T cells from these filarial-infected asymptomatic patients show the following: a muted/anergic parasite-specific lymphoproliferative response 50– 52; an increased parasite-specific IL-4/IFN-γ ratio 46; dysfunctional antigen-presenting cells (APCs) 53, 54; expanded natural Treg (nTreg) cells expressing CTLA-4, PD-1, and GITR (molecules associated with regulatory functions on nTreg cells) 55; and elevated IL-10 levels 48, 56. In contrast, infected patients with progressive and often symptomatic infection, such as elephantiasis, fail to suppress (or be tolerant to) filarial antigen-driven inflammation. This relative immune hyper-responsiveness is associated with microfilarial clearance but also consequent morbidity 56. Furthermore, anthelmintic therapy that leads to clearance of the microfilariae or in vitro blockade of IL-10 can result in a recovery of many of the parasite antigen-specific responses, suggesting that they were actively inhibited in the presence of the parasites or of circulating parasite antigens 56, 57.
Traditionally, it has been shown that, beyond attenuating parasite-specific response, helminths can suppress the immunity to bystander pathogens or to vaccines 7, 58. It is known that the induction of the regulatory response by helminths is associated with the downmodulation of Th1 response 3, 59, 60, considered crucial for the immunological control of viral, bacterial, or protozoal infections ( Figure 1). Immuno-epidemiological studies suggest that coincident infection with helminths has a strong potential to significantly influence the course of viral or protozoan infections, especially in those infections where protective immunity depends on a strong Th1/Th17 immune response 61– 63. In addition, several recent studies have provided insight into how helminths and helminth-derived molecules (ES products) regulate some of the inflammatory responses that underlie allergic, autoimmune, or metabolic disorders.
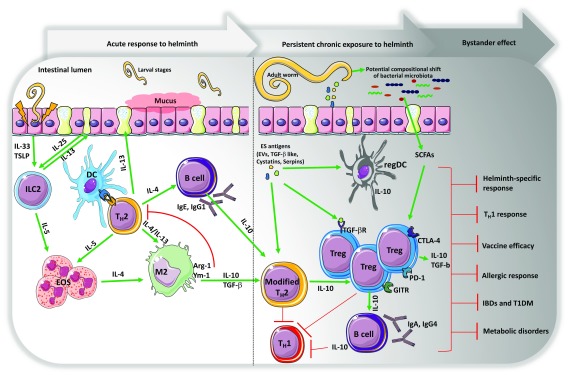
Figure 1. Acuteness and chronicity of helminth infection drive distinct immune profiles.
Early in infection, normally during the larval migration through the lungs or intestinal mucosa, prior to adult worm development and establishment, epithelial cells secrete a group of alarmins—thymic stromal lymphopoietin (TSLP) and interleukin-33 (IL-33), including IL-25-producing tuft cells—that promote the activation and differentiation of type 2 innate lymphoid cells (ILC2) and polyfunctional CD4 T helper 2 (Th2) cells, leading to the secretion of a myriad of cytokines, including IL-4, IL-5, and IL-13. These type 2-associated cytokines result in goblet cell hyperplasia, mucus hyper-secretion, peripheral and tissue eosinophilia, and differentiation of M2 macrophages and also induce high antigen-specific IgG1 and IgE levels. Helminth early/acute responses generally associate with an allergy-like response. The persistent exposure to helminth parasites and helminth-derived excretory/secretory (ES) antigens over the course of the infection lead to a modified type 2 response resulting in a significant modulation of T helper 1 (Th1) response—IL-2 and interferon-gamma (IFN-γ)—and also induce the expansion of natural regulatory T (nTreg) cells expressing CTLA-4, PD-1, GITR, and regulatory dendritic cells (regDCs) and monocytes, which are all sources of IL-10. This same response drives B-cell class-switching to IgG4. Chronic infection with helminth also alters the composition of intestinal bacterial communities leading to more microbial-derived short chain fatty acids (SCFAs) that also activate and promote the expansion of Treg cells. Collectively, this new regulatory environment is the signature for the establishment of an asymptomatic chronic long-standing infection, characterized by a muted/anergic parasite-specific lymphoproliferative response but also a suppressed immunity to bystander pathogens, allergens, vaccines, or non-related inflammatory, autoimmune—inflammatory bowel diseases (IBDs) and type 1 diabetes (T1DM)—or metabolic diseases. DC, dendritic cell; EOS, eosinophil; EV, extracellular vesicle; TGF-β, transforming growth factor beta.
Helminth-derived excretory/secretory products: the era of the extracellular vesicles
Helminth-induced immune responses have long been postulated to be directed at the ES products from living parasite stages during the infection. Some of the soluble proteins, lipids, and carbohydrates present in the ES products have been shown to have immunomodulatory activity 64, 65. The list of helminth-derived immunomodulatory molecules that evoke a regulatory phenotype among innate and adaptive immune cells has been increasing over the last decade 9, 10, 41, 64– 66.
The relatively recent discovery of extracellular vesicles (EVs) secreted by helminths has suggested a new paradigm in the study of host–parasite interaction 67, 68. EVs are released from most cell types and from a diverse group of pathogens, including parasitic helminths 69, 70. At homeostasis, EVs represent a mechanism by which cell-to-cell communication occurs through the transfer of genetic material, proteins, and lipids 68. In parasitic infections, EVs can function by transmitting signals between parasites, from parasite to host cells, or from the host to the environment 68.
