ABSTRACT
The exact pathophysiology of osteonecrosis of the femoral head (ONFH) is still unknown. There is evidence to suggest that in ON there is decreased population and altered function of the mesenchymal stem cells (MSCs) of the femoral head. This could influence both the actual occurrence of ON itself and the repair process that follows. Hence, in such an environment it only is rational to consider the use of cell-based treatments to potentially regenerate lost or damaged bone. The aim of this review is to provide an up-to-date, evidence-based information in the use of cell therapies in the treatment of nontraumatic ONFH and the use of hip arthroscopy in the field.
INTRODUCTION
Nontraumatic osteonecrosis of the femoral head (ONFH) typically affects relatively young, active patients (aged 20–40 years) and frequently follows an unrelenting course resulting in considerable loss of function [1]. It occurs when the cells of the trabecular bone spontaneously die due to a vascular insult to the femoral head blood supply. The exact pathophysiology of this bone infarct is not thoroughly understood and various ‘incriminating’ factors have been identified. However, the final outcome is the adjacent articular cartilage collapse and subsequent osteoarthritis in approximately 60% of even asymptomatic patients [2, 3].
Treatment is based on a number of parameters, such as lesion characteristics (size, the presence of collapse, acetabular involvement), patient’s age and comorbidities [2, 4]. The optimal treatment modality has not yet been identified. Several algorithms of medical and surgical treatments have been developed to delay its progression, with variable success [5].
Surgically, total hip replacement (THR) is the most frequent intervention for postcollapse treatment, and core decompression (CD) is commonly performed for symptomatic, precollapse cases [6]. Historically, THR for osteonecrosis (ON) had poor results, attributed to the young and active character of the patients. During the 1980s and early 1990s, studies reported high failure rates [7, 8]. More recent reports and systematic reviews show that the introduction of newer implants and better surgical technique, consistently deliver better results [9, 10]. Nevertheless, since we are dealing with mostly young patients the possibility of failure and revision of the THR constitutes a reality.
As a result, there has been an increased focus on early interventions for ONFH aimed at preservation of the native articulation. During early stage disease, the most common joint preserving procedure performed is CD or percutaneous drilling aiming to increase blood flow to the necrotic area by reducing the intraosseous pressure, thus alleviating pain and improving function [5, 6]. Adjunctive techniques such as cell-based therapies have been described in an attempt to improve CD outcomes. This treatment strategy is based on the hypothesis that the harvested cells injected or embedded into the necrotic zone of the femoral head will repopulate the lesion, restore the local cell population and enhance regeneration and remodeling [11, 12].
The aim of this review is to provide an up-to-date, evidence-based guide to the use of cell therapy in the treatment of nontraumatic ON of the femoral head.
PATHOPHYSIOLOGY OF ON
Most of the theories regarding the mechanism of spontaneous ONFH point toward alterations in intravascular blood flow, leading to decreased oxygenation, toxicity and cellular death. There are several recognized conditions and environmental insults that predispose patients to ON such as high-dose corticosteroid administration, alcohol abuse, hemoglobinopathy, Gaucher disease and coagulopathies [1, 13, 14].
A number of papers that have studied the exogenous insult of alcohol and corticosteroid administration suggest that they have a profound effect on bone marrow stromal cell differentiation, blood supply and oxygenation of the femoral head [15–21]. Use of corticosteroids may deviate bone marrow stromal cells into the adipocytic pathway as opposed to the osteoblastic pathway [22–24]. A clinical study has also shown a decreased osteogenic differentiation in cells harvested from patients with corticosteroid or alcohol-associated ONFH [15]. Particular attention in this setting has been paid to the multipotent mesenchymal stem cells (MSCs), their ability to multiply and their capability to differentiate into various cellular types, such as osteoblasts, osteocytes, chondrocytes and adipocytes [12].
In ONFH, the decreased population and altered function of the MSCs may influence the two different events in the pathogenesis of ON; the actual occurrence of ON itself and the bone repair process that follows.
