ABSTRACT
To investigate iliotibial band (ITB) diameter thickness at the greater trochanter in patients requiring iliotibial band release who have failed conservative modalities, in comparison to an asymptomatic patient population. A total of 68 subjects were selected to be reviewed using T2 axial plane MRI. The ITB diameter thickness was measured in 34 subjects who underwent surgical ITB release, and compared with a match-paired asymptomatic hip cohort consisting of 34 subjects. ITB diameter thickness was measured at the thickest location for each subject twice by two different examiners. Inter/intra class correlation coefficient was determined for ITB measurement technique accuracy, and the presence of recalcitrant proximal hip pain was evaluated. Interclass correlation coefficient with 95% confidence was measured to be 0.953. The average thickness for ITB surgical release subjects was measured to be 5.61 ± 2.10 mm, and for asymptomatic subjects 3.77 ± 0.79 mm (P < 0.001). The results of this study demonstrate a statistically significant positive relationship of an increased diameter thickness in the ITB in symptomatic patients who failed conservative therapy and underwent surgical intervention for treatment.
INTRODUCTION
Pain, tenderness or weakness associated with abnormal iliotibial band (ITB) anatomy and kinematics is a common occurrence at the lateral hip. The conditions associated with pain at hip and knee joint locations are attributed to many factors and have been studied independently. Prior classification of laterally based hip pain was referred as ‘trochanteric bursitis’. In recent years this term has been substituted with greater trochanteric pain syndrome (GTPS). GTPS now encompasses several hip pathologies including trochanteric bursitis, gluteus medius and minimus tendinopathy, and externa coxa saltans [1–4]. Pain at the level of the hip joint resulting from the ITB can be associated with GTPS and is addressed with conservative modalities including steroid injections, anti-inflammatory medicines and physical therapy treatment [2]. Failed cases of conservative treatment for ITB-related GTPS require surgical intervention [5].
Birnbaum et al.’s detailed comprehensive analysis describes the ITB as a ‘thickening of the fascia originating from the tensor facia latae and gluteus maximus’ [6]. The superficial and deep layers of the ITB encase the tensor facia latae, with the deep layer attaching to the hip joint capsule through the tensor facia latae fibers. The ITB converges with the gluteus maximum tendon at the linea aspera portion of the femur. ITB inserts into Gerdy’s tubercle at the head of the fibula and patella [6]. ITB strain resulting from increased hip abduction and knee external rotation may present as distal hip pain due to the extended relationship of the ITB insertion at Gerdy’s tubercle [7]. The pain symptoms can be further associated with weak muscular control and stiffness in the knee joint [7].
Anatomical deviations that span the length of the iliotibial tract from the pelvis to knee directly influence kinematic responses. ITB thickness in symptomatic patients is not reported currently in the literature, although references do cite increased thickness as a cause of snapping hip and GTPS [5]. The current study aims to determine increased ITB thickness as a causative factor for recalcitrant GTPS. The hypothesis states patients with symptomatic lateral hip pain who have failed conservative treatment and undergone an ITB release surgery will have an increased diameter thickness at the level of the posterior facet of the greater trochanter, compared with asymptomatic patients at the same level of the hip.
MATERIALS AND METHODS
The ITB diameter thickness of subjects who failed conservative treatment modalities and underwent surgical intervention were examined and compared to a match-paired asymptomatic population. Increased ITB thickness subjects were identified through surgical records of patients treated between 2009 and 2016. Surgical records were retrospectively reviewed for the presence of lateral hip pain, snapping hip syndrome or GTPS. Further inclusion criteria included patients that had failed conservative treatment and underwent surgery to release the proximal ITB tract. Subjects were excluded from the cohort if they presented with previous hip trauma or fracture, prior hip surgery, hip dysplasia or incomplete MRI or medical record. In total, 34 subjects were identified as meeting inclusionary criteria. The asymptomatic subjects (n = 34) were match paired to control for gender, age, height and BMI. In total, 68 subjects were included for analysis.