In general, it is felt that helminth EVs have immunoregulatory effects on host cells 71, 72. For a group of helminths, the analysis of the composition of these EVs has identified proteins previously described in ES products along with microRNAs (miRNAs), a highly conserved group of small, non-coding RNA molecules that can control gene expression. Among the proteins identified as components of helminth EVs are cysteine protease inhibitors (cystatins), serine protease inhibitors (serpins), metabolic enzymes such as enolase, GAPDH, and aldolase, and the well-known exosome components Hsp70, Hsp90, and annexins 73.
Recently, it has been shown that EVs secreted by both the parasite and the host can influence the outcome of an infection. With an experimental murine model for a chronic helminth infection ( H. polygyrus), it was shown that EVs secreted by the H. polygyrus are internalized by murine macrophages and, as a consequence of this internalization, suppress the activation of both M1 and M2 macrophages 72. In contrast, with the infective stage of the filarial parasite Brugia malayi, it has been shown that these parasites secrete EVs containing parasite protein and miRNAs, which are also internalized by macrophages but which elicit/induce macrophage (M1) activation 74. Finally, with H. polygyrus and rodent filarial nematode Litomosoides sigmodontis, it was shown that these parasites secrete EVs containing miRNAs, which when administered prior to allergic sensitization in an experimental allergy-asthma model in mice actually suppressed the allergen-induced type 2 innate immune response in vivo 71.
Notwithstanding the data demonstrating EV-induced suppression of host inflammation and immune response, some groups have advocated the use of helminth-derived EVs for the identification of targets to be used in vaccines against some helminth infections 69, 73, 75. Indeed, EVs isolated from the ES products of Trichuris muris (a whipworm of mice) can induce protective immunity, reducing about 60% of parasite burden, in a murine model when administered as a vaccine without adjuvant, generating a strong EV-specific antibody response 76. Moreover, helminth-derived EVs induced protection to H. polygyrus larval challenge in mice 72.
Interestingly, there has been a suggestion that helminth-derived EVs could be used as therapeutics to regulate inflammation in the context of allergic, autoimmune, and metabolic disorders 71, 77, 78. As suggested by Siles-Lucas et al. 78, specific molecules from helminth exosomes could be delivered in artificial exosomes to host cells with the aim of regulating pathologic inflammatory responses. How to target specific cells, to stabilize these EVs, and to find the correct dosage are challenges that will need to be addressed.
Allergic diseases and helminth infection
Allergies are inflammatory disorders that result generally from inappropriate immune responses to environmental allergens. Allergic sensitization or atopy is driven by allergen-specific responses initiated by CD4 + Th2 cells that ultimately drive the production of allergen-specific IgE 79. Although the hygiene hypothesis suggests that the lack of exposure in children early in their development to helminth parasites or other microbial products (as seen in high- and middle-income countries) may drive the increased incidence of allergic diseases seen in these countries, there are conflicting sets of studies in humans and in experimental models 80– 83 that have called this particular hypothesis into question. Leonardi-Bee et al. 84 demonstrated, in a meta-analysis, that chronic infection by the hookworm Necator americanus protects against asthma but that A. lumbricoides infection aggravates the clinical symptoms of this allergic condition. Interestingly, children living in a helminth-endemic region of Ecuador had a lower risk of allergies when compared with non-parasitized children in the same region 85. Moreover, repetitive anthelmintic treatment in endemic areas has been shown to increase the prevalence of allergen skin test reactivity in children 86.
The differences among these studies likely reflect differences in the timing of parasite infection in relationship to immune maturation or sensitization, although the species of the helminth, the intensity of the helminth infection, or the nature of the allergic disease assessed (or a combination of these) may also play a role in driving the outcomes seen. The most compelling explanation relates to the relative acuteness of the helminth parasitic infection, with early exposure to helminths driving an enhanced allergic inflammatory response 32 and long-term chronic infections attenuating the host allergic response 58.
Among the various hypotheses put forward to explain the modulatory influence of helminth infection on allergic effector responses in humans and murine models, the IL-10-induced suppression of Th2-effector responses and the expansion of natural and parasite-induced Treg cells 9, 87, 88 have been the leading candidates. One possible mechanism is the IL-10-induced inhibition of IgE signaling (key players in allergic diseases) in basophils 89, 90. Over the last decade, it has been shown that, in human parasitic infection and in experimental models of helminth infection, helminth parasites can induce B cells to differentiate into IL-10-producing regulatory B cells that may play a role in the suppression of the immune response that leads to an expansion of Treg cells 91, 92.
Other studies have suggested that helminths potentiate the functional effect of Treg cells by the secretion of parasite-derived TGF-β mimics. Helminth-derived TGF-β-like molecules can bind to TGF-β receptors and trigger FoxP3 + Treg cell expansion 93– 96. These data notwithstanding, new data suggest (based on H. polygyrus infection in mice) that the suppression of the type 2 allergic immune response in helminths is driven by a Hp-secreted protein (HpARI) that actively inhibits IL-33 release, thereby inhibiting the allergic response 97.
As reviewed recently, the ability of helminths to induce parasite-reactive Treg cells and IL-10 production may occur through parasite ES products 64. In addition, these helminth-derived products likely modulate bystander inflammatory responses, particularly the development of allergy 9, 10, 64. The molecular basis of this suppression has yet to be defined.
