ABSTRACT
Hip arthroscopy patients can experience significant post-operative pain. Many strategies to combat this pain have been explored including nerve blocks, which can be costly. An alternative option for pain management is local infiltration analgesia (LIA) which has been studied in hip and knee arthroplasty, but its ability to decrease pain in the setting of hip arthroscopy remains uncertain. A prospective randomized controlled trial of 74 patients who underwent hip arthroscopy at a single medical center was performed. Thirty-seven patients received a 20-ml extracapsular injection of 0.25% bupivacaine-epinephrine under direct arthroscopic visualization after capsular closure while 37 from the control group received no injection. Primary outcome measures were both maximum and discharge numeric rating scale (NRS) pain scores while in the post-anesthesia care unit (PACU). The LIA group had a statistically significant decrease in the maximum PACU NRS score (6.16 versus 7.35, P = 0.009), however this did not reach the level of minimal clinically important difference of 1.5. There was an insignificant difference in discharge PACU pain scores. This is the first randomized controlled trial studying extracapsular LIA in hip arthroscopy. While LIA offers an uncomplicated and low-cost approach to post-operative pain management, this specific technique did not reduce pain to a clinically significant level.
INTRODUCTION
Hip arthroscopy is a growing field in orthopedic surgery that has gained momentum in recent years as a treatment for an array of hip pathologies with a low rate of complications [1, 2]. Longitudinal studies have demonstrated favorable outcomes in the treatment of femoroacetabular impingement, borderline hip dysplasia and labral tears [3, 4]. However, many patients experience significant pain post-operatively [5, 6]. Acute pain after surgical procedures can hinder patient outcomes as brief periods of pain can induce neuronal remodeling and sensitization precipitating chronic pain and psychological distress [7–9].
Many injection strategies to combat pain associated with hip arthroscopy have been explored. These include femoral nerve blocks, fascia iliaca compartment blocks (FICBs) and paravertebral blocks. Nerve blocks have been shown to decrease post-operative pain and opioid use [5, 6, 10, 11]; however, they can be costly as they frequently require separate procedural and equipment fees billed by the anesthesiologist. Furthermore, nerve blocks have been shown to increase the rate of patient falls. Rarely, they can also carry the risk of residual parasthesias and permanent nerve damage [12–15].
One way to circumvent these limitations is local infiltration analgesia (LIA), a technique that has been described for post-operative pain management in orthopedic modalities like knee arthroscopy, total knee arthroplasty (TKA) and total hip arthroplasty (THA) [16–25]. LIA has been studied to a limited extent in the setting of hip arthroscopy [26–30]; however, its efficacy is unclear due to variable described LIA injection sites and limited study designs.
This study utilizes an extracapsular LIA technique which localizes analgesic to the hip capsule following hip arthroscopy. The capsule was targeted as it is known to have a high concentration of nociceptive nerve fibers which are traumatized during hip arthroscopic procedures and may contribute to post-operative pain [31–33]. The extracapsular LIA technique was researched previously by the authors of the present study in a case-control study which found that hip arthroscopy patients who received an extracapsular LIA injection had a significantly decreased rate of post-operative nerve blocks (56–35%) as compared with the control group (P = 0.027) [34]. These results motivated the current prospective study.
The purpose of this randomized controlled study is to compare the efficacy of an extracapsular LIA in reducing immediate postoperative pain. Secondary outcome measures include post-anesthesia care unit (PACU) opioid consumption and data from patient recorded pain dairies. We hypothesize that the LIA group will have lower maximum pain scores in the immediate postoperative period and lower pain scores at the time of discharge.
MATERIALS AND METHODS
This was a single-center prospective randomized controlled study. The participants, anesthesiologists and PACU ancillary staff were blinded to group assignments. The surgeon administering the LIA was not blinded. Participants were randomized to the LIA or non-LIA group and stratified by current use of narcotic pain medication. Patients were invited to participate in the study if they were undergoing any unilateral hip arthroscopy procedure during the study period. Patients undergoing bilateral hip procedures were excluded. All surgeries were performed by the senior author (SKA).