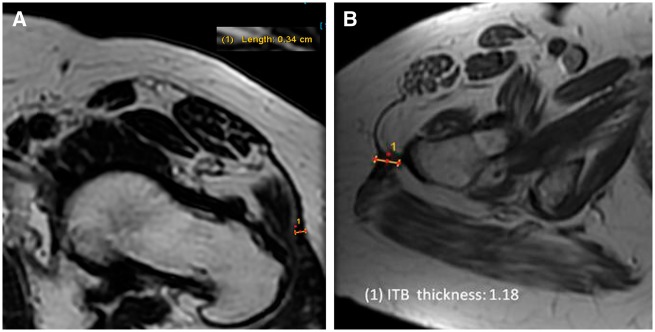
ITB diameter thickness was measured in the axial plane of MRIs. The ITB measurement location was considered as the region of greatest thickness between the most proximal images of the greater trochanter to the most proximal sequence showing the lesser trochanter (Fig. 1). The MRI’s for both subject groups were examined and repeat measurements taken for inter/intra class correlation (ICC) analysis. Two examiners independently assessed ITB thickness measurements for both subject groups. The averages of the two sets were used for statistical analysis. An ICC coefficient analysis was performed for the collected values to determine the validity of the measurements. The images were then reviewed to document the location of the greatest thickness as anterior, lateral or posterior.
Fig. 1.
ITB measurement. The ITB measurement location was considered as the region of greatest thickness between the most proximal images of the greater trochanter to the most proximal sequence showing the lesser trochanter. (A) Asymptomatic subject measurement. ITB thickness was measured to be 0.34 cm. (B) Increased ITB thickness measurement. ITB thickness was measured to be 1.18 cm.
Statistical analysis was performed with SPSS statistical software package (version 22.0) (IBM, Armonk, NY, USA). A two-tailed Student t-test assuming unequal variance was used to compare differences between groups. Pearson’s correlation coefficients were utilized to assess relationship between demographics and ITB thickness.
RESULTS
The 34 ITB release subjects included 27 females and 7 males, and the 34 asymptomatic patients included 25 females and 9 males. The average age of the ITB release subjects that complained of proximal hip pain and failed conservative treatment was 55.7 years, and average age of asymptomatic patients was 51 years. The average height of the ITB release subjects was 65.79 inches, and average height of asymptomatic patients was 67.03 inches. The average weight and BMI of the individuals that had lateral hip pain and failed conservative treatment was 171.9 lbs and 27.7, respectively, and average weight and BMI of asymptomatic patients was 177.67 lbs and 27.61, respectively. No fatty atrophy of the gluteus medius or minimus muscles was observed when reviewing MRI.
An ICC coefficient with 95% confidence interval was found to be 0.953. ICC coefficient yielded high correlation results for examiner one and examiner two (0.95 and 0.91) when measuring ITB diameter thickness in MRIs on the asymptomatic group.
The average value of diameter thickness for the ITB in the symptomatic test group was 5.61 ± 2.10 mm (SEM: 0.36). The average value for the asymptomatic control group was 3.77 ± 0.79 mm (SEM: 0.13). A two-tailed t-test for equality assuming unequal variance was found to be significant for ITB thickness with a value of P < 0.001 (Table I). Subject demographic data and ITB thickness were analysed and no correlations between demographic measures and ITB thickness were determined statistically significant.