Accepting the premise that an important part of the underlying pathology in ON is cell deficiency, the next rational step is to consider the use of cell-based treatments to enhance the regeneration of lost or damaged bone.
USE OF STEM CELL THERAPY IN ON
Although clinical experience has shown that dead bone may be replaced by living bone, the osteogenic potential for repair in ON is low. A decrease in osteogenic stem cells in the femoral head has been observed beneath the necrotic lesion up to the intertrochanteric region which might account for the insufficient creeping substitution in bone remodeling of the femoral head after ON. This can explain the fact that although reconstruction and repair has been observed after CD, it is usually slow and inadequate [23, 24].
Even though, MSCs act via not-completely understood multifaceted pathways, it seems that they perform two separate functions that can influence the natural history of ON: (i) secretion of a wide spectrum of factors with anti-inflammatory, antiapoptotic, proangiogenic, proliferative or chemo attractive capacities, and (ii) initiating the differentiation process for functional tissue restoration [25].
In clinical practice, a common source for MSCs is bone marrow mononuclear cells (BMMCs) due to (i) their ease of harvest (iliac crest), (ii) their abundance and (iii) their marked osteogenic properties and interaction with angiogenic cytokines that elicit the formation of new blood vessels [25–28]. Tracking studies of BMMCs implanted directly into the necrotic area in ON showed 56% of installed cells remained in the implantation site 24 h after implantation. Similar studies in animal models also demonstrated the survival and multiplications of these cells up to 12 weeks postimplantation [29–31].
In 2002, Hernigou pioneered the technique of injecting MSCs combined with standard CD into the area of necrosis introducing the basic science of biology in ON [32]. In a study of 189 hips (116 patients), MSCs (in the form of concentrated iliac crest bone marrow) were injected through a CD tract into the area of necrosis. Patients with early (precollapse) disease had excellent results at 5–10 years of clinical follow-up, with only 9 of 145 hips requiring THR [33]. He also reported an association between the outcome of ONFH and the quantity of cells transplanted into the femoral head and recommended a specific minimum number of cell transplantation [11, 33, 34]. A total of 35 000 MSCs should be the target number to load in an osteonecrotic femoral head in order to re-establish the same number of MSCs as in a normal femoral head [25].
BONE MARROW HARVESTING AND PREPARATION TECHNIQUE
The most common place to collect bone marrow is either the anterior or posterior part of the iliac crest depending on the patient positioning and surgeon preference. Collection of bone marrow from the iliac crest can be accomplished by the use of a single beveled aspirating needle. A number of such systems are available commercially.
The highest quality of bone marrow aspiration (number of stem/progenitor cells) is when the aspirate is in small volumes (1–2 ml) and from different locations since, when a greater volume is drawn from any single area the peripheral blood infiltrates and dilutes the aspirate [35]. Technically, in order to achieve this, the needle is turned during successive aspirations thereby affording access to the largest possible space. After one full turn, the needle is slowly moved toward the surface and the process is repeated.
The pooled aspirates (the volume can range between 30 and 120 ml) is filtered to separate cellular aggregates and fat. The aspirated material should be reduced in volume in order to increase the stem cell concentration. This is done with centrifugation, which separates the red blood cells (nonnucleated cells) and plasma in such a way as to retain only the nucleated cells: mononuclear stem cells, monocytes and lymphocytes. Removing the nonnucleated cells reduces the aspirate to a concentrated myeloid suspension of stem cells which can be used for reinjection.
INTRAOSSEOUS INJECTION OF MESENCHYMAL STEM CELLS
CD is the most common procedure performed for small- or medium-sized lesions, especially at the precollapse stage [14, 36]. It is a generic term that is often accompanied with supplemental procedures (vascularized or nonvascularized graft, injection of cells, graft, electrical stimulation, etc.) [37].