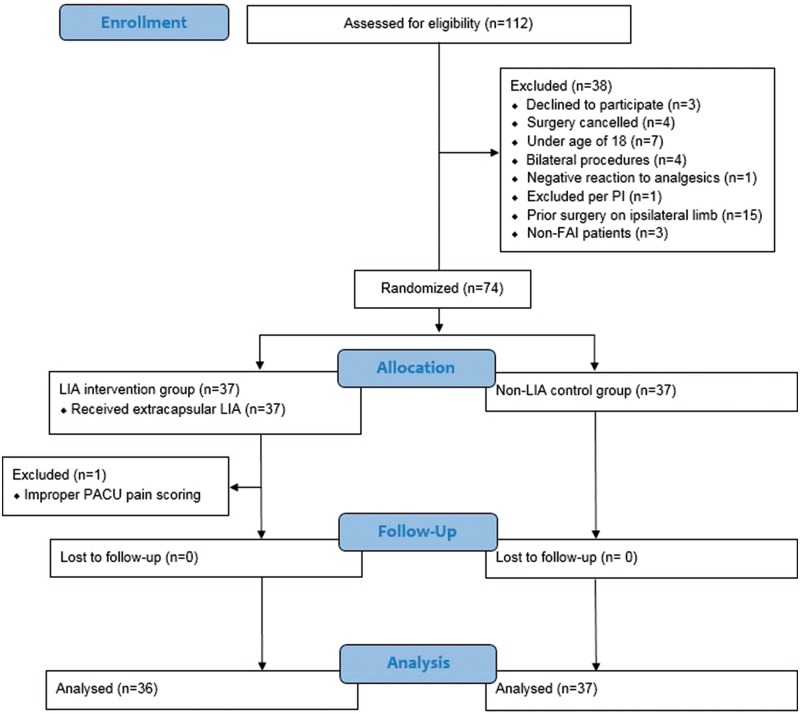
After receiving approval from the institutional review board, eligible patients were recruited either at preoperative clinic appointments or in the preoperative area on the day of surgery. A total of 74 patients were enrolled in the study; one was excluded due to incorrect pain documentation in the PACU. Thirty-six patients were assigned to the intervention group while 37 patients were assigned to the control group (Fig. 1).
Fig. 1.
CONSORT flow diagram of the progress of patients in the study from enrollment to analysis.
In the LIA group, once the interportal capsulotomy was fully closed, a spinal needle was arthroscopically placed in the space just anterior to the capsule (Fig. 2). Excess saline was then suctioned out of the pericapsular space and all portals were closed. After portal closure, 20 ml of 0.25% bupivacaine-epinephrine (1:200 000) was injected into the extracapsular space. Participants in the control group did not receive any injection into the extracapsular space.
Fig. 2.
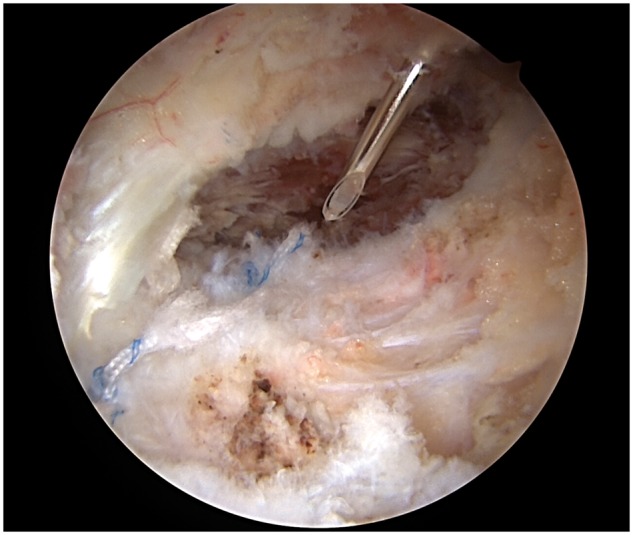
LIA administration via spinal needle placed just anterior to the hip capsule.
To quantify patients’ subjective pain, a numeric rating scale (NRS) of pain (0–10 scale) was used. As soon as the patient was awake, aware and responsive following surgery, the PACU nursing staff documented the patient’s NRS pain score. The nursing staff was asked to chart pain scores with each of their interactions with the patient, at the time of administration of opioids, after the opioids were perceived to have taken effect and at the time of discharge from the facility.
PACU opioid use was documented by the nursing staff and total post-operative consumption was calculated based on morphine milligram equivalents (MME) described by the Centers for Disease Control and Prevention [35, 36].
