Abstract
Patient: Female, 80
Final Diagnosis: Aortoesophageal fistula
Symptoms: Hematemesis
Medication: —
Clinical Procedure: Thoracic endovascular aortic repair
Specialty: General and Internal Medicine
Objective:
Rare disease
Background:
Hemetemesis is rarely caused by an aorta-esophageal fistula with thoracic aorta aneurysm in patients. This uncommon etiology, AEF/TAA, can potentially rupture and cause death if left untreated. Thoracic endovascular aorta repair places a stent-graft to seal the aneurysm and cover the fistulous track. Open surgical repair is associated with high risk of morbidity and mortality; therefore, TEVAR is a much safer alternative to it. However, recurrent or persistent infection remains a major concern with TEVAR for AEF.
Case Report:
We present a rare case of an 80-year-old woman who presented with complaints of hemetemesis and epigastric pain. The patient underwent a computerized tomography scan, highlighting a TAA and AEF. A stent was placed in the descending thoracic aorta via endovascular approach and a subsequent EGD was negative for any residual bleeding. Methicillin-resistant Staphylococcus aureus was isolated from the patient’s sputum cultures and she was treated with a prolonged course of antibiotics. She presented to the hospital a few weeks later with new-onset hematemesis. Workup identified an AEF. The patient was high risk for open surgical repair due to her comorbid conditions; therefore, an esophageal stent was placed. She was diagnosed with AEF secondary to an infected endovascular thoracic aorta stent.
Conclusions:
Patients who are high risk for open surgical repair from immediate rupture of TAA with AEF can benefit from use of the TEVAR approach. The stent itself is a foreign body; therefore, the risk of infection persists. AEF is a rare but potentially fatal complication of the infected thoracic aortic stent itself.
MeSH Keywords: Aortic Aneurysm, Esophageal Fistula, Hematemesis
Background
Aortoesophageal fistula (AEF), an infrequent etiology of hemetemesis, was first reported by Dubreuil in 1818 [1] as an entity caused by foreign body ingestion, malignancy, or post-operative trauma. It is now known that AEF is often due to penetrating injuries and ruptured thoracic aortic aneurysms (TAA). While TAA can be repaired by open surgical methods, thoracic endovascular aorta repair (TEVAR) is an alternative approach that can be used in high-risk patients. It places a stent-graft to seal the aneurysm and cover the fistulous track. Paradoxically, this innovative attempt to correct TAA, a known cause of aortoesophageal fistula, can itself lead to the formation of an AEF. Here, we report one such case of recurrent AEF 6 weeks after TEVAR.
Case Report
We present a case of an 80-year-old woman with comorbid conditions of atrial fibrillation, chronic obstructive pulmonary disease, hypertension, coronary artery disease, deep venous thrombosis, pulmonary embolism, a right-lung lower-lobe non-malignant mass, and hiatal hernia was transferred to the Emergency Department (ED) with complaints of hematemesis, nausea, epigastric pain, and vomiting. An esophagogastroduodenoscopy (EGD) was performed, which showed a mass that was compressing the distal third of the esophagus, and an ulcer was present. A computerized tomography (CT) scan of the chest identified a TAA measuring 3.5×5.5×6 cm that was compressing the esophagus, and an AEF was also identified. A stent was placed using the TEVAR approach in the descending thoracic aorta, and a repeat EGD was negative for residual bleeding or any leakage of contrast from the site of AEF. Methicillin-resistant Staphylococcus aureus (MRSA) was isolated from sputum cultures, blood cultures remained negative, and stool studies were positive for Clostridium difficile.
The patient was started empirically on antibiotics (ertapenem, oral fluconazole, and vancomycin) for 2 weeks, and then discharged to complete a total 4-week antimicrobial course. She presented to the ED a few weeks later with complaints of back pain due to a recent fall, recurrent hematemesis, and weight loss. She was diagnosed with sepsis due to elevated white blood cell count and MRSA-positive blood cultures and was treated with daptomycin. A CT scan of the chest was repeated, which revealed extraluminal air around the thoracic graft (Figure 1), indicating the stent had been infected. AEF was identified using an EGD, which showed contrast leaking from the esophagus into the aorta (Figure 2). The patient was high risk for open surgical repair due to comorbid conditions; therefore, an esophageal stent was positioned to cover the whole esophagus, after which no further leakage of contrast into the aorta was visualized (Figure 3). The patient was diagnosed with sepsis secondary to an infected thoracic stent, leading to an AEF. The patient died 3 months after the thoracic aorta stent was placed.
Figure 1.