Recently, a novel mechanism underlying the helminth suppression of the allergic response has been suggested that implicates an interaction between helminth-derived proteins and the local microbiome 98. This concept stems from the “barrier regulation hypothesis of allergy” whereby, in the healthy state, a microbiome replete with mucosa-associated taxa stimulates the intestinal mucosa (mediated by IL-22) to produce a protective mucous layer and to produce anti-microbial peptides 99 that, in turn, regulate the abundance of particular bacterial communities. These bacteria-induced barrier-protective functions reduce the ability of allergens to cross the epithelial barrier 99. Compositional shifts within bacterial communities through dietary changes or antibiotic use can induce alterations in these bacteria-induced barrier-protective responses, thereby driving allergen-induced ILC2- or Th2-associated inflammation or both 100. A slight variation on this theme suggests that in an environment with chronic microbial exposure, the lung and gut microbiome stimulates the formation of regulatory dendritic cells that promote the differentiation of allergy-specific Treg cells that suppress allergen-induced Th2-associated inflammation 79.
Whether it is the helminth infection per se or helminth-derived proteins, changes in microbial composition/abundance/diversity appear to contribute indirectly to the modulation of the allergic response in the host 100. Indeed, it has been shown that chronic infection with H. polygyrus altered the intestinal bacterial communities 101 and, in so doing, increased the amount of microbial-derived short chain fatty acids (SCFAs) that in turn suppressed house dust mite-induced allergic inflammation 98.
Helminth infections and autoimmune and metabolic disorders
Epidemiologic evidence demonstrates that while the prevalence of helminth infections is declining worldwide, the prevalence of autoimmune diseases—including IBDs and type 1 diabetes (T1DM)—and metabolic disorders is increasing rapidly. This phenomenon has led many to infer that there is a relationship between exposure to helminth infection and protection from autoimmune diseases—for example, Crohn’s disease (CD), ulcerative colitis (UC), and multiple sclerosis—and metabolic disorders. But how helminths regulate the group of varied inflammatory disorders, autoimmune diseases, and metabolic disorders remains unknown.
Using experimental model approaches, many authors have shown that helminth infection itself or treatment with helminth ES products is sufficient to suppress inflammation in numerous models of inflammatory diseases, including the dextran sodium sulfate (DSS)-induced colitis model in mice. ES products of Ancylostoma ceylanicum (human, cat, dog, and rodent hookworm) 102, A. caninum (dog hookworm) 103, Trichinella spiralis (carnivorous animal roundworm) 104, and S. japonicum (human blood fluke) have each been shown to attenuate the severity of DSS-induced colitis in mice 105. In addition, EVs of N. brasiliensis and T. muris 77 and the recombinant B. malayi protein rBmALT2 and cystatin 106, 107 have been shown to modulate colitis in experimental animal models. A common aspect of all of these studies has been the presence of increased levels of Th2-associated and regulatory cytokines (IL-10 and TGF-β) and a concomitant reduction in the inflammatory cytokines IL-6, IL-1β, IFN-γ, and IL-17a, known to be associated with the colitis-induced pathology. Concomitantly, two major species of helminths have been tested in more than 10 placebo-controlled clinical trials that have looked at Trichuris suis ova for the treatment of active UC and CD 108 or infection with N. americanus for the treatment of celiac disease in humans 11, 109, 110. As recently reviewed by Smallwood et al. 111, the results of the clinicals trials in humans are still controversial depending on the nature of the IBD or parasite evaluated, but, for some of them, there was some clinical improvement 108, 109, 112.
It has been shown that helminth infection can prevent T1DM based on the non-obese diabetic (NOD) mouse model. The data suggest that the immune switch from a Th1 to either a Th2 or a regulatory response is the primary mechanism through which T1DM is ameliorated 12, 113. In addition, it has been shown that helminth-derived proteins inhibit the initiation of autoreactive T-cell responses and prevent diabetes in the NOD mouse model 114. Interestingly, it has been postulated that the presence of these type 2 or regulatory cells in the pancreas of NOD mice has to take place before the bulk of beta cell mass is compromised by autoimmune attack 115. With a filarial infection in IL-4-deficient NOD mice, it was demonstrated that, despite the absence of a type 2 immune shift, filarial infection in IL-4-deficient NOD mice prevented the onset of T1DM and was accompanied by increases in CD4 +CD25 +Foxp3 + Treg cells 40. Moreover, blocking TGF-β signaling prevented the beneficial effect of helminth infection on T1DM, suggesting that skewing the immune response to a Th2 and regulatory environment could elicit suppression of the diabetogenic Th1 response.
Finally, when investigators evaluated the beneficial impact of helminth on protecting against the development of metabolic disorders, including obesity and dyslipidemia, commonly associated with insulin resistance and type 2 diabetes, parasite‐induced IL‐10 and the type 2 immune responses seem to act to improve insulin sensitivity 116, thereby ameliorating the metabolic syndrome (MetS)-associated morbidity 117. In this context, it has been shown that helminths have an important beneficial role by skewing this inflammatory response toward one with IL-4-producing eosinophils, M2 macrophages, and Treg cells that maintain insulin signaling and sensitivity 118.
Future directions
Helminths are potent regulators of type 1 immune response induced by bystander pathogens or inflammatory disorders or both. Understanding the mechanisms underlying this interaction and identifying the potential molecular targets are the current challenges and areas that need to be investigated further to develop novel strategies to prevent or delay allergic, inflammatory, autoimmune, or metabolic disorders in humans.