Table I:
Mean ITB diameter thickness
| Average thickness (mm) | Standard deviation | t-test | |
|---|---|---|---|
| ITB release (n = 34) | 5.61 | ±2.10 | P < 0.001 |
| Asymptomatic (n = 20) | 3.77 | ±0.79 |
DISCUSSION
This retrospective study was intended to investigate the relationship between increased ITB thickness and recalcitrant GTPS, and ITB thickness location. ITB thickness was investigated in this cohort because they had failed conservative treatment modalities. This factor may be attributed to an increased ITB density that is too thick to be addressed through physical therapy and injections alone. The average thickness for subjects who underwent ITB release was 5.61 mm, whereas the asymptomatic group thickness was 3.77 mm, as measured with MRI at the posterior facet. Statistical significance was observed between the ITB release and control group (P < 0.001). The location of greatest ITB thickness was also investigated by assigning a designation of lateral, anterior or posterior for each subject in both sample groups. The greatest ITB thickness was located laterally in 23 out of 34 subjects (67.6%), 20.5% anteriorly and 11.7% posteriorly in the control group measured. The ITB release group displayed similar results with 41% of greatest ITB thickness located laterally and 29% located both anteriorly and posteriorly. The results from the study confirm the initial hypothesis that patients with symptomatic hip pain have increased ITB diameter thickness.
About 73.7% of patients who underwent endoscopic ITB release complained of pain, weakness or tears in the gluteal and abductor muscles. The synergistic responsibilities of the surrounding musculotendinous structures around the hip joint are responsible for alleviating stress in osseous structures. Muscle fatigue and weakness may lead to overuse and pain of surrounding tissue. The presence of weakness or failure in musculature surrounding the hip joint may require the ITB to absorb increased loading [8]. Excessive loading to femoro-pelvic osseous components through increased ITB stress can further influence muscular weakness [9]. Reflex inhibition is usually attributed to pain, and has been described as a possible cause of muscle weakness [10]. This inhibition may indicate a cause and effect relationship cycle between friction, pain, muscle wasting and accessory muscle compensation.
GTPS encompasses gluteus medius/minimus tears, greater trochanteric bursitis and external coxa sultans [11]. The several symptoms that comprise GTPS help support a causal theory of hip weakness increasing friction between the ITB and the greater trochanter. Posterior ITB thickening contributes to snapping hip, as it is positioned at the posterior section of the greater trochanter [5]. A study by Strauss et al. found that the repetitive snapping of the ITB in external coxa sultans and overuse of the ITB can result in a thickened ITB and trochanteric bursitis [2]. Trochanteric bursitis and thickened ITB are both associated with inflammation brought on by repetitive rubbing and friction to the anterior/lateral/or posterior facet by the ITB [12]. During the gait cycle snapping is exacerbated during flexion when the ITB is maximally stretched [13] and slides anteriorly over the greater trochanter, and repeats extension as the ITB moves posteriorly. Repetitive motions may lead to secondary issues including trochanteric bursitis [5, 14].
Diagnostic procedure for hip pain should account the five levels of the hip joint including the osseous, musculotendinous, neurovascular, capsulolabral and kinematic chain [15]. The length of the IT tract extends from the iliac crest to Gerdy’s tubercle in the knee, and any abnormalities associated to either level can accelerate the development of pain symptoms above the pelvis or below the knee. GTPS is more commonly observed in the female population [2, 4, 16–18]. The ITB stabilizes the hip and knee to resist knee internal rotation and hip adduction [7, 19]. Fredericson et al. reported female runners with ITBS have an increased peak trunk ipsilateral flexion during the stance phase of running, when compared with those without ITBS [20]. Proper alignment of the trunk can influence the orientation of the pelvis which can directly affect the functionality of the abductor muscles [20]. Noehren et al. revealed differences in trunk biomechanics and biomechanical orientations of the pelvis and hip in weight bearing positions in patients with and without ITBS [21]. Recent investigations support this kinematic relationship and suggest ITB related contributions to low back pain [4, 17].
Physical therapy is an effective treatment for ITB pain, primarily in cases of decreased muscular function and control. Weakness in the gluteal or tensor facia latae muscles precipitates hip internal rotation resulting in increased strain in the ITB and consequent friction with the greater trochanter during movement [22]. A study conducted on ITB releases for snapping hip patients reported favorable outcomes through gluteal and abductor muscle strengthening [12]. An additional study started patients on a 6-week abductor strengthening physical therapy regimen and reported no pain in 91% of subjects at 6-month follow up [20]. These outcomes further support the proposal of ITB absorbing added stabilization responsibility as a compensatory mechanism to muscle weakness. Nonoperative treatment has an average success rate of 54% in treating ITBS, and is often the initial consideration to alleviate friction and associated ITB inflammation and tendinopathies [23]. Further studies should address conservative treatment failure as a predictor to surgical treatment.
In the case of failed conservative treatment for ITB pain, surgical intervention is recommended. Surgical release/lengthening relieves pain through alleviating excessive ITB tension and compression of the posterior facet; as this region is innervated by the superficial inferior gluteal nerves [24]. The senior author recommends ITB lengthening with a transverse release at the thickest region of the ITB. Z-plasty of the ITB is a surgical treatment for external coxa sultans and is performed in the thickened portion of the band, anterior to the center of the greater trochanter. The post-operative ITB is thinner and elongated. The Z-plasty investigation attributes the elimination of pain to the patients having a longer and thinner ITB [24]. The ITB release can also be performed endoscopically, as described by Ilizaliturri et al. Of the 10 patients reported using endoscopic ITB release, 100% reported relief of pain and 91% resolution of snapping symptoms. Resultant data suggests an evaluation of the ITB with dynamic assessment is necessary intraoperatively.
Limitations of this study include its retrospective nature, and the subject population is solely from one clinic. The variables of pain diagnosis and associated symptoms were not included in this study as the primary evaluation included the relationship between ITB thickness and pain. Ober’s test was not performed on all subjects when assessing tenderness at the posterior facet. The authors also suggest investigating the root cause of ITB pain, specifically whether ITB thickness is a primary symptom or a reactionary response to biomechanical abnormality. The authors also recommend evaluating osseous density and potential inferior nerve webbing.
CONCLUSION
The results of this study demonstrate a statistically significant positive relationship of an increased diameter thickness in the ITB in symptomatic patients who failed conservative therapy and underwent surgical intervention for treatment.
ACKNOWLEDGEMENTS
The authors are thankful for ongoing research support from the Baylor Scott & White Research Institute.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
The authors report no source of funding for this retrospective study.
REFERENCES
- 1. Redmond JM, Chen AW, Domb BG.. Greater trochanteric pain syndrome. J Am Acad Orthop Surg 2016; 24: 231–40. [DOI] [PubMed] [Google Scholar]
- 2. Strauss EJ, Nho SJ, Kelly BT.. Greater trochanteric pain syndrome. Sports Med Arthrosc 2010; 18: 113–9. [DOI] [PubMed] [Google Scholar]
- 3. Mallow M, Nazarian L.. Greater trochanteric pain syndrome diagnosis and treatment. Phys Med Rehabil Clin N Am 2014; 25: 279–89. [DOI] [PubMed] [Google Scholar]
- 4. Segal NA, Felson DT, Torner JC. et al. Greater trochanteric pain syndrome: epidemiology and associated factors. Arch Phys Med Rehabil 2007; 88: 988–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ilizaliturri VM, Martinez-Escalante FA, Chaidez PA, Camacho-Galindo J.. Endoscopic iliotibial band release for external snapping hip syndrome. Arthrosc J Arthrosc Relat Surg 2006; 22: 505–10. [DOI] [PubMed] [Google Scholar]
- 6. Birnbaum K, Siebert CH, Pandorf T. et al. Anatomical and biomechanical investigations of the iliotibial tract. Surg Radiol Anat 2004; 26: 433–46. [DOI] [PubMed] [Google Scholar]
- 7. Aderem J, Louw QA.. Biomechanical risk factors associated with iliotibial band syndrome in runners: a systematic review. BMC Musculoskelet Disord 2015; 16: 356.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fairclough J, Hayashi K, Toumi H. et al. The functional anatomy of the iliotibial band during flexion and extension of the knee: implications for understanding iliotibial band syndrome. J Anat 2006; 28: 309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Phillips D, Case LE, Griffin D. et al. Physical therapy management of infants and children with hypophosphatasia. Mol Genet Metab 2016; 119: 2–7. [DOI] [PubMed] [Google Scholar]
- 10. Stokes M, Young A.. The contribution of reflex inhibition to arthrogenous muscle weakness. Clin Sci (Lond) 1984; 67: 7–14. [DOI] [PubMed] [Google Scholar]
- 11. Craig RA, Gwynne Jones DP, Oakley AP. et al. Iliotibial band Z-lengthening for refractory trochanteric bursitis (greater trochanteric pain syndrome). ANZ J Surg 2007; 77: 996–8. [DOI] [PubMed] [Google Scholar]
- 12. Zini R, Munegato D, De Benedetto M. et al. Endoscopic iliotibial band release in snapping hip. Hip Int 2013; 23: 225–32. [DOI] [PubMed] [Google Scholar]
- 13. Eng CM, Arnold AS, Lieberman DE. et al. The capacity of the human iliotibial band to store elastic energy during running. J Biomech 2015; 48: 3341–8. [DOI] [PubMed] [Google Scholar]
- 14. Allen WC, Cope R.. Coxa saltans: the snapping hip revisited. J Am Acad Orthop Surg 1995; 3: 303–8. [DOI] [PubMed] [Google Scholar]
- 15. Martin HD, Palmer IJ.. History and physical examination of the hip: the basics. Curr Rev Musculoskelet Med 2013; 6: 219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams BS, Cohen SP.. Greater trochanteric pain syndrome: a review of anatomy, diagnosis and treatment. Anesth Analg 2009; 108: 1662–70. [DOI] [PubMed] [Google Scholar]
- 17. Tortolani PJ, Carbone JJ, Quartararo LG.. Greater trochanteric pain syndrome in patients referred to orthopedic spine specialists. Spine J 2002; 2: 251–4. [DOI] [PubMed] [Google Scholar]
- 18. Bird P, Oakley S, Shnier R. et al. Prospective evaluation of magnetic resonance imaging and physical examination finding in patients with greater trochanteric pain syndrome. Arthritis Rheum 2001; 44: 2138–45. [DOI] [PubMed] [Google Scholar]
- 19. Ferber R, Noehren B, Hamill J. et al. Competitive female runners with a history of iliotibial band syndrome demonstrate atypical hip and knee kinematics. J Orthop Sports Phys Ther 2010; 40: 52–8. [DOI] [PubMed] [Google Scholar]
- 20. Fredericson M, Cookingham CL, Chaudhari AM. et al. Hip abductor weakness in distance runners with iliotibial band syndrome. Clin J Sport Med 2000; 10: 169–75. [DOI] [PubMed] [Google Scholar]
- 21. Noehren B, Davis I, Hamill J.. Prospective study of the biomechanical factors associated with iliotibial band syndrome. Clin Biomech 2007; 22: 951–6. [DOI] [PubMed] [Google Scholar]
- 22. Ekman EF, Pope T, Martin DF. et al. Magnetic resonance imaging of iliotibial band syndrome. Am J Sports Med 1994; 22: 851–4. [DOI] [PubMed] [Google Scholar]
- 23. Holmes JC, Pruitt AL, Whalen NJ.. Iliotibial band syndrome in cyclists. Am J Sports Med 1993; 21: 419–24. [DOI] [PubMed] [Google Scholar]
- 24. Provencher MT, Hofmeister EP, Muldoon MP.. The surgical treatment of external coxa saltans (the snapping hip) by Z-plasty of the iliotibial band. Am J Sports Med 2004; 32: 470–6. [DOI] [PubMed] [Google Scholar]