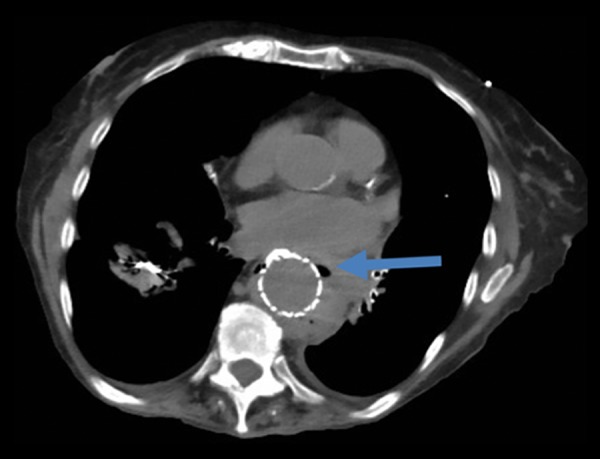
Computed tomography of chest showing extraluminal air (arrow) around the thoracic aorta stent graft.
Figure 2.
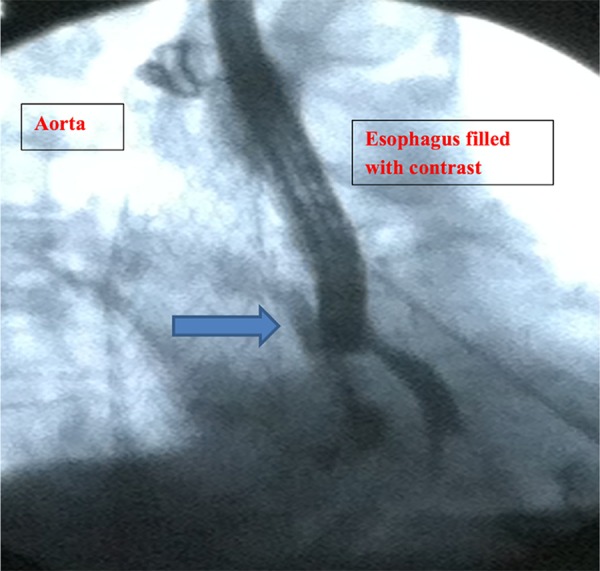
Esophagogastroduodenoscopy showing contrast leaking (arrow) from esophagus into aorta under fluoroscopy.
Figure 3.
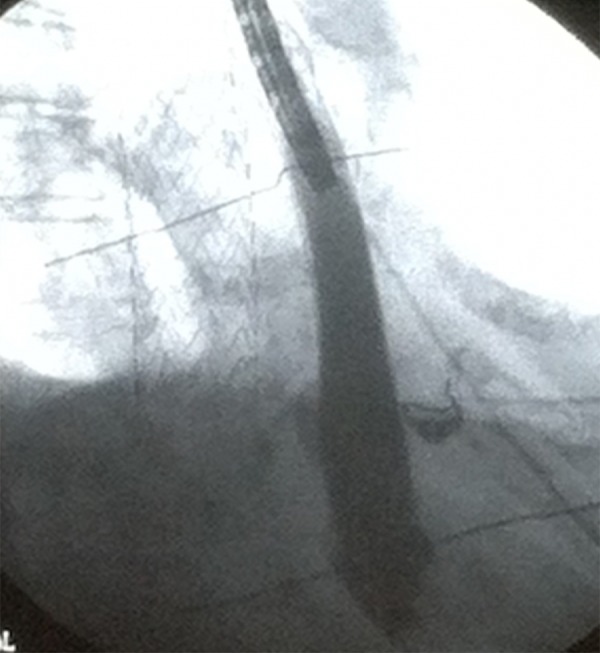
Esophagogastroduodenoscopy showing esophageal wall stent and no further extravasation of contrast from esophagus into aorta.
Discussion
The estimated yearly incidence rate of thoracic aortic aneurysms (TAAs) is 6 cases per 100 000 population [2–4]. Until recently, the only effective treatment method was open aneurysmal repair, with strict prerequisites for surgical candidacy and high risk due to the nature of the surgical technique. TEVAR is a novel, less-invasive treatment method based on the principle of segmental exclusion with an endoluminal prosthesis [5–7].
AEF is an infrequent but a lethal complication of TEVAR; recent studies assess that the incidence of AEF secondary to TEVAR is 1.9–5% [8,9].
There are several potential hypotheses regarding the pathophysiologic mechanisms of AEF formation secondary to TEVAR. The most common cause of AEF status after TEVAR is thought to involve the direct erosion of the aorta secondary to the self-expanding force of the stent graft, which ultimately leads to pressure necrosis of the esophageal wall. The stent graft may also cover, and in-turn blocks, the aortic side branches carrying blood to the esophagus, leading to ischemic necrosis. Finally, the stent graft may become infected, and, as the graft continues to expand, may transmit this infection to the esophagus, leading to wall erosion [8,10].