Abbreviations
AAM, alternatively activated macrophages; CD, Crohn’s disease; DSS, dextran sodium sulfate; ES, excretory/secretory; EV, extracellular vesicle; IBD, inflammatory bowel disease; IFN-γ, interferon-gamma; IL, interleukin; ILC2, innate lymphoid cell type 2; miRNA, microRNA; NOD, non-obese diabetic; nTreg, natural regulatory T; T1DM, type 1 diabetes; TGF-β, transforming growth factor-beta; Treg, regulatory T; UC, ulcerative colitis
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Padraic Fallon, Department of Clinical Medicine, Trinity College Dublin, Dublin, Ireland
Rick M. Maizels, Wellcome Centre for Molecular Parasitology, Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow, G12 8TA, UK
Funding Statement
This work was supported by the Division of Intramural Research of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Hotez PJ, Brindley PJ, Bethony JM, et al. : Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118(4):1311–21. 10.1172/JCI34261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nutman TB: Looking beyond the induction of Th2 responses to explain immunomodulation by helminths. Parasite Immunol. 2015;37(6):304–13. 10.1111/pim.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maizels RM, McSorley HJ: Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol. 2016;138(3):666–75. 10.1016/j.jaci.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Harnett W, Harnett MM: Lymphocyte hyporesponsiveness during filarial nematode infection. Parasite Immunol. 2008;30(9):447–53. 10.1111/j.1365-3024.2008.01045.x [DOI] [PubMed] [Google Scholar]
- 5. Babu S, Nutman TB: Helminth-Tuberculosis Co-infection: An Immunologic Perspective. Trends Immunol. 2016;37(9):597–607. 10.1016/j.it.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Metenou S, Kovacs M, Dembele B, et al. : Interferon regulatory factor modulation underlies the bystander suppression of malaria antigen-driven IL-12 and IFN-γ in filaria-malaria co-infection. Eur J Immunol. 2012;42(3):641–50. 10.1002/eji.201141991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sabin EA, Araujo MI, Carvalho EM, et al. : Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis. 1996;173(1):269–72. 10.1093/infdis/173.1.269 [DOI] [PubMed] [Google Scholar]
- 8. Cooper PJ, Espinel I, Paredes W, et al. : Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J Infect Dis. 1998;178(4):1133–8. 10.1086/515661 [DOI] [PubMed] [Google Scholar]
- 9. Daniłowicz-Luebert E, O'Regan NL, Steinfelder S, et al. : Modulation of specific and allergy-related immune responses by helminths. J Biomed Biotechnol. 2011;2011:821578. 10.1155/2011/821578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Erb KJ: Can helminths or helminth-derived products be used in humans to prevent or treat allergic diseases? Trends Immunol. 2009;30(2):75–82. 10.1016/j.it.2008.11.005 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. McSorley HJ, Gaze S, Daveson J, et al. : Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLoS One. 2011;6(9):e24092. 10.1371/journal.pone.0024092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zaccone P, Cooke A: Helminth mediated modulation of Type 1 diabetes (T1D). Int J Parasitol. 2013;43(3–4):311–8. 10.1016/j.ijpara.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 13. Chen Z, Andreev D, Oeser K, et al. : Th2 and eosinophil responses suppress inflammatory arthritis. Nat Commun. 2016;7:11596. 10.1038/ncomms11596 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Hotez PJ, Alvarado M, Basáñez MG, et al. : The global burden of disease study 2010: Interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8(7):e2865. 10.1371/journal.pntd.0002865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spillmann RK: Pulmonary ascariasis in tropical communities. Am J Trop Med Hyg. 1975;24(5):791–800. 10.4269/ajtmh.1975.24.791 [DOI] [PubMed] [Google Scholar]
- 16. BEAVER PC, DANARAJ TJ: Pulmonary ascariasis resembling eosinophilic lung; autopsy report with description of larvae in the bronchioles. Am J Trop Med Hyg. 1958;7(1):100–11. 10.4269/ajtmh.1958.7.100 [DOI] [PubMed] [Google Scholar]
- 17. Dold C, Holland CV: Ascaris and ascariasis. Microbes Infect. 2011;13(7):632–7. 10.1016/j.micinf.2010.09.012 [DOI] [PubMed] [Google Scholar]
- 18. LOFFLER W: Transient lung infiltrations with blood eosinophilia. Int Arch Allergy Appl Immunol. 1956;8(1–2):54–9. 10.1159/000228268 [DOI] [PubMed] [Google Scholar]
- 19. Gazzinelli-Guimarães PH, Gazzinelli-Guimarães AC, Silva FN, et al. : Parasitological and immunological aspects of early Ascaris spp. infection in mice. Int J Parasitol. 2013;43(9):697–706. 10.1016/j.ijpara.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 20. Nogueira DS, Gazzinelli-Guimarães PH, Barbosa FS, et al. : Multiple exposures to ascaris suum induce tissue injury and mixed Th2/Th17 immune response in mice. PLoS Negl Trop Dis. 2016;10(1):e0004382. 10.1371/journal.pntd.0004382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Midttun HLE, Acevedo N, Skallerup P, et al. : Ascaris Suum Infection Downregulates Inflammatory Pathways in the Pig Intestine In Vivo and in Human Dendritic Cells In Vitro. J Infect Dis. 2018;217(2):310–9. 10.1093/infdis/jix585 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Paterson JC, Garside P, Kennedy MW, et al. : Modulation of a heterologous immune response by the products of Ascaris suum. Infect Immun. 2002;70(11):6058–67. 10.1128/IAI.70.11.6058-6067.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Urban JF, Jr, Noben-Trauth N, Donaldson DD, et al. : IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8(2):255–64. 10.1016/S1074-7613(00)80477-X [DOI] [PubMed] [Google Scholar]