Patients were sent home with a pain dairy with entries for the first 7 days post-operatively, and one entry at the end of each of the following 3 weeks. The daily entries included an average daily NRS pain score, opioid use and satisfaction with pain control using a Likert-type scale (very unsatisfied, somewhat unsatisfied, neutral, somewhat satisfied and very satisfied). The weekly entries included overall weekly NRS pain score and satisfaction with pain control. Diaries were returned to the study team via self-addressed envelopes or in person during post-operative follow-up appointments.
Patients were also asked to document if they perceived a distinct increase in pain during the first 48 h postoperatively. If a distinct increase in pain was experienced, the patients were asked to give an NRS pain rating both before and after the increase in pain occurred. Patients were instructed to document this in their pain diary at the time the rebound pain occurred in order to minimize recall bias.
Based on the available literature, the minimal clinically important difference (MCID) for the NRS was estimated to be 1.5 points [37–40]. To detect a 1.5-point difference between our LIA and non-LIA arms with 80% power at a 0.05 significance level, we would need 68 participants using a pooled standard deviation of 2.2, which was estimated from the Xing et al. study of femoral nerve blocks in hip arthroscopy patients [41]. This calculation was based on a two-tailed z-test incorporating one interim and one final look at the data using the O'Brien and Fleming method [42].
Demographics, rate of rebound pain, PACU pain scores, opioid consumption and time-to-discharge were compared between intervention and control groups. Continuous variables were compared using a t-test or Wilcoxon–Mann–Whitney test. Categorical variables were described in frequency and percentage, and were compared using a chi-square test. The data collected from the pain diaries were analysed using generalized linear mixed effect model which tested for a difference in NRS pain scores, satisfaction scores and daily opioid consumption between LIA vs non-LIA groups at baseline and over time. All statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
There were no significant differences in sex, age, BMI or laterality of surgery between LIA and control groups. Demographic characteristics can be found in Table I. The distribution of surgical procedures were very similar between the two groups (LIA/control): 19/19 patients underwent primary femoral osteochondroplasty with labral repair, 9/8 patients underwent femoracetabuloplasty, 6/6 patients underwent femoral osteochondroplasty without labral repair and 2/4 patients underwent revision femoral osteochondroplasty. All patients underwent complete capsular repair.
Table III.
Comparison between LIA and non-LIA at baseline and over time from generalized linear mixed effect models
| Outcomes | Regression used | Comparison between LIA vs n-LIA |
||
|---|---|---|---|---|
| Estimate (95% CI) at baseline | P-value at baseline | P-value over time | ||
| NRS | Linear mixed effect model | Coefficient = −0.31, 95% CI = (−1.35, 0.73) | 0.56 | 0.98 |
| Satisfaction (very satisfied versus other) | Logistic mixed effect model | Odds ratio = 0.93, 95% CI = (0.26, 3.37) | 0.97 | 0.97 |
| Opioid consumption | Negative binomial mixed effect model | Coefficient = 0.77, 95% CI = (0.42, 1.43) | 0.41 | 0.53 |
Table I.
Patient demographics (N = 73)
| Total | LIA (N = 36) | Non-LIA (N = 37) | P-values | |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 21 (28.8) | 11 (30.6) | 10 (27.0) | 0.739a |
| Female | 52 (71.2) | 25 (69.4) | 27 (73.0) | |
| Age at surgery, mean (SD) | 34.7 (10.0) (range 19–58) | 36.3 (10.9) (range 19–58) | 33.3 (8.9) (range 19–54) | 0.085b |
| BMI, mean (SD) | 25.6 (5.1) (range 18.0–41.2) | 24.7 (4.5) (range 19.0–39.6) | 26.4 (5.6) (range 18.0–41.2) | 0.141b |
| Side of surgery, n (%) | ||||
| Left | 35 (47.9) | 17 (47.2) | 18 (48.6) | 0.903a |
| Right | 38 (52.1) | 19 (52.8) | 19 (51.4) |
Chi-square test.
Two sample t test.
There was a statistically significant decrease in the maximum PACU NRS score in the LIA group versus the non-LIA group (6.16 versus 7.35, P = 0.009). There was no significant difference in discharge PACU NRS pain scores. While our study was not powered specifically to measure secondary outcomes, the non-LIA group did have a longer median PACU duration (97 min versus 118 min, P = 0.09) and higher median PACU opioid consumption (10 MME versus 14 MME, P = 0.09); these factors may have been influential in the similar mean PACU discharge pain score (3.36 versus 3.86 P = 0.18). Rebound pain, and other pain diary variables of interest also showed no difference between groups. Results can be found in Tables II and III.