Chiari described the typical syndrome of AEF as a triad of midthoracic pain or dysphagia, a small self-limiting arterial hemorrhage, followed by an interval free of symptoms, then ultimately leading to overt exsanguination [11,12]. The initial midthoracic pain experienced in AEF can be explained by distension or localized dissection of the aortic wall with esophageal perforation and mediastinitis. Several theories have been postulated regarding the self-limiting arterial hemorrhage and the succeeding lucid interval: 1) temporary occlusion of the fistula due to arterial wall spasm, 2) intravascular hypotension due to initial hemorrhage, and 3) occlusion of the fistula by periaortic hematoma, which is later consumed by infection or digested by gastric contents. Most patients present with massive volumes of bright red blood associated with spontaneous hematemesis.
Endoscopy has been cited as the most sensitive and specific test for diagnosing AEF [13.] In our experience, a diagnosis was made with the help of a chest CT showing air bubbles trapped between the thoracic aorta and esophagus (Figure 1). An EGD was performed to confirm the existence of an AEF. While the initial EGD showed only an ulcer, a second EGD performed at the presentation of the AEF demonstrated extravasation of dye from the esophagus into the aorta (Figure 2). We diagnosed AEF using the combination of patient history (prior TEVAR, hematemesis, and weight loss due to dysphagia) and diagnostic studies (findings of air bubbles on chest CT, with extravasation of dye into aorta on EGD). Moreover, AEF should be suspected in a patient with fever and increased serum inflammatory markers with a history of TEVAR [8].
The mortality rate of AEF secondary to TEVAR is extremely high with either conservative (88.3–100%) or surgical treatment (63.6%) [8,9]. However, the definitive treatment remains surgical debridement, resection of the esophagus, and reconstruction of aorta and esophagus using grafts [14,15]. Operative management of AEF is frequently complicated by mediastinitis, sepsis, and hemorrhage [16]. Recently, TEVAR was utilized for treatment of post-surgical AEF [17]. In our case, open surgical repair would have been extremely high risk due to the patient’s poor general health and multiple comorbidities. The patient was treated with a full-wall esophageal stent (Figure 4) in addition to the previously placed stent in the thoracic aorta to occlude the bleeding. Upon review of the literature, TEVAR is best suited for emergency treatment of AEF for hemodynamic stabilization for possible palliation vs. bridging technique until patient is able to tolerate a more definitive open surgical method, as it does contain risk of infection [18].
Figure 4.
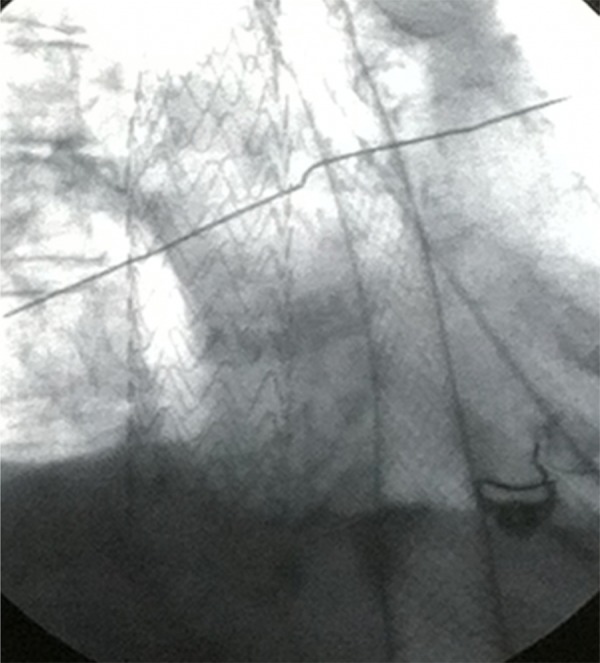
Esophageal wall stent and thoracic aorta stent visualized under fluoroscopy.
However, conservative management involves decreasing stress on the esophageal lesion with treatment by proton pump inhibitors and enteral feeding via percutaneous gastrostomy. While antibiotic treatment is indicated in cases complicated by mediastinitis, prolonged courses have been associated with elevated mortality [19]. Despite these efforts, the outcome of conservative management is almost invariably fatal (88.3–100%) due to complications, as was the case in our patient.
Conclusions
AEF is a known and fatal complication of TEVAR, and this case reiterates its mortality in high-risk patients who are deemed inappropriate for open aneurysmal repair. This case also demonstrates the occurrence of AEF after TEVAR for TAA, indicating the need for open repair of AEF/TAA as a more definitive therapy. It would also be prudent to suspect AEF in a patient presenting with hematemesis and a history of TEVAR. We report a survival time of 12 weeks after the initial TEVAR for AEF/TAA.
Footnotes
Conflicts of interest
None.