- 24. Camberis M, Le Gros G, Urban J, Jr: Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr Protoc Immunol. 2003;Chapter 19:Unit 19.12. 10.1002/0471142735.im1912s55 [DOI] [PubMed] [Google Scholar]
- 25. Sorobetea D, Svensson-Frej M, Grencis R: Immunity to gastrointestinal nematode infections. Mucosal Immunol. 2018;11(2):304–15. 10.1038/mi.2017.113 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Maizels RM, Pearce EJ, Artis D, et al. : Regulation of pathogenesis and immunity in helminth infections. J Exp Med. 2009;206(10):2059–66. 10.1084/jem.20091903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cortés A, Muñoz-Antoli C, Esteban JG, et al. : Th2 and Th1 Responses: Clear and Hidden Sides of Immunity Against Intestinal Helminths. Trends Parasitol. 2017;33(9):678–93. 10.1016/j.pt.2017.05.004 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Salazar-Castañon VH, Legorreta-Herrera M, Rodriguez-Sosa M: Helminth parasites alter protection against Plasmodium infection. Biomed Res Int. 2014;2014:913696. 10.1155/2014/913696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. von Moltke J, Ji M, Liang HE, et al. : Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529(7585):221–5. 10.1038/nature16161 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Gerbe F, Sidot E, Smyth DJ, et al. : Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529(7585):226–30. 10.1038/nature16527 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Grencis RK, Worthington JJ: Tuft cells: a new flavor in innate epithelial immunity. Trends Parasitol. 2016;32(8):583–5. 10.1016/j.pt.2016.04.016 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Gazzinelli-Guimarães PH, Bonne-Année S, Fujiwara RT, et al. : Allergic Sensitization Underlies Hyperreactive Antigen-Specific CD4 + T Cell Responses in Coincident Filarial Infection. J Immunol. 2016;197(7):2772–9. 10.4049/jimmunol.1600829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Babu S, Nutman TB: Immunology of lymphatic filariasis. Parasite Immunol. 2014;36(8):338–46. 10.1111/pim.12081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allen JE, Maizels RM: Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11(6):375–88. 10.1038/nri2992 [DOI] [PubMed] [Google Scholar]
- 35. Pearce EJ, MacDonald AS: The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2(7):499–511. 10.1038/nri843 [DOI] [PubMed] [Google Scholar]
- 36. King CL, Kumaraswami V, Poindexter RW, et al. : Immunologic tolerance in lymphatic filariasis. Diminished parasite-specific T and B lymphocyte precursor frequency in the microfilaremic state. J Clin Invest. 1992;89(5):1403–10. 10.1172/JCI115729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maizels RM, Yazdanbakhsh M: Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3(9):733–44. 10.1038/nri1183 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Metenou S, Nutman TB: Regulatory T cell subsets in filarial infection and their function. Front Immunol. 2013;4:305. 10.3389/fimmu.2013.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Metenou S, Coulibaly YI, Sturdevant D, et al. : Highly heterogeneous, activated, and short-lived regulatory T cells during chronic filarial infection. Eur J Immunol. 2014;44(7):2036–47. 10.1002/eji.201444452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grainger JR, Smith KA, Hewitson JP, et al. : Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med. 2010;207(11):2331–41. 10.1084/jem.20101074 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Navarro S, Pickering DA, Ferreira IB, et al. : Hookworm recombinant protein promotes regulatory T cell responses that suppress experimental asthma. Sci Transl Med. 2016;8(362):362ra143. 10.1126/scitranslmed.aaf8807 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Babu S, Kumaraswami V, Nutman TB: Alternatively activated and immunoregulatory monocytes in human filarial infections. J Infect Dis. 2009;199(12):1827–37. 10.1086/599090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Regan NL, Steinfelder S, Venugopal G, et al. : Brugia malayi microfilariae induce a regulatory monocyte/macrophage phenotype that suppresses innate and adaptive immune responses. PLoS Negl Trop Dis. 2014;8(10):e3206. 10.1371/journal.pntd.0003206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Passos LS, Gazzinelli-Guimarães PH, Oliveira Mendes TA, et al. : Regulatory monocytes in helminth infections: insights from the modulation during human hookworm infection. BMC Infect Dis. 2017;17(1):253. 10.1186/s12879-017-2366-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nutman TB, Kumaraswami V: Regulation of the immune response in lymphatic filariasis: perspectives on acute and chronic infection with Wuchereria bancrofti in South India. Parasite Immunol. 2001;23(7):389–99. 10.1046/j.1365-3024.2001.00399.x [DOI] [PubMed] [Google Scholar]
- 46. King CL, Mahanty S, Kumaraswami V, et al. : Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J Clin Invest. 1993;92(4):1667–73. 10.1172/JCI116752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taylor MD, LeGoff L, Harris A, et al. : Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol. 2005;174(8):4924–33. 10.4049/jimmunol.174.8.4924 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Mahanty S, Nutman TB: Immunoregulation in human lymphatic filariasis: The role of interleukin 10. Parasite Immunol. 1995;17(8):385–92. 10.1111/j.1365-3024.1995.tb00906.x [DOI] [PubMed] [Google Scholar]