Table II.
Clinical characteristics comparison between LIA versus non-LIA groups (N = 73)
| Variables | LIA (N = 36) | Non-LIA (N = 37) | P-value |
|---|---|---|---|
| Maximum PACU NRS score, mean (SD) | 6.19 (1.97) | 7.35 (1.69) | 0.009a |
| Median (IQR) | 6.50 (5.00, 7.50) | 7.00 (6.00, 8.00) | |
| Discharge PACU NRS score, mean (SD) | 3.36 (1.61) | 3.86 (1.57) | 0.18a |
| Median (IQR) | 4.00 (2.50, 4.50) | 4.00 (3.00, 5.00) | |
| PACU opioid use (MME), mean (SD) | 11.91 (8.50) | 15.11 (9.92) | |
| Median (IQR) | 10.00 (5.00, 18.30) | 15.00 (7.50, 21.70) | 0.19b |
| Total opioid use (MME), Mean (SD) | 37.86 (13.37) | 42.67 (17.27) | 0.19a |
| Median (IQR) | 38.00 (28.35, 48.30) | 40.00 (30.80, 56.70) | |
| Total PACU time, mean (SD) | 124.47 (67.01) | 136.78 (52.48) | |
| Median (IQR) | 97.00 (86.00, 142.00) | 118.00 (101.00, 182.00) | 0.09b |
| Perceived postoperative increase in pain, n (%) | 0.58c | ||
| Yes | 15 (45.45) | 14 (38.89) | |
| No | 18 (54.55) | 22 (61.11) | |
| NRS score prior to perceived increase in pain, mean (SD) | 3.67 (1.95) | 4.57 (2.21) | 0.25a |
| Median (IQR) | 3.00 (2.00, 5.00) | 4.00 (3.00, 7.00) | |
| NRS score after perceived increase in pain, mean (SD) | 6.13 (1.46) | 7.57 (1.65) | 0.019a |
| Median (IQR) | 6.00 (5.00, 7.00) | 7.50 (7.00, 9.00) |
Two sample t-test.
Wilcoxon–Mann–Whitney test.
Chi-square test.
DISCUSSION
The findings of this study support our hypothesis that administration of extracapsular LIA would decrease PACU pain scores in hip arthroscopy patients, although the decrease did not reach the MCID for the NRS score for pain in previously reported literature [39, 41].
LIA has been used in a diverse array of surgical settings; however, there has been limited research into the efficacy of LIA in hip arthroscopy. In 2007, Morgenthaler et al. published findings from a randomized controlled trial of a small cohort of 26 hip arthroscopy patients in which 13 received an intra-articular 20 ml 0.25% bupivacaine and 13 received placebo normal saline. Visual analogue scale (VAS) pain scores were recorded at 0.5, 4, 12, 16 and 20 h post-operatively. The mean of these scores was found to be 17.5 in the LIA group and 27.5 in the control group (P = 0.05) [30].
There has been concern regarding intra-articular LIA injections due to the potential chondrotoxicity of analgesic medicine [43]. Consequently, Baker et al. compared intra-articular injection with portal injection of 10 ml of 0.25% bupivacaine in a 2011 study. The analgesic efficacy of these two LIA strategies was shown to be similar; the only significant differences between groups were that the portal group required more rescue analgesia initially after surgery, but had lower VAS scores at 6 h after surgery [26]. However, the lack of control group limits the ability to characterize the analgesic effect of LIA.
In 2017, Garner et al. carried out further research into portal LIA in the setting of hip arthroscopy, this time comparing the analgesic effect of 40 ml of 0.125% levobupivacaine LIA to the FICB. They found that LIA group had significantly less pain in the 1st hour postoperatively and consumed less opioids than patients who had received FICB [27]. While FICB has been shown to significantly reduce pain in hip arthroscopy patients [44], and is therefore a valuable comparison for LIA, the lack of a control group makes it difficult to quantify the analgesic effect of LIA.