References:
- 1.Dubreuil O. Observation sur la perforation de l’oesophage et de l’aorta thoracique par une portion d’os avale: Avee de refiexions. J Univ Sci Med. 1818;9:357–63. [in French] [Google Scholar]
- 2.Bickerstaff LK, Pairolero PC, Hollier LH, et al. Thoracic aortic aneurysms: A population-based study. Surgery. 1982;92(6):1103–8. [PubMed] [Google Scholar]
- 3.Pressler V, McNamara JJ. Aneurysm of the thoracic aorta. Review of 260 cases. J Thorac Cardiovasc Surg. 1985;89(1):50–54. [PubMed] [Google Scholar]
- 4.Pressler V, McNamara JJ. Thoracic aortic aneurysm: natural history and treatment. J Thorac Cardiovasc Surg. 1980;79(4):489–98. [PubMed] [Google Scholar]
- 5.Fann JI, Miller DC. Endovascular treatment of descending thoracic aortic aneurysms and dissections. Surg Clin North Am. 1999;79(3):551–74. doi: 10.1016/s0039-6109(05)70024-4. [DOI] [PubMed] [Google Scholar]
- 6.Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340(20):1539–45. doi: 10.1056/NEJM199905203402003. [DOI] [PubMed] [Google Scholar]
- 7.Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340(20):1546–52. doi: 10.1056/NEJM199905203402004. [DOI] [PubMed] [Google Scholar]
- 8.Eggebrecht H, Mehta RH, Dechene A, et al. Aortoesophageal fistula after thoracic aortic stent-graft placement: A rare but catastrophic complication of a novel emerging technique. JACC Cardiovasc Interv. 2009;2(6):570–76. doi: 10.1016/j.jcin.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Eggebrecht H, Baumgart D, Radecke K, et al. Aortoesophageal fistula secondary to stent-graft repair of the thoracic aorta. J Endovasc Ther. 2004;11(2):161–67. doi: 10.1583/03-1114.1. [DOI] [PubMed] [Google Scholar]
- 10.Hance KA, Hsu J, Eskew T, Hermreck AS. Secondary aortoesophageal fistula after endoluminal exclusion because of thoracic aortic transection. J Vasc Surg. 2003;37(4):886–88. doi: 10.1067/mva.2003.159. [DOI] [PubMed] [Google Scholar]
- 11.Gavens E, Zaidi Z, Al-Jundi W, Kumar P. Aortoesophageal fistula after endovascular aortic aneurysm repair of a mycotic thoracic aneurysm. Int J Vasc Med. 2011;2011:649592. doi: 10.1155/2011/649592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiari H. Ueber Fremdkorpeverletzung des oesophagus mit aortenperforation. Ber Klin Wochenschr. 1914;51:7–9. [in German] [Google Scholar]
- 13.Akaraviputh T, Sriprayoon T, Prachayakul V, Sakiyalak P. Endoscopic diagnosis of secondary aortoesophageal fistula. Endoscopy. 2008;40(Suppl. 2):E90. doi: 10.1055/s-2007-995549. [DOI] [PubMed] [Google Scholar]
- 14.Czerny M, Zimpfer D, Fleck T, et al. Successful treatment of an aortoesophageal fistula after emergency endovascular thoracic aortic stent-graft placement. Ann Thorac Surg. 2005;80(3):1117–20. doi: 10.1016/j.athoracsur.2004.02.136. [DOI] [PubMed] [Google Scholar]
- 15.Girdauskas E, Falk V, Kuntze T, et al. Secondary surgical procedures after endovascular stent grafting of the thoracic aorta: Successful approaches to a challenging clinical problem. J Thorac Cardiovasc Surg. 2008;136(5):1289–94. doi: 10.1016/j.jtcvs.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 16.Iguchi A, Miyazaki S, Akimoto H, et al. Successful management of secondary aortoesophageal fistula with graft infection. Thorac Cardiovasc Surg. 2001;49(2):126–28. doi: 10.1055/s-2001-11707. [DOI] [PubMed] [Google Scholar]
- 17.Shiraishi S, Watarida S, Matsubayashi K, et al. Successful management of an aortoesophageal fistula resulting from an aneurysm of the thoracic aorta with a covered stent. J Cardiovasc Surg (Torino) 2002;43(1):95–98. [PubMed] [Google Scholar]
- 18.Dalio MB, Dezotti NR, Ribeiro MS, et al. Aortogastric fistula due to a penetrating atherosclerotic aortic ulcer. Ann Vasc Surg. 2015;29(8):1659e21–5. doi: 10.1016/j.avsg.2015.06.080. [DOI] [PubMed] [Google Scholar]
- 19.Canaud L, Ozdemir BA, Bee WW, et al. Thoracic endovascular aortic repair in management of aortoesophageal fistulas. J Vasc Surg. 2014;59(1):248–54. doi: 10.1016/j.jvs.2013.07.117. [DOI] [PubMed] [Google Scholar]