- 49. Satoguina JS, Weyand E, Larbi J, et al. : T regulatory-1 cells induce IgG4 production by B cells: role of IL-10. J Immunol. 2005;174(8):4718–26. 10.4049/jimmunol.174.8.4718 [DOI] [PubMed] [Google Scholar]
- 50. Ottesen EA, Weller PF, Heck L: Specific cellular immune unresponsiveness in human filariasis. Immunology. 1977;33(3):413–21. [PMC free article] [PubMed] [Google Scholar]
- 51. Piessens WF, McGreevy PB, Piessens PW, et al. : Immune responses in human infections with Brugia malayi: specific cellular unresponsiveness to filarial antigens. J Clin Invest. 1980;65(1):172–9. 10.1172/JCI109648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nutman TB, Kumaraswami V, Ottesen EA: Parasite-specific anergy in human filariasis. Insights after analysis of parasite antigen-driven lymphokine production. J Clin Invest. 1987;79(5):1516–23. 10.1172/JCI112982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Semnani RT, Keiser PB, Coulibaly YI, et al. : Filaria-induced monocyte dysfunction and its reversal following treatment. Infect Immun. 2006;74(8):4409–17. 10.1128/IAI.01106-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Semnani RT, Law M, Kubofcik J, et al. : Filaria-induced immune evasion: suppression by the infective stage of Brugia malayi at the earliest host-parasite interface. J Immunol. 2004;172(10):6229–38. 10.4049/jimmunol.172.10.6229 [DOI] [PubMed] [Google Scholar]
- 55. Metenou S, Dembele B, Konate S, et al. : At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J Immunol. 2010;184(9):5375–82. 10.4049/jimmunol.0904067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. King CL, Nutman TB: Regulation of the immune response in lymphatic filariasis and onchocerciasis. Immunol Today. 1991;12(3):A54–8. 10.1016/S0167-5699(05)80016-7 [DOI] [PubMed] [Google Scholar]
- 57. Henry NL, Law M, Nutman TB, et al. : Onchocerciasis in a nonendemic population: clinical and immunologic assessment before treatment and at the time of presumed cure. J Infect Dis. 2001;183(3):512–6. 10.1086/318088 [DOI] [PubMed] [Google Scholar]
- 58. Smits HH, Yazdanbakhsh M: Chronic helminth infections modulate allergen-specific immune responses: Protection against development of allergic disorders? Ann Med. 2007;39(6):428–39. 10.1080/07853890701436765 [DOI] [PubMed] [Google Scholar]
- 59. Salgame P, Yap GS, Gause WC: Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol. 2013;14(11):1118–26. 10.1038/ni.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gazzinelli-Guimarães PH, de Freitas LF, Gazzinelli-Guimarães AC, et al. : Concomitant helminth infection downmodulates the Vaccinia virus-specific immune response and potentiates virus-associated pathology. Int J Parasitol. 2017;47(1):1–10. 10.1016/j.ijpara.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 61. Gabrie JA, Rueda MM, Rodríguez CA, et al. : Immune Profile of Honduran Schoolchildren with Intestinal Parasites: The Skewed Response against Geohelminths. J Parasitol Res. 2016;2016:1769585. 10.1155/2016/1769585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Metenou S, Dembele B, Konate S, et al. : Filarial infection suppresses malaria-specific multifunctional Th1 and Th17 responses in malaria and filarial coinfections. J Immunol. 2011;186(8):4725–33. 10.4049/jimmunol.1003778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Metenou S, Babu S, Nutman TB: Impact of filarial infections on coincident intracellular pathogens: Mycobacterium tuberculosis and Plasmodium falciparum. Curr Opin HIV AIDS. 2012;7(3):231–8. 10.1097/COH.0b013e3283522c3d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hewitson JP, Grainger JR, Maizels RM: Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Mol Biochem Parasitol. 2009;167(1):1–11. 10.1016/j.molbiopara.2009.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McSorley HJ, Hewitson JP, Maizels RM: Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol. 2013;43(3–4):301–10. 10.1016/j.ijpara.2012.11.011 [DOI] [PubMed] [Google Scholar]
- 66. Wu Z, Wang L, Tang Y, et al. : Parasite-Derived Proteins for the Treatment of Allergies and Autoimmune Diseases. Front Microbiol. 2017;8:2164. 10.3389/fmicb.2017.02164 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Coakley G, Buck AH, Maizels RM: Host parasite communications-Messages from helminths for the immune system: Parasite communication and cell-cell interactions. Mol Biochem Parasitol. 2016;208(1):33–40. 10.1016/j.molbiopara.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Coakley G, Maizels RM, Buck AH: Exosomes and Other Extracellular Vesicles: The New Communicators in Parasite Infections. Trends Parasitol. 2015;31(10):477–89. 10.1016/j.pt.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Riaz F, Cheng G: Exosome-like vesicles of helminths: implication of pathogenesis and vaccine development. Ann Transl Med. 2017;5(7):175. 10.21037/atm.2017.03.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schorey JS, Cheng Y, Singh PP, et al. : Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015;16(1):24–43. 10.15252/embr.201439363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Buck AH, Coakley G, Simbari F, et al. : Erratum: Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat Commun. 2015;6:8772. 10.1038/ncomms9772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Coakley G, McCaskill JL, Borger JG, et al. : Extracellular Vesicles from a Helminth Parasite Suppress Macrophage Activation and Constitute an Effective Vaccine for Protective Immunity. Cell Rep. 2017;19(8):1545–57. 10.1016/j.celrep.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Eichenberger RM, Sotillo J, Loukas A: Immunobiology of parasitic worm extracellular vesicles. Immunol Cell Biol. 2018;96(7):704–713. 10.1111/imcb.12171 [DOI] [PubMed] [Google Scholar]