Also in 2017, Shlaifer et al. published research comparing intra-articular to periacetabular LIA injection in hip arthroscopy patients. At the early stages of the hip arthroscopy procedure, one group received a 20 ml intra-articular injection of 0.5% bupivacaine while the other group received a 20 ml periacetabular injection of 0.5% bupivacaine. During the closing stages, both groups received an additional 20 ml intra-articular injection of 0.5% bupivacaine. The two different LIA groups had similar post-operative pain with the periacetabular group having improved pain at 30 min and 18 h compared with the intra-articular group [29]. The analgesic effect of intra-articular or periacetabular LIA is difficult to quantify as no control group was included in the study design.
The authors of the present study published a retrospective case-control study in 2018, which compared the rate of post-operative femoral nerve blocks in hip arthroscopy patients who received an extracapsular LIA versus those with no injection. The LIA group had a 22% decrease in the rate of nerve blocks compared with controls (P = 0.027). This study was limited by its retrospective design and the lack of pain score data.
The limited study design of the previous research, as well as the variability in LIA technique employed, spurred the current prospective study of postoperative pain scores in hip arthroscopy patients who received extracapsular LIA. The findings of this study are concurrent with previous research suggesting that LIA reduces post-operative pain in hip arthroscopy patients.
The challenges and limitations of this study characterize some of the difficulties in using patient-reported pain as a primary outcome measure. Pain itself is multidimensional in nature. While the NRS 0-10 scale is useful in quantifying pain, it only measures a single dimension of pain and consequently may be an inadequate tool for fully characterizing and measuring patients’ pain [9]. The measurement of this pain was carried out by many different members of the PACU staff leaving the data subject to measurement bias. Additionally, patients must be below a certain threshold of pain before meeting PACU discharge criteria; the incentive to have patients report pain below the discharge threshold to facilitate discharge may influence how pain measurements are reported by patients or gathered and recorded by clinicians. Other limitations include the surgeon being unblinded to the administration of LIA. Lastly, patients in the study were cared for by different anesthesiologists, creating further potential for variability in intraoperative and perioperative pain management.
This is the first randomized controlled trial studying extracapsular LIA versus no injection in hip arthroscopy. In this study, an injection of 20-ml of bupivacaine-epinephrine demonstrated improvements in patient reported PACU pain scores (P = 0.009), shorter PACU stays (P = 0.09) and less PACU opioid use (P = 0.09). While LIA offers an uncomplicated and low-cost approach to post-operative pain management, the clinical improvements regarding the MCID could be questioned and requires further scientific investigation.
ACKNOWLEDGEMENTS
This investigation was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8UL1TR000105 (formerly UL1RR025764).
FUNDING
This work was supported by the LS Peery Foundation through grant [90371].
CONFLICT OF INTEREST STATEMENT
SKA is a consultant for Stryker Medical. TGM is a consultant for Arthrex.
REFERENCES
- 1. Bozic KJ, Chan V, Valone FH. 3rd et al. Trends in hip arthroscopy utilization in the United States. J Arthroplasty 2013; 28: 140–3. [DOI] [PubMed] [Google Scholar]
- 2. Maradit Kremers H, Schilz SR, Van Houten HK. et al. Trends in utilization and outcomes of hip arthroscopy in the United States between 2005 and 2013. J Arthroplasty 2017; 32: 750–5. [DOI] [PubMed] [Google Scholar]
- 3. Menge TJ, Briggs KK, Dornan GJ. et al. Survivorship and outcomes 10 years following hip arthroscopy for femoroacetabular impingement: labral debridement compared with labral repair. J Bone Joint Surg Am 2017; 99: 997–1004. [DOI] [PubMed] [Google Scholar]
- 4. Byrd JW, Jones KS.. Hip arthroscopy for labral pathology: prospective analysis with 10-year follow-up. Arthroscopy 2009; 25: 365–8. [DOI] [PubMed] [Google Scholar]
- 5. Lee EM, Murphy KP, Ben-David B.. Postoperative analgesia for hip arthroscopy: combined L1 and L2 paravertebral blocks. J Clin Anesth 2008; 20: 462–5. [DOI] [PubMed] [Google Scholar]
- 6. Ward JP, Albert DB, Altman R. et al. Are femoral nerve blocks effective for early postoperative pain management after hip arthroscopy? Arthroscopy 2012; 28: 1064–9. [DOI] [PubMed] [Google Scholar]
- 7. Carr DB, Goudas LC.. Acute pain. Lancet 1999; 353: 2051–8. [DOI] [PubMed] [Google Scholar]
- 8. Katz J, Seltzer Z.. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother 2009; 9: 723–44. [DOI] [PubMed] [Google Scholar]
- 9. Chapman CR, Vierck CJ.. The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain 2017; 18: 359 e1–e38. [DOI] [PubMed] [Google Scholar]
- 10. Dold AP, Murnaghan L, Xing J. et al. Preoperative femoral nerve block in hip arthroscopic surgery: a retrospective review of 108 consecutive cases. Am J Sports Med 2014; 42: 144–9. [DOI] [PubMed] [Google Scholar]
- 11. Potter MQ, Sun GS, Fraser JA. et al. Psychological distress in hip arthroscopy patients affects postoperative pain control. Arthroscopy 2014; 30: 195–201. [DOI] [PubMed] [Google Scholar]
- 12. Auroy Y, Benhamou D, Bargues L. et al. Major complications of regional anesthesia in France: the SOS Regional Anesthesia Hotline Service. Anesthesiology 2002; 97: 1274–80. [DOI] [PubMed] [Google Scholar]
- 13. Liguori GA. Complications of regional anesthesia: nerve injury and peripheral neural blockade. J Neurosurg Anesthesiol 2004; 16: 84–6. [DOI] [PubMed] [Google Scholar]
- 14. Jeng CL, Torrillo TM, Rosenblatt MA.. Complications of peripheral nerve blocks. Br J Anaesth 2010; 105(Suppl. 1): i97–107. [DOI] [PubMed] [Google Scholar]
- 15. Sharma S, Iorio R, Specht LM. et al. Complications of femoral nerve block for total knee arthroplasty. Clin Orthop Relat Res 2010; 468: 135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baverel L, Cucurulo T, Lutz C. et al. Anesthesia and analgesia methods for outpatient anterior cruciate ligament reconstruction. Orthop Traumatol Surg Res 2016; 102: S251–S55. [DOI] [PubMed] [Google Scholar]
- 17. Niemelainen M, Kalliovalkama J, Aho AJ. et al. Single periarticular local infiltration analgesia reduces opiate consumption until 48 hours after total knee arthroplasty. A randomized placebo-controlled trial involving 56 patients. Acta Orthop 2014; 85: 614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reilly KA, Beard DJ, Barker KL. et al. Efficacy of an accelerated recovery protocol for Oxford unicompartmental knee arthroplasty–a randomised controlled trial. Knee 2005; 12: 351–7. [DOI] [PubMed] [Google Scholar]
- 19. Busch CA, Shore BJ, Bhandari R. et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am 2006; 88: 959–63. [DOI] [PubMed] [Google Scholar]
- 20. Bianconi M, Ferraro L, Traina GC. et al. Pharmacokinetics and efficacy of ropivacaine continuous wound instillation after joint replacement surgery. Br J Anaesth 2003; 91: 830–5. [DOI] [PubMed] [Google Scholar]
- 21. Andersen KV, Pfeiffer-Jensen M, Haraldsted V. et al. Reduced hospital stay and narcotic consumption, and improved mobilization with local and intraarticular infiltration after hip arthroplasty: a randomized clinical trial of an intraarticular technique versus epidural infusion in 80 patients. Acta Orthop 2007; 78: 180–6. [DOI] [PubMed] [Google Scholar]
- 22. Kerr DR, Kohan L.. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery: a case study of 325 patients. Acta Orthop 2008; 79: 174–83. [DOI] [PubMed] [Google Scholar]
- 23. Murphy TP, Byrne DP, Curtin P. et al. Can a periarticular levobupivacaine injection reduce postoperative opiate consumption during primary hip arthroplasty? Clin Orthop Relat Res 2012; 470: 1151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bech RD, Ovesen O, Lindholm P. et al. Local anesthetic wound infiltration for pain management after periacetabular osteotomy. A randomized, placebo-controlled, double-blind clinical trial with 53 patients. Acta Orthop 2014; 85: 141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zoric L, Cuvillon P, Alonso S. et al. Single-shot intraoperative local anaesthetic infiltration does not reduce morphine consumption after total hip arthroplasty: a double-blinded placebo-controlled randomized study. Br J Anaesth 2014; 112: 722–8. [DOI] [PubMed] [Google Scholar]
- 26. Baker JF, McGuire CM, Byrne DP. et al. Analgesic control after hip arthroscopy: a randomised, double-blinded trial comparing portal with intra-articular infiltration of bupivacaine. Hip Int. 2011; 21: 373–7. [DOI] [PubMed] [Google Scholar]
- 27. Garner M, Alshameeri Z, Sardesai A. et al. A prospective randomized controlled trial comparing the efficacy of fascia iliaca compartment block versus local anesthetic infiltration after hip arthroscopic surgery. Arthroscopy 2017; 33: 125–32. [DOI] [PubMed] [Google Scholar]
- 28. Redmond JM, Gupta A, Stake CE. et al. Clinical results of hip arthroscopy for labral tears: a comparison between intraoperative platelet-rich plasma and bupivacaine injection. Arthroscopy 2015; 31: 445–53. [DOI] [PubMed] [Google Scholar]
- 29. Shlaifer A, Sharfman ZT, Martin HD. et al. Preemptive analgesia in hip arthroscopy: a randomized controlled trial of preemptive periacetabular or intra-articular bupivacaine in addition to postoperative intra-articular bupivacaine. Arthroscopy.2017; 33: 118–24. [DOI] [PubMed] [Google Scholar]
- 30. Morgenthaler K, Bauer C, Ziegeler S. et al. Intra-articular bupivacaine following hip joint arthroscopy. Effect on postoperative pain. Anaesthesist 2007; 56: 1128–32. [DOI] [PubMed] [Google Scholar]
- 31. Birnbaum K, Prescher A, Hessler S. et al. The sensory innervation of the hip joint – an anatomical study. Surg Radiol Anat 1997; 19: 371–5. [DOI] [PubMed] [Google Scholar]
- 32. Haversath M, Hanke J, Landgraeber S. et al. The distribution of nociceptive innervation in the painful hip: a histological investigation. Bone Joint J 2013; 95-B:770–6. [DOI] [PubMed] [Google Scholar]
- 33. Kampa RJ, Prasthofer A, Lawrence-Watt DJ. et al. The internervous safe zone for incision of the capsule of the hip. A cadaver study. J Bone Joint Surg Br 2007; 89: 971–6. [DOI] [PubMed] [Google Scholar]
- 34. Philippi MT, Kahn TL, Adeyemi TF. et al. Extracapsular local infiltration analgesia in hip arthroscopy: a retrospective study. J Hip Preserv Surg 2018; 5: 60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain – United States, 2016. JAMA 2016; 315: 1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Broglio K. Approximate Dose Conversions for Commonly Used Opioids In: Post T. (ed.). Waltham, MA: UpToDate, 2017. https://www.uptodate.com/contents/image?imageKey=PALC%2F111207&topicKey=PALC%2F2814&source=see_link. Accessed: 14 February 2017. [Google Scholar]
- 37. Kelly AM. Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain? Acad Emerg Med 1998; 5: 1086–90. [DOI] [PubMed] [Google Scholar]
- 38. Sloman R, Wruble AW, Rosen G. et al. Determination of clinically meaningful levels of pain reduction in patients experiencing acute postoperative pain. Pain Manag Nurs 2006; 7: 153–8. [DOI] [PubMed] [Google Scholar]
- 39. Todd KH, Funk KG, Funk JP. et al. Clinical significance of reported changes in pain severity. Ann Emerg Med 1996; 27: 485–9. [DOI] [PubMed] [Google Scholar]
- 40. Salaffi F, Stancati A, Silvestri CA. et al. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain 2004; 8: 283–91. [DOI] [PubMed] [Google Scholar]
- 41. Xing JG, Abdallah FW, Brull R. et al. Preoperative femoral nerve block for hip arthroscopy: a randomized, triple-masked controlled trial. Am J Sports Med 2015; 43: 2680–7. [DOI] [PubMed] [Google Scholar]
- 42. O'Brien PC, Fleming TR.. A multiple testing procedure for clinical trials. Biometrics 1979; 35: 549–56. [PubMed] [Google Scholar]
- 43. Webb ST, Ghosh S.. Intra-articular bupivacaine: potentially chondrotoxic? Br J Anaesth 2009; 102: 439–41. [DOI] [PubMed] [Google Scholar]
- 44. Krych AJ, Baran S, Kuzma SA. et al. Utility of multimodal analgesia with fascia iliaca blockade for acute pain management following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc 2014; 22: 843–7. [DOI] [PubMed] [Google Scholar]