Retrograde CD can be technically demanding, requiring biplanar imaging for proper placement of the core drill. Hip arthroscopy can supplement fluoroscopic-assisted retrograde drilling, by guiding the accurate placement of the tip of the drill into the area of chondral softening or irregularity or the ‘ballottable’ segment of the femoral head, which corresponds to the underlying necrotic lesion [38].
A modification to the retrograde drilling was proposed by Mont where the CD is performed through a window at the femoral head–neck junction (trapdoor technique) [39]. However, this procedure requires an extensive dissection, and it is also technically more difficult than a standard CD [37].
In a less invasive fashion, drilling can be guided arthroscopically under direct visualization by inserting the drill in the peripheral compartment thought the anterior or an auxiliary portal in the direction of the necrotic lesion under image guidance (Fig. 1) [38, 40]. Following the drilling, the thin hip arthroscopy nitinol guidewire can be inserted in the femoral head following the CD track and then, over it, the cannulated arthroscopic needle. This ensures the accuracy of the placement of the injected MSCs in the necrotic lesion. Backflow of the injected medium is not observed since the fluid diffuses to surrounding cancellous bone of the femoral head. During the injection time, the pressure in the femoral head can rise, but a normal pressure pattern is restored once the injection is finished [25]. Anecdotally, if excision of the cam deformity is done in conjunction with the CD drilling, overflow of the injected fluid can be observed from the exposed cancellous bone of the osteoplasty site after the injection of the first 10–15 ml, allowing the osteoplasty to act as a release ‘valve’ to the increased pressure.
Fig. 1.
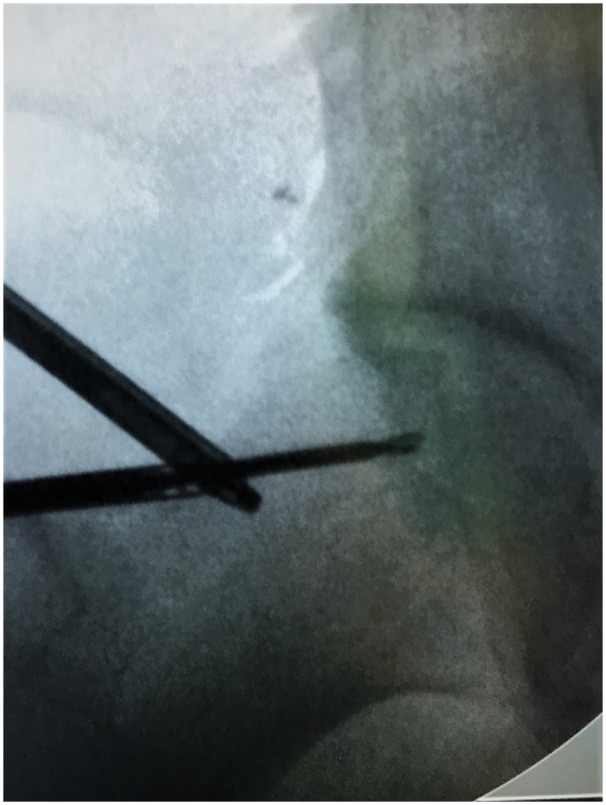
Image intensifier view of CD under arthroscopic guidance.
DISCUSSION: LITERATURE-REPORTED OUTCOMES FOLLOWING CELL-BASED TREATMENT
Nontraumatic ONFH typically affects relatively young, active patients where the consequences of the disease could severely impact their leisure activities and work status. There is consequently great interest in trying to preserve the FH whenever possible. Unfortunately, there is no consensus among surgeons regarding the optimal operative management for these patients. Numerous procedures have been proposed to treat this condition, including CD, vascularized grafts and osteotomy [41].
The reason for this cacophony is that there are so many uncontrolled variables (diagnosis associated with ON, age, comorbidities, etc.) that no appropriately powered randomized trials comparing different surgical techniques exist. Another difficulty to allow safe conclusions to be drawn is that patients are evaluated using different classifications systems [3, 31] preoperatively and evaluation outcomes postintervention.
The use of cell therapy as a supplement to the surgical techniques in the treatment of ON came from the recognition that the capacity of osteoblastic cells and MSCs pool are significantly reduced in the proximal femur [23, 24]. Hence supplementing the femoral head with viable multipotent cells could be considered as an appropriate pathophysiological approach for the treatment of this condition. In 2002, Hernigou pioneered the technique of injecting MSCs combined with standard CD into the area of necrosis introducing the basic science of biology in ON [32]. Since his work, a number of cell therapy options can be found in the literature.
Bone morphogenetic proteins (BMPs)
In 2004, Lieberman et al. were the first to report a retrospective evaluation of 15 patients (17 hips) with symptomatic ON of the hip treated with CD combined with an allogeneic antigen-extracted, autolysed fibular allograft and 50 mg of partially purified human BMP and noncollagenous protein [42]. The results were encouraging but there was no comparative group and therefore the exact therapeutic impact of BMP on the overall outcome cannot be verified.
A large case series (39 hips) on the use of BMPs in ONFH was published by Seyler et al. [43]. They used the trap door technique to make a window at the head–neck junction to remove the necrotic bone and to pack the excavated area with autologous cancellous bone graft, marrow and OP-1(BMP 7). The overall early clinical success rate was 67% after a mean follow-up period of 36 months. The size of the lesion and the staging of ONFH had a significant influence on the survival of the hips in their series.
In 2014, Sun et al. evaluated clinical outcomes of impacted bone graft with or without human-recombinant BMP-2 for ONFH on 42 patients (72 hips) [44]. After a mean follow-up of 6.3 years, the survival rate of the FH was 64.1% in the group treated with the bone graft alone and 69.7% for those patients treated with bone graft + BMP-2. No statistical difference was found.
Platelet-rich plasma (PRP)
A small study (3 patients) was published in the use of PRP and bone grafting for the ONFH treatment [40]. Arthroscopic CD was achieved by drilling through the base of the head and then 10 ml of ‘liquid PRP’ was delivered into the necrotic area. In cases with advanced stage ON, full debridement of the necrotic lesion was carried out by a window in the head and neck junction and autologous bone graft mixed with PRP was grafted into the necrotic area. Hemostasis and enhanced healing was obtained by placing autologous fibrin membranes over the cortical window opened in the base of the femoral head. All three patients reported a significant reduction in pain intensity by >60% on a VAS scale and a return to activities of daily living by 5 months.
Peripheral blood stem cells (PBSCs)
A recent randomized clinical trial (55 patients and 89 hips) described the use of mechanical support treatment (porous tantalum rod implantation) in combination, for the treatment group, of intra-arterial delivery via medial circumflex femoral artery of PBSCs [45, 46]. At 36 months, compared with the control group, combination treatment significantly improved the functional scores, had better survival for conversion to THR and better radiological progression. The authors concluded that targeted intra-arterial infusion of PBSCs is capable of enhancing the efficacy of biomechanical support in the treatment of ONFH.
Βone marrow mononuclear cells
In clinical practice the most common source of cell therapies are BMMCs due to their ease of harvest (iliac crest) and their abundance [25–28]. Equally, the most common joint preserving procedure performed for ON is CD [5, 6]. Hence the combination of the two is naturally the most researched and best published. There are a number of studies that use BMMC therapy with level of evidence of III or higher [29, 34, 47–54]. These studies for the treatment group report variations in the source of cells, method of cell processing, cell characterization, quantitative and qualitative assessment of the cells used, surgical method of implantation, adjuvant therapies (i.e. use of structural graft), patient cohorts (age, etiology of ON), ONFH classification and the outcome measures used [3, 12, 31, 55].
The clinical effectiveness of a procedure is usually analyzed by the use of a patient-reported outcome (PRO), imaging and the endpoint which—in this case—is the conversion to a THR.
A recent systematic review, including 11 studies with a level of evidence III or higher, concluded that the use of cell treatments for ON has been reported to be safe and suggest improved clinical outcomes with a lower rate of deterioration [3].
PROs
Improvements in one or more PRO were reported for cell-therapy groups when compared with noncell therapy groups. It seems that cell therapy with CD showed improvement in mHHS, VAS and WOMAC scores when compared with CD alone [3].
Imaging
There are many variations of the MR signal during the creeping regeneration in the absence of collapse; furthermore, when scaffolds are used, their presence remains visible in the femoral head for a long time, and act as an artifact limiting the ability of the MR to evaluate the exact repair. Therefore traditionally, most clinical studies report as an imaging outcome measure the absence of collapse during the evolution of ON [25, 56, 57].
Cell-based therapies have structural modifying effect measured both by MRI and radiographs with decreased rate of ON progression or even in some cases, restoration of original MR signal of a living bone marrow [3]. In a recent review by Piuzzi, from a total of 93 out of 380 hips (24.5%) that belonged to the treatment group and received cell therapy showed radiographic progression compared with 98 of 245 hips (40%) of the control group [3].
Endpoint
In most studies, success or failure is determined mainly by the endpoint of patient undergoing a THR. THR conversion reported lower rates in the cell-therapies treatment groups [3]. These reports should be considered positively and even promising [29, 47, 49–51, 53, 54] despite the fact that the decision to offer THR (surgeon bias) and the decision to accept THR (patient bias) are subjective decisions that can be influenced by a number of factors.
CONCLUSION
In conclusion, a definitive pathogenetic mechanism for ONFH remains elusive. But, since an important part of the underlying pathology in ON is cell deficiency, it is rational to consider the use of cell-based treatments to potentially regenerate lost or damaged bone. The current reports are positive and even promising on the use of cell-based treatments in bone regeneration.
It seems that the outcome is as one would expect best at the early and certainly precollapse stages of ON. Cell therapies, particularly when employed at early (precollapse) stages of ONFH, improve clinical results and the survivorship of the native hip, reducing the need for hip replacement.
The debate still remains on the ideal source, the lack of standardization and optimization of the harvested cells, their processing, method of transplantation and even method of surgical delivery. The abundance of different cell-based treatments and our ability to control the behavior of the cells after implantation naturally raises concerns on their long-term safety. In our review, none of the studies reported any major adverse events but the quality of the evidence remains inadequate with long-term safety data still required.
It is the author’s belief that the use of cell-based therapies constitutes good clinical practice since it is safe, adds minimal surgical time and difficulty, very little morbidity, this of the donor site and potentially can influence only positively the outcome of the chosen surgical intervention. We believe that there is enough evidence that cell therapy should not be considered experimental but rather a developing technique.
FUNDING
No direct or indirect funding was received by any of the authors regarding this manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Lieberman JR, Berry DJ, Mont MA. et al. Osteonecrosis of the hip: management in the 21st century. Instr Course Lect 2003; 52: 337–55. [PubMed] [Google Scholar]
- 2. Mont MA, Zywiel MG, Marker DR. et al. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am 2010; 92: 2165–70. [DOI] [PubMed] [Google Scholar]
- 3. Piuzzi NS, Chahla J, Schrock JB. et al. Evidence for the use of cell-based therapy for the treatment of osteonecrosis of the femoral head: a systematic review of the literature. J Arthroplasty 2017; 32: 1698–708. [DOI] [PubMed] [Google Scholar]
- 4. Chughtai M, Piuzzi NS, Khlopas A. et al. An evidence-based guide to the treatment of osteonecrosis of the femoral head. Bone Joint J 2017; 99-B: 1267–79. [DOI] [PubMed] [Google Scholar]
- 5. Mont MA, Cherian JJ, Sierra RJ. et al. Nontraumatic osteonecrosis of the femoral head: where do we stand today? A ten-year update. J Bone Joint Surg 2015; 97: 1604–27. [DOI] [PubMed] [Google Scholar]
- 6. McGrory BJ, York SC, Iorio R. et al. Current practices 283 of AAHKS members in the treatment of adult osteonecrosis of the femoral head. J Bone Joint Surg Am 2007; 89: 1194–204. [DOI] [PubMed] [Google Scholar]
- 7. Cornell CN, Salvati EA, Pellicci PM.. Long-term follow-up of total hip replacement in patients with osteonecrosis. Orthop Clin North Am 1985; 16: 757–69. [PubMed] [Google Scholar]
- 8. Chandler HP, Reineck FT, Wixson RL. et al. Total hip replacement in patients younger than thirty years old: a five-year follow-up study. J Bone Joint Surg Am 1981; 63: 1426–34. [PubMed] [Google Scholar]
- 9. Issa K, Pivec R, Kapadia BH. et al. Osteonecrosis of the femoral head. The total hip replacement solution. Bone Joint J 2013; 95-B: 46–50. [DOI] [PubMed] [Google Scholar]
- 10. De Kam DC, Busch VJ, Veth RP, Schreurs BW.. Total hip arthroplasties in young patients under 50 years: limited evidence for current trends. A descriptive literature review. Hip Int 2011. Sep-Oct; 21: 518–25. [DOI] [PubMed] [Google Scholar]
- 11. Hernigou P, Manicom O, Poignard A. et al. Core decompression with marrow stem cells. Oper Tech Orthop 2004; 14: 68–74. [Google Scholar]
- 12. Papakostidis C, Tosounidis TH, Jones E. et al. The role of “cell therapy” in osteonecrosis of the femoral head. A systematic review of the literature and meta-analysis of 7 studies. Acta Orthop 2016 Feb; 87: 72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones LC, Hungerford DS.. Osteonecrosis: etiology, diagnosis, and treatment. Curr Opin Rheumatol 2004; 16: 443–9. [DOI] [PubMed] [Google Scholar]
- 14. Mont MA, Jones LC, Hungerford DS.. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am 2006; 88: 1117–32. [DOI] [PubMed] [Google Scholar]
- 15. Suh KT, Kim SW, Roh HL. et al. Decreased osteogenic differentiation of mesenchymal stem cells in alcohol-induced osteonecrosis. Clin Orthop Relat Res 2005; (431): 220–5. [DOI] [PubMed] [Google Scholar]
- 16. Cui Q, Wang Y, Saleh KJ. et al. Alcohol-induced adipogenesis in a cloned bone-marrow stem cell. J Bone Joint Surg Am 2006; 88: 148–54. [DOI] [PubMed] [Google Scholar]
- 17. Drescher W, Bünger MH, Weigert K. et al. Methylprednisolone enhances contraction of porcine femoral head epiphyseal arteries. Clin Orthop Relat Res 2004; 423: 112–7. [DOI] [PubMed] [Google Scholar]
- 18. Drescher W, Li H, Lundgaard A. et al. Endothelin1-induced femoral head epiphyseal artery constriction is enhanced by long-term corticosteroid treatment. J Bone Joint Surg Am 2006; 88: 173–9. [DOI] [PubMed] [Google Scholar]
- 19. Yin L, Li YB, Wang YS.. Dexamethasone-induced adipogenesis in primary marrow stromal cell cultures: mechanism of steroid induced osteonecrosis. Chin Med J 2006; 119: 581–8. [PubMed] [Google Scholar]
- 20. Lee JS, Roh HL, Kim CH. et al. Alterations in the differentiation ability of mesenchymal stem cells in patients with nontraumatic osteonecrosis of the femoral head: comparative analysis according to the risk factor. J Orthop Res 2006; 24: 604–9. [DOI] [PubMed] [Google Scholar]
- 21. Wang GJ, Cui Q.. The pathogenesis of steroid-induced osteonecrosis and the effect of lipid-clearing agents on this mechanism In: JR Urbaniak, JP Jones (eds). Osteonecrosis: Etiology, Diagnosis, and Treatment. Rosemont, IL: American Academy of Orthopaedic Surgeons, 1997, 159–66. [Google Scholar]
- 22. Wang GJ, Cui Q, Balian G.. The pathogenesis and prevention of steroid induced osteonecrosis. Clin Orthop Relat Res 2000; 370: 295–310. [DOI] [PubMed] [Google Scholar]
- 23. Hernigou P, Beaujean F.. Abnormalities in the bone marrow of the iliac crest in patients who have osteonecrosis secondary to corticosteroid therapy or alcohol abuse. J Bone Joint Surg Am 1997; 79: 1047–53. [DOI] [PubMed] [Google Scholar]
- 24. Hernigou P, Beaujean F, Lambotte JC.. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br 1999; 81: 349–55. [DOI] [PubMed] [Google Scholar]
- 25. Hernigou P, Trousselier M, Roubineau F. et al. Stem cell therapy for the treatment of hip osteonecrosis: a 30-year review of progress. Clin Orthop Surg 2016 Mar; 8: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bianco P, Riminucci M, Gronthos S. et al. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 2001; 19: 180–92. [DOI] [PubMed] [Google Scholar]
- 27. Conway EM, Collen D, Carmeliet P.. Molecular mechanisms of blood vessel growth. Cardiovasc Res 2001; 49: 507–21. [DOI] [PubMed] [Google Scholar]
- 28. Murphy MB, Moncivais K, Caplan AI.. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 2013; 45: e54.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gangji V, De Maertelaer V, Hauzeur JP.. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone 2011; 49: 1005–9. [DOI] [PubMed] [Google Scholar]
- 30. Yan Z, Hang D, Guo C, Chen Z.. Fate of mesenchymal stem cells transplanted to osteonecrosis of femoral head. J Orthop Res 2009; 27: 442–6. [DOI] [PubMed] [Google Scholar]
- 31. Alshameeri Z, McCaskie A.. The role of orthobiologics in hip preservation surgery. J Hip Preserv Surg 2015 Dec; 2: 339–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hernigou P, Beaujean F.. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res 2002; 405: 14–23. [DOI] [PubMed] [Google Scholar]
- 33. Hernigou P, Poignard A, Zilber S. et al. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop 2009; 43: 40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sen RK, Tripathy SK, Aggarwal S. et al. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis. A randomized control study. J Arthroplasty 2012; 27: 679–86. [DOI] [PubMed] [Google Scholar]
- 35. Hernigou P, Homma Y, Flouzat Lachaniette CH. et al. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop 2013; 37: 2279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marker DR, Seyler TM, Ulrich SD. et al. Do modern techniques improve core decompression outcomes for hip osteonecrosis? Clin Orthop Relat Res 2008; 466: 1093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petrigliano FA, Lieberman JR.. Osteonecrosis of the hip: novel approaches to evaluation and treatment. Clin Orthop Relat Res 2007; 465: 53–62. [DOI] [PubMed] [Google Scholar]
- 38. Papavasiliou A, Yercan HS, Koukoulias N.. The role of hip arthroscopy in the management of osteonecrosis. J Hip Preserv Surg 2014; 1: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mont MA, Etienne G, Ragland PS.. Outcome of nonvascularized bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res 2003; 417: 84–92. [DOI] [PubMed] [Google Scholar]
- 40. Guadilla J, Fiz N, Andia I, Sánchez M.. Arthroscopic management and platelet-rich plasma therapy for avascular necrosis of the hip. Knee Surg Sports Traumatol Arthrosc 2012; 20: 393–8. [DOI] [PubMed] [Google Scholar]
- 41. Lieberman JR, Engstrom SMBS, Meneghini MR, SooHoo NF.. Which factors influence preservation of the osteonecrotic femoral head? Clin Orthop Relat Res 2012; 470: 525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lieberman JR, Conduah A, Urist MR.. Treatment of osteonecrosis of the femoral head with core decompression and human bone morphogenetic protein. Clin Orthop Relat Res 2004; 429: 139–45. [DOI] [PubMed] [Google Scholar]
- 43. Seyler TM, Marker DR, Ulrich SD. et al. Nonvascularized bone grafting defers joint arthroplasty in hip osteonecrosis. Clin Orthop Relat Res 2008; 466: 1125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun W, li Z, Gao f. et al. Recombinant human bone morphogenetic protein-2 in debridement and impacted bone graft for the treatment of femoral head osteonecrosis. PLoS One 2014; 9: e100424.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mao Q, Jin H, Liao F. et al. The efficacy of targeted intra-arterial delivery of concentrated autologous bone marrow containing mononuclear cells in the treatment of osteonecrosis of the femoral head: a five-year follow-up study. Bone 2013; 57: 509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mao Q, Wang W, Xu T. et al. Combination treatment of biomechanical support and targeted intra-arterial infusion of peripheral blood stem cells mobilized by granulocyte-colony stimulating factor for the osteonecrosis of the femoral head: a randomized controlled clinical trial. J Bone Miner Res 2015; 30: 647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu Y, Liu S, Su X.. Core decompression and implantation of bone marrow mononuclear cells with porous hydroxylapatite composite filler for the treatment of osteonecrosis of the femoral head. Arch Orthop Trauma Surg 2013; 133: 125–33. [DOI] [PubMed] [Google Scholar]
- 48. Lim YW, Kim YS, Lee JW, Kwon SY.. Stem cell implantation for osteonecrosis of the femoral head. Exp Mol Med 2013; 45: e61.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ma Y, Wang T, Liao J. et al. Efficacy of autologous bone marrow buffy coat grafting combined with core decompression in patients with avascular necrosis of femoral head: a prospective, double-blinded, randomized, controlled study. Stem Cell Res Ther 2014; 5: 115.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pepke W, Kasten P, Beckmann N. et al. Core decompression and autologous bone marrow concentrate for treatment of femoral head osteonecrosis: a randomized prospective study. Orthop Rev 2016; 8: 6162.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rastogi S, Sankineani SR, Nag HL. et al. Intralesional autologous mesenchymal stem cells in management of osteonecrosis of femur: a preliminary study. Musculoskelet Surg 2013; 97: 223–8. [DOI] [PubMed] [Google Scholar]
- 52. Tabatabaee RM, Saberi S, Parvizi J. et al. Combining concentrated autologous bone marrow stem cells injection with core decompression improves outcome for patients with early-stage osteonecrosis of the femoral head: a comparative study. J Arthroplasty 2015; 30: 11–5. [DOI] [PubMed] [Google Scholar]
- 53. Yamasaki T, Yasunaga Y, Ishikawa M. et al. Bone-marrow derived mononuclear cells with a porous hydroxyapatite scaffold for the treatment of osteonecrosis of the femoral head: a preliminary study. J Bone Joint Surg Br 2010; 92: 337–41. [DOI] [PubMed] [Google Scholar]
- 54. Zhao D, Cui D, Wang B. et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone 2012; 50: 325–30. [DOI] [PubMed] [Google Scholar]
- 55. Houdek MT, Wyles CC, Martin JR. et al. Stem cell treatment for avascular necrosis of the femoral head: current perspectives. Stem Cells Cloning 2014; 7: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hernigou P, Lambotte JC.. Bilateral hip osteonecrosis: influence of hip size on outcome. Ann Rheum Dis 2000; 59: 817–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hernigou P, Lambotte JC.. Volumetric analysis of osteonecrosis of the femur: anatomical correlation using MRI. J Bone Joint Surg Br 2001; 83: 672–5. [DOI] [PubMed] [Google Scholar]