- 74. Zamanian M, Fraser LM, Agbedanu PN, et al. : Release of Small RNA-containing Exosome-like Vesicles from the Human Filarial Parasite Brugia malayi. PLoS Negl Trop Dis. 2015;9(9):e0004069. 10.1371/journal.pntd.0004069 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Sotillo J, Pearson M, Potriquet J, et al. : Extracellular vesicles secreted by Schistosoma mansoni contain protein vaccine candidates. Int J Parasitol. 2016;46(1):1–5. 10.1016/j.ijpara.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 76. Shears RK, Bancroft AJ, Hughes GW, et al. : Extracellular vesicles induce protective immunity against Trichuris muris. Parasite Immunol. 2018;40(7):e12536. 10.1111/pim.12536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Eichenberger RM, Ryan S, Jones L, et al. : Hookworm Secreted Extracellular Vesicles Interact With Host Cells and Prevent Inducible Colitis in Mice. Front Immunol. 2018;9:850. 10.3389/fimmu.2018.00850 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Siles-Lucas M, Morchon R, Simon F, et al. : Exosome-transported microRNAs of helminth origin: new tools for allergic and autoimmune diseases therapy? Parasite Immunol. 2015;37(4):208–14. 10.1111/pim.12182 [DOI] [PubMed] [Google Scholar]
- 79. Lambrecht BN, Hammad H: The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. 2017;18(10):1076–83. 10.1038/ni.3829 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Hagel I, Cabrera M, Hurtado MA, et al. : Infection by Ascaris lumbricoides and bronchial hyper reactivity: an outstanding association in Venezuelan school children from endemic areas. Acta Trop. 2007;103(3):231–41. 10.1016/j.actatropica.2007.06.010 [DOI] [PubMed] [Google Scholar]
- 81. Wördemann M, Diaz RJ, Heredia LM, et al. : Association of atopy, asthma, allergic rhinoconjunctivitis, atopic dermatitis and intestinal helminth infections in Cuban children. Trop Med Int Health. 2008;13(2):180–6. 10.1111/j.1365-3156.2007.01988.x [DOI] [PubMed] [Google Scholar]
- 82. van den Biggelaar AH, van Ree R, Rodrigues LC, et al. : Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 2000;356(9243):1723–7. 10.1016/S0140-6736(00)03206-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. van der Werff SD, Twisk JW, Wördemann M, et al. : Deworming is not a risk factor for the development of atopic diseases: a longitudinal study in Cuban school children. Clin Exp Allergy. 2013;43(6):665–71. 10.1111/cea.12129 [DOI] [PubMed] [Google Scholar]
- 84. Leonardi-Bee J, Pritchard D, Britton J: Asthma and current intestinal parasite infection: systematic review and meta-analysis. Am J Respir Crit Care Med. 2006;174(5):514–23. 10.1164/rccm.200603-331OC [DOI] [PubMed] [Google Scholar]
- 85. Cooper PJ, Chico ME, Bland M, et al. : Allergic symptoms, atopy, and geohelminth infections in a rural area of Ecuador. Am J Respir Crit Care Med. 2003;168(3):313–7. 10.1164/rccm.200211-1320OC [DOI] [PubMed] [Google Scholar]
- 86. Endara P, Vaca M, Chico ME, et al. : Long-term periodic anthelmintic treatments are associated with increased allergen skin reactivity. Clin Exp Allergy. 2010;40(11):1669–77. 10.1111/j.1365-2222.2010.03559.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yazdanbakhsh M, Wahyuni S: The role of helminth infections in protection from atopic disorders. Curr Opin Allergy Clin Immunol. 2005;5(5):386–91. 10.1097/01.all.0000182541.52971.eb [DOI] [PubMed] [Google Scholar]
- 88. Mitre E, Chien D, Nutman TB: CD4 + (and not CD25 +) T cells are the predominant interleukin-10-producing cells in the circulation of filaria-infected patients. J Infect Dis. 2008;197(1):94–101. 10.1086/524301 [DOI] [PubMed] [Google Scholar]
- 89. Larson D, Hübner MP, Torrero MN, et al. : Chronic helminth infection reduces basophil responsiveness in an IL-10-dependent manner. J Immunol. 2012;188(9):4188–99. 10.4049/jimmunol.1101859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Larson D, Cooper PJ, Hübner MP, et al. : Helminth infection is associated with decreased basophil responsiveness in human beings. J Allergy Clin Immunol. 2012;130(1):270–2. 10.1016/j.jaci.2012.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hussaarts L, van der Vlugt LE, Yazdanbakhsh M, et al. : Regulatory B-cell induction by helminths: implications for allergic disease. J Allergy Clin Immunol. 2011;128(4):733–9. 10.1016/j.jaci.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 92. Tian F, Hu X, Xian K, et al. : B10 cells induced by Schistosoma japonicum soluble egg antigens modulated regulatory T cells and cytokine production of T cells. Parasitol Res. 2015;114(10):3827–34. 10.1007/s00436-015-4613-x [DOI] [PubMed] [Google Scholar]
- 93. Johnston CJC, Smyth DJ, Kodali RB, et al. : A structurally distinct TGF-β mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat Commun. 2017;8(1):1741. 10.1038/s41467-017-01886-6 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Gomez-Escobar N, Lewis E, Maizels RM: A novel member of the transforming growth factor-beta (TGF-beta) superfamily from the filarial nematodes Brugia malayi and B. pahangi. Exp Parasitol. 1998;88(3):200–9. 10.1006/expr.1998.4248 [DOI] [PubMed] [Google Scholar]
- 95. Maizels RM, Gomez-Escobar N, Gregory WF, et al. : Immune evasion genes from filarial nematodes. Int J Parasitol. 2001;31(9):889–98. 10.1016/S0020-7519(01)00213-2 [DOI] [PubMed] [Google Scholar]
- 96. Smyth DJ, Harcus Y, White MPJ, et al. : TGF-β mimic proteins form an extended gene family in the murine parasite Heligmosomoides polygyrus. Int J Parasitol. 2018;48(5):379–85. 10.1016/j.ijpara.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Osbourn M, Soares DC, Vacca F, et al. : HpARI Protein Secreted by a Helminth Parasite Suppresses Interleukin-33. Immunity. 2017;47(4):739–751.e5. 10.1016/j.immuni.2017.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Zaiss MM, Rapin A, Lebon L, et al. : The Intestinal Microbiota Contributes to the Ability of Helminths to Modulate Allergic Inflammation. Immunity. 2015;43(5):998–1010. 10.1016/j.immuni.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 99. Wesemann DR, Nagler CR: The Microbiome, Timing, and Barrier Function in the Context of Allergic Disease. Immunity. 2016;44(4):728–38. 10.1016/j.immuni.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 100. McCoy KD, Köller Y: New developments providing mechanistic insight into the impact of the microbiota on allergic disease. Clin Immunol. 2015;159(2):170–6. 10.1016/j.clim.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Reynolds LA, Finlay BB, Maizels RM: Cohabitation in the Intestine: Interactions among Helminth Parasites, Bacterial Microbiota, and Host Immunity. J Immunol. 2015;195(9):4059–66. 10.4049/jimmunol.1501432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cançado GG, Fiuza JA, de Paiva NC, et al. : Hookworm products ameliorate dextran sodium sulfate-induced colitis in BALB/c mice. Inflamm Bowel Dis. 2011;17(11):2275–86. 10.1002/ibd.21629 [DOI] [PubMed] [Google Scholar]
- 103. Ferreira I, Smyth D, Gaze S, et al. : Hookworm excretory/secretory products induce interleukin-4 (IL-4) + IL-10 + CD4 + T cell responses and suppress pathology in a mouse model of colitis. Infect Immun. 2013;81(6):2104–11. 10.1128/IAI.00563-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yang X, Yang Y, Wang Y, et al. : Excretory/secretory products from Trichinella spiralis adult worms ameliorate DSS-induced colitis in mice. PLoS One. 2014;9(5):e96454. 10.1371/journal.pone.0096454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wang L, Yu Z, Wan S, et al. : Exosomes Derived from Dendritic Cells Treated with Schistosoma japonicum Soluble Egg Antigen Attenuate DSS-Induced Colitis. Front Pharmacol. 2017;8:651. 10.3389/fphar.2017.00651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Khatri V, Amdare N, Yadav RS, et al. : Brugia malayi abundant larval transcript 2 protein treatment attenuates experimentally-induced colitis in mice. Indian J Exp Biol. 2015;53(11):732–9. [PubMed] [Google Scholar]
- 107. Khatri V, Amdare N, Tarnekar A, et al. : Brugia malayi cystatin therapeutically ameliorates dextran sulfate sodium-induced colitis in mice. J Dig Dis. 2015;16(10):585–94. 10.1111/1751-2980.12290 [DOI] [PubMed] [Google Scholar]
- 108. Summers RW, Elliott DE, Urban JF, Jr, et al. : Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128(4):825–32. 10.1053/j.gastro.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 109. Croese J, Giacomin P, Navarro S, et al. : Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J Allergy Clin Immunol. 2015;135(2):508–16. 10.1016/j.jaci.2014.07.022 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 110. Daveson AJ, Jones DM, Gaze S, et al. : Effect of hookworm infection on wheat challenge in celiac disease--a randomised double-blinded placebo controlled trial. PLoS One. 2011;6(3):e17366. 10.1371/journal.pone.0017366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Smallwood TB, Giacomin PR, Loukas A, et al. : Helminth Immunomodulation in Autoimmune Disease. Front Immunol. 2017;8:453. 10.3389/fimmu.2017.00453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Summers RW, Elliott DE, Qadir K, et al. : Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003;98(9):2034–41. 10.1111/j.1572-0241.2003.07660.x [DOI] [PubMed] [Google Scholar]
- 113. Hübner MP, Shi Y, Torrero MN, et al. : Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-β. J Immunol. 2012;188(2):559–68. 10.4049/jimmunol.1100335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lund ME, O'Brien BA, Hutchinson AT, et al. : Secreted proteins from the helminth Fasciola hepatica inhibit the initiation of autoreactive T cell responses and prevent diabetes in the NOD mouse. PLoS One. 2014;9(1):e86289. 10.1371/journal.pone.0086289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zaccone P, Hall SW: Helminth infection and type 1 diabetes. Rev Diabet Stud. 2012;9(4):272–86. 10.1900/RDS.2012.9.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tang CL, Liu ZM, Gao YR, et al. : Schistosoma Infection and Schistosoma-Derived Products Modulate the Immune Responses Associated with Protection against Type 2 Diabetes. Front Immunol. 2018;8:1990. 10.3389/fimmu.2017.01990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wiria AE, Hamid F, Wammes LJ, et al. : Infection with soil-transmitted helminths is associated with increased insulin sensitivity. PLoS One. 2015;10(6):e0127746. 10.1371/journal.pone.0127746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hussaarts L, García-Tardón N, van Beek L, et al. : Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J. 2015;29(7):3027–39. 10.1096/fj.14-266239 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation