ABSTRACT
This review aims to determine whether platelet-rich plasma (PRP) has any role in improving clinical outcomes in patients with symptomatic greater trochanteric pain syndrome (GTPS). A search of NICE healthcare database advanced search (HDAS) via Athens (PubMed, MEDLINE, CINAHL, EMBASE and AMED databases) was conducted from their year of inception to April 2018 with the keywords: ‘greater trochanteric pain syndrome’ or ‘GTPS’ or ‘gluteus medius’ or ‘trochanteric bursitis’ and ‘platelet rich plasma’ (PRP). A quality assessment was performed using the JADAD score for RCTs and MINORS for non-RCT studies. Five full-text articles were included for analysis consisting of three RCTs and two case series. We also identified four additional studies from published conference abstracts (one RCT and three case series). The mean age in 209 patients was 58.4 years (range 48–76.2 years). The majority of patients were females and the minimum duration of symptoms was three months. Diagnosis was made using ultrasound or MRI. Included studies used a variety of outcome measures. Improvement was observed during the first 3 months after injection. Significant improvement was also noted when patients were followed up till 12 months post treatment. PRP seems a viable alternative injectable option for GTPS refractory to conservative measures. The current literature has revealed that PRP is relatively safe and can be effective. Considering the limitations in these studies, more large-sample and high-quality randomized clinical trials are required in the future to provide further evidence of the efficacy for PRP as a treatment in GTPS.
Systematic Review Registration
PROSPERO CRD42017080662
Level of Evidence
Level I, systematic review of Level I studies.
INTRODUCTION
Greater trochanteric pain syndrome (GTPS), alternatively known as trochanteric bursitis, is a painful condition that commonly affects middle-aged women [1]. It is characterized by pain over the lateral aspect of the hip. Recently the understanding of the nature of the disease has evolved. Gluteal tendinopathy is believed to be the main contributory factor rather than bursal inflammation. There are numerous studies reporting little evidence of bursal inflammation in GTPS but found gluteal tendon tendinopathy more commonly associated with GTPS [1–4].
GTPS has also been associated with low back pain, knee osteoarthritis and iliotibial band syndrome. No relation has been found between the occurrence of GTPS and obesity, age or race [5, 6]. Raman et al. suggested that GTPS is commonly misdiagnosed, successfully remediable cause of pain in rheumatoid arthritis (RA) and that specific examination for its presence should be a routine in all patients with RA, especially those with hip pain [7]. Recently Pozzi et al. [8], evaluated the incidence of GTPS in patients who underwent magnetic resonance arthrography of the hip for a suspected femoroacetabular impingement syndrome. They concluded that GTPS was more frequently observed in patients with normal hip morphology than in patients with FAI, particularly in patients under 40 years of age.
GTPS normally settles with conservative treatments such as relative rest and anti-inflammatory medication in the majority of patients [9–11]. If conservative measures fail then progressively more invasive treatment options including shockwave therapy, corticosteroid injections (CSI), PRP and surgery may be required [12, 13].
Lately PRP has become very popular among the orthopaedic community as a minimally invasive way of enhancing tissue healing in different conditions including rotator cuff repair [14, 15], patellar tendinopathy [16, 17], knee osteoarthritis [18], lateral epicondylitis [19], osteochondral lesions of the talus [20] and other orthopaedic conditions. It has been postulated that PRP promotes soft tissue healing by delivering a higher than normal concentration of platelets and therefore increased concentration of platelet derived growth factors to the diseased area [21]. This has been shown in various studies [22–24].
The use of PRP in treating GTPS has become more prevalent in recent times. The purpose of this review article is to summarize the existing knowledge on the role of PRP in GTPS.
MATERIALS AND METHODS
A search of NICE healthcare database advanced search (HDAS) via Athens (PubMed, MEDLINE, CINAHL, EMBASE and AMED databases) was conducted from their year of inception to April 2018 with the keywords: ‘greater trochanteric pain syndrome’ or ‘GTPS’ or ‘gluteus medius’ or ‘trochanteric bursitis’ and ‘“platelet-rich plasma’.
Broad search keywords were used rather than specific terms to ensure no articles were missed. There was no language limit and all the relevant published articles or abstracts were included. The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) methodology guidance was employed [25].
Abstracts from the search were reviewed for relevant articles by two authors (MA and EO). If a decision regarding relevance could not be made from reviewing the title and abstract alone then the full-text article was reviewed with author AM. All the references listed in the relevant articles were also reviewed for any other papers not found in the initial search. Studies were included if they reported clinical, functional and imaging outcomes of patients treated with PRP for GTPS. Due to lack of studies, we did not set a minimum follow-up period. Case reports, reviews, studies on animals and technical notes were excluded.
Once relevant articles were identified, data was extracted using a standardized form for each of the following: Author, year of publication, study design, sample size, demographics, diagnostic test, injection technique, outcome measures and follow-up frequency. RCTs were accompanied by lower quality non-randomized studies. Published conference abstracts were also included due to the paucity of evidence available but reported separately to full-text articles. Due to the heterogeneity of the included data, a meta-analysis could not be conducted, therefore all data was reported descriptively.
Quality assessment
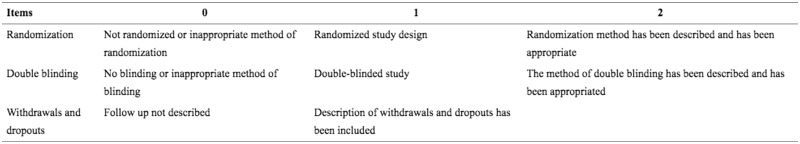
This review utilized the JADAD quality evaluation scale [26] to rank the quality of the included randomized controlled trials. The studies ranked 1–2 points were low-quality studies whereas studies with 3–5 points were high-quality studies (Fig. 1).
Fig. 1.
JADAD quality evaluation scale.
The non-RCT studies were assessed using the MINORS score [27], which serves as a methodological index for non-randomized studies. It has 12 domains for which non-comparative studies use the first 8 domains. Each domain is scored out of 2 with ideal scores being at least 16 for non-comparative studies and 24 for comparative studies. The investigators discussed scores where a more than two-point difference was recorded, until an agreement was reached.
RESULTS
Search results
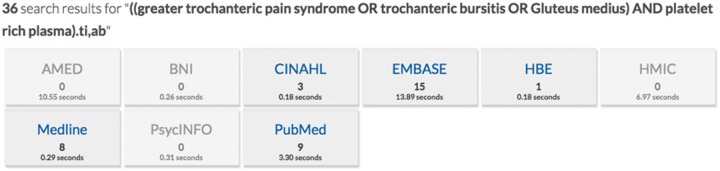
The search threads used in the NICE HDAS database resulted in 36 articles (Fig. 2).
Fig. 2.
Search results.
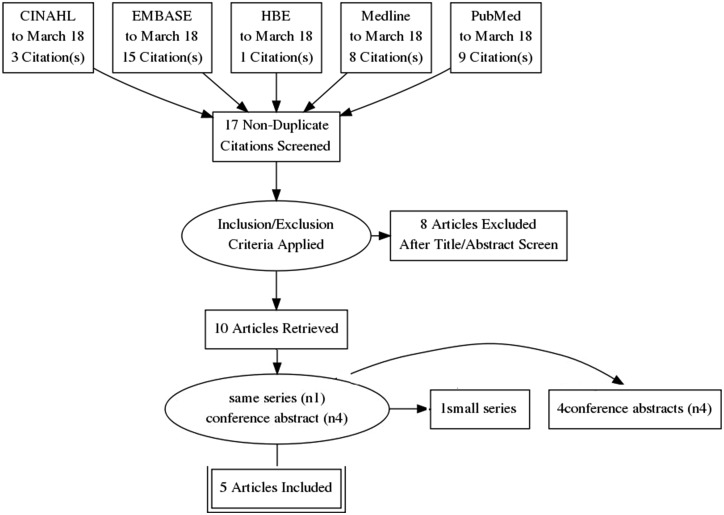
Abstracts were manually screened excluding the duplicates then records which were non-clinical/human or unrelated to GTPS or PRP. Reviews, operative technique, small case series (less than 5 patients) and case reports (Fig. 3) were excluded.
Fig. 3.
Study selection process.
Summary of studies
In total, five studies were included for analysis consisting of three RCTs [28–30] and two case series [31, 32]. We also identified four additional studies from published conference abstracts (one RCT [34] and three case series [33, 35, 36]).
The full-text articles included 102 hips, with sample sizes ranging from 10 to 40 patients, with a mean age ranging from 48 to 60 years and majority of patients were female (Table I).
Table I.
Description of included articles
| Author-year | Type of study | Control group | No. of hips in the PRP group | Male/ female | Mean age | Diagnostic test | Duration of symptoms | Mean FU | Outcome measures | Methodology assessment |
|---|---|---|---|---|---|---|---|---|---|---|
| Fitzpatrick et al. 2018 [28] | RCT | Steroid injection | 40 | 01:09 | 60 | US/MRI | 14 months | 12 weeks | mHHS, PASS | JADAD score: 5 |
| Ribeiro et al. 2016 [29] | RCT | Steroid injection | 10 | 3:7 | 49.8 | MRI | Minimum 3 months | 2 months | FEPS, HHS, WOMAC | JADAD score: 5 |
| Jacobson et al. 2016 [30] | RCT | Fenestration | 15 | 1:14 | 53 | US | Minimum 3 months | 3 months | Pain score estimate | JADAD score: 1 |
| Mautner et al. 2013 [31] | Case series | N/A | 16 | Not reported available | 48 | MRI | 18 months | 15 months | VAS, improvement of symptoms | MINORS score: 6/16 |
| Lee et al.2016 [32] | Case series | N/A | 21 | 4:17 | 48 | US | Minimum 3 months | 19.7 months | mHHS, HOS-ADL, HOS-Sport, iHOT-33 | MINORS score: 13/16 |
The conference abstracts included 147 hips, with sample sizes ranging from 10 to 85 patients, with a mean age ranging from 60 to 76.2 years and majority of patients were female (Table II).
Table II.
Description of included abstracts
| Author-year | Type of study | Control | No. of hips in the PRP group | Male/ female | Mean age | Diagnostic test | Duration of symptoms | Mean FU | Outcome measures |
|---|---|---|---|---|---|---|---|---|---|
| Blucher et al. 2015 [33] | Case series | N/A | 85 | 1:4 | 60 | MRI/US | Minimum 3 months | Not reported | EQ-5D, VAS, HOOS |
| Monto 2014 [34] | RCT | Steroid injection | 20 | 5:15 | 66 | MRI/US | 11 months | 12 months | HHS, WOMAC |
| Rajeev et al. 2016 [35] | Case series | N/A | 32 | 12:20 | 76.2 | Not reported | Minimum 6 months | 12 months | HHS, VAS |
| LaSalle et al. 2013 [36] | Case series | N/A | 10 | 1:9 | 64.7 | MRI | 12 weeks | 10.2 months | VAS, NASS, FRI |
Diagnosis
Diagnosis of GTPS was made either by ultrasound (US) [28, 30, 32] or MRI [28, 29, 31]. In all studies, patients had more than 3 months duration of symptoms and had failed conservative management. The mean follow-up ranged from two months to 19.7 months.
The conference abstracts used MRI and US as diagnostic modalities except in one study by Rajeev et al. [35]. Patients had more than 12 weeks duration of symptoms and failed conservative management. The mean follow-up was reported in three studies [34–36] to range between 10.2 and 12 months.
Outcome measures
The full-text articles used a variety of outcome measures including a pain score, Harris Hip Score (HHS), Western Ontario McMaster Index (WOMAC), Facial Expressions Pain Scale (FEPS), Visual Analogue Scale (VAS), modified Harris Hip Score (mHHS), Hip Outcome Score–Activities of Daily Living (HOS-ADL) subscale, Hip Outcome Score–Sport (HOS-Sport)-Specific sub-scale, the International Hip Outcome Tool–33 (iHOT-33) and the Patient Acceptable Symptom State (PASS) (Table I).
The outcome measures utilized in the conference abstracts included the HHS, WOMAC, VAS, EQ-5D, North American Spine Society (NASS) patient satisfaction index, Hip Disability and Osteoarthritis Outcome Scores (HOOS) and Functional Rating Index (FRI) (Table II).
PRP INJECTION
The PRP preparation, volume of blood drawn and volume of PRP were poorly documented in all conference abstract. There were different PRP systems used in these studies such as the Zimmer Biomet GPS III, Magellan, Harvest Tech, Fanem Excels II and Arthrex ACP (Tables III and IV).
Table III.
PRP preparation
| Author-year | Volume of blood drawn | Volume of PRP | PRP preparation | Injection technique | Complications | PRP system |
|---|---|---|---|---|---|---|
| Fitzpatrick et al. 2018 [28] | 55 ml | 6–7 ml | Centrifuged for 15 min. 9.3× platelet increase over baseline and 5× white blood cell increase over baseline. used straight away | No buffering agent was added. Local anaesthetic was administered, and then 6–7 ml was injected into the affected area of the tendon in 5–6 passes using US guidance | Pain | GPS III kit (Zimmer Biomet) |
| Ribeiro et al. 2016 [29] | 60 ml | 3–4 ml | Centrifuged for 15 min, platelet concentration of PRP was 9.23 × 106 U/μl. used straight away | US guided, the most painful point was identified by palpation. injected into the trochanteric bursa and around it, according to the size of the affected area | None | Fanem Excelsa II |
| Jacobson et al. 2016 [30] | 60 ml | 7–10 ml | Centrifuged at up to 2650 rpm for approximately 14 min. Leukocyte-rich sample, concentration 4–6 times, used straight away | US guided, needle was inserted into the deepest aspect of the tendon abnormality, and the PRP was injected as the needle was withdrawn through the abnormal tendon segment | None | Harvest Tech |
| Mautner et al. 2013 [31] | Variable | Variable | Not reported | US guidedDid not mention site of injection | None | Not reported |
| Lee et al. 2016 [32] | 25 ms | 4 ml | Leukocyte-rich, used straight away | US guided, into the hypoechoic and tender regions overlying the greater trochanter (Figs 1 and 2). A needle tenotomy technique followed, consisting of 6–9 needles passes through the hypoechoic regions of the gluteus medius tendon | None | Magellan |
Table IV.
PRP preparation
| Author-year | Amount of blood drawn | Amount of PRP | Injection technique | Complications | PRP system |
|---|---|---|---|---|---|
| Blucher et al. 2015 [33] | Not available | Not available | Blind | Not reported | Not reported |
| Monto 2014 [34] | Not available | Not available | US guided | Not reported | Not reported |
| Rajeev et al. 2016 [35] | Not available | Not available | Blind | Not reported | Arthrex ACP |
| LaSalle et al. 2013 [36] | Not available | Not available | US guided | Not reported | Not mentioned |
FULL ARTICLE RESULTS
Fitzpatrick’s double-blind RCT compared the effect of single US-guided PRP injections with corticosteroid in the treatment of gluteal tendinopathy [28]. Each arm included 40 patients with a mean age of 60 years and mean duration of symptoms of 14 months. Follow-up period intervals were 2, 6 and 12 weeks. PRP demonstrated a significant advantage compared with corticosteroid groups over 12 weeks (mean mHHS 74.05 ± 13.92 versus 67.13 ± 16.04, respectively, P = 0.048). There were no significant differences between the groups at 2 weeks (mean mHHS, PRP: 65.23 ± 11.60 versus corticosteroid: 66.95 ± 15.14) or 6 weeks (PRP: 68.79 ± 13.33 versus corticosteroid: 69.51 ± 14.78). When considering the minimum clinically important difference (MCID), 82% in the PRP group achieved improvement compared to 56.7% in the corticosteroid group (P = 0.016).
Ribeiro et al.’s [29] double-blind randomized prospective comparative study compared the efficacy of US-guided PRP injections against corticosteroid (Triamcinolone) in 20 hips with GTPS. Outcomes were assessed at baseline, 10, 30 and 60 days using the FEPS, HHS and WOMAC questionnaires. Inter-group analysis demonstrated no significant differences between the two treatment arms at any time point with any of the outcome measures. Intra-group comparisons demonstrated significant improvements in the HHS in the corticosteroid group at 10 and 60 days (pre-10 days: mean difference 20.8, P = 0.03; pre-60 days: mean difference 22.3, P = 002) and at each follow-up period for the FEPS (pre-10 days: mean difference 2.1, P = 0.004; pre-30 days: mean difference −2.1, P = 0.004; pre-60 days: mean difference 2.9, P = 0.0001. The PRP group showed no statistical improvement in any of the outcome measures up to 2 months.
In Jacobson et al.’s study [30] 30 patients were randomized equally to compare the efficacy of US-guided PRP injection against percutaneous tendon fenestration for treatment of GTPS. The fenestration group received 20–30 passes of the needle and the PRP group received a maximum of 10 passes. Patients answered a series of questions using a scale of 0–10 with regard to hip symptoms including level of pain, pain interfering with general activity, pain interfering with walking, pain interfering with climbing stairs and pain interfering with sleeping. Pain scores were recorded at baseline, week 1, week 2 and 3 months after treatment. The fenestration group demonstrated mean pain scores of 32.4 (SD 10.2, range 8–49) at baseline, 16.8 (SD 11.5, range 0–34) at 1 week and 15.2 (SD 10.8, range 0–34) at 2 weeks. The PRP group demonstrated mean pain scores of 31.4 (SD 7.3, range 11–41) at baseline, 25.5 (SD 8.8, range 9–40.5) at 1 week and 19.4 (SD 10.3, range 4–42) at 2 weeks. The authors reported significant pain score improvements comparing baseline with 1 and 2 weeks follow-up (P < 0.0001) with no difference between the groups (P = 0.1623). At 3 months 71% and 79% improvements in the fenestration and PRP groups respectively, with significant improvements in pain scores in both groups. No significant difference between the treatments were identified (P >.99).
Mautner et al. [31] evaluated the efficacy of US-guided PRP injections in a retrospective cross-sectional study for chronic tendinopathies refractory to conventional treatments in 180 patients. Their main outcome measures were perceived improvement in symptoms at least 6 months after treatment, VAS, functional pain and overall patient satisfaction. Mean follow-up for all patients including those with gluteus tendinopathy, was 15 months (± 6 months) following PRP injection. The study included 16 patients with gluteus medius tendinopathy out of the 180 patients. The remaining patients had a variety of tendinopathies affecting other tendons such as patella, Achilles, lateral epicondyle, rotator cuff and others. Although the study did not report specific outcome measure values separately for gluteus medius tendinopathy, they found that 81% of patients with gluteus medius tendinopathy had moderate improvement to complete resolution of symptoms at a mean follow-up of 15 months. The authors presented their main comparative results by combining the VAS scores of all 180 patients. Sixty percent of the 180 patients received only one injection, 30% received two injections and 10% received three or more injections. Seventy-five percent had a perceived decrease in VAS, from 7.0 to 1.8 (5.2, SD 2.7, 95% confidence interval 5.65–4.86, P 0.0001). Ninety-five percent had no pain at rest and 68% reported no pain during activities. Eighty-five percent were satisfied with PRP injection.
Lee et al. [32] reported a prospective case series evaluating the efficacy of US-guided PRP injections with needle tenotomy in GTPS. Their injection method consisted of PRP injection into the tendon followed by needle tenotomy (6–9 passes). The 21 patients included in this series had symptoms longer than 3 months and their symptoms were refractory to other treatments. All participants were assessed at baseline and post-injection with four outcome measures including mHHS, HOS-ADL, HOS-Sport and iHOT-33. The mean follow-up was 19.7 months (range 12.1–32.3 months). The mean improvements from baseline to post-injection follow-up were 56.73 (range 35.20–73.70)–74.17 (range 42.90–95.70) for mHHS, 68.93 (range 20.59–100.00)–84.14 (range 48.53–100.00) for HOS-ADL, 45.54 (5.56–94.40)–66.72 (range 28.13–100.00) for HOS-Sport and 34.06 (range 6.45–74.06)–66.33 (range 19.60–94.60) for iHOT-33. The improvements in all outcome measures were clinically and statistically significant (P< 0.001).
CONFERENCE ABSTRACTS RESULTS
The conference abstracts included one randomized trial and three case series which were non-peer reviewed and presented limited data but were included in this review to assist when making inferences and drawing conclusions. We found generally that information about PRP preparation, amount of injected PRP and complications following injections were not included.
Blucher et al. [33] reported their prospective single surgeon case series of 85 patients with recalcitrant GTPS to assess whether PRP injections improved their symptoms and to evaluate PRP effects in relation to quality of life and daily activities. Gluteal tendinopathy and trochanteric bursitis were proven radiologically with either MRI or US, however they did not report the relative proportions of either. Pain scores (0–10), EQ-5D Health Domain, HOOS, Utility and VAS scores were collected at baseline and following PRP injection. The duration of symptoms ranged from 3 to 120 months. Twenty percent of patients reported moderate and 78% severe symptoms. Pain scores improved from 8.1 at baseline to 4.6 post-injection (P < 0.0001). Sixty-nine percent of patients reported successful outcomes. Both EQ-5D Utility and EQ-5D VAS scores improved after the PRP injection and the proportion of reported level II (some problems) and III (extreme problems) decreased significantly for each of the EQ-5D dimensions at the last follow-up (P < 0.001). HOOS scores increased significantly (P < 0.01) in all groups after treatment.
Monto R [34] compared US-guided PRP injection with cortisone injection in the treatment of severe cases of GTPS. Forty patients who had failed a minimum of 6 months of conservative treatment were randomized into a two-arm blinded study. The results in group 1 (cortisone) demonstrated an improvement from a mean baseline HSS of 52 (range 43–54)–75 (range 62–84) at 3 months post injection but worsened to 68 (range 54–84) at 6 months and 59 (range 53–77) at 12 months. The trend was similar with the WOMAC scores, with a baseline of 58 (54–66), 83 (range 61–87) at 3 months, 68 (range 54–84) at 6 months and 63 (range 58–79) at 12 months. Group 2 (PRP) demonstrated sustained improvements from a mean baseline HSS of 51 (range 49–53)–84 (range 77–92) at 3 months and 87 (range 82–92) at 6 months and 87 (range 81–92) at 12 months. This was reflected in the WOMAC scores with improvements from a mean baseline of 59 (range 55–61)–91 (range 80–97) at 3 months, 90 (range 83–97) at 6 months and 89 (range 83–96) at 12 months. They reported a statistical significance of P = 0.001.
Rajeev et al. [35] prospectively assessed the outcomes of PRP injection in a 32 patient case series with severe GTPS following total hip replacement. Patients had a minimum of 6 months of conservative treatment. Using HHS and VAS, patients were evaluated at baseline, 3 months, 6 months and 1 year following PRP injection. The pre-treatment HHS were 54 (range 48–60) and VAS was 7.8 (7–8). The post-treatment HHS initially improved to 78 (62–84) and VAS of 4.5 (3–5) at 3 months. The HHS after 6 months were 72 (64–80) and VAS 5.4 (5–6). At 1 year,, the HHS dropped to 68 (54–74) and VAS improved 6.7 (5–8).
LaSalle et al.’s [36] abstract retrospectively reported the efficacy of US-guided PRP injections in patients with radiologically (MRI) proven gluteus medius or minimus tears, tendinosis or degeneration. Ten patients had more than 12 weeks unsuccessful conservative treatment. The main outcome measures included VAS, FRI and NASS. The mean duration of pain was 46 months (range 8–120 months) and mean follow-up 10.2 months (range 6–26 months). The mean VAS was 8.10 (SD 1.7) at baseline and 3.8 (SD 2.7) post-injection (P = 0.002). Overall patient satisfaction was 80% (as measured by NASS). Two patients reported no improvement. Of the eight patients who reported improvement, their mean FRI score was 62.4 (out of 100) at baseline and 21.3 six months post-injection (P = 0.001). The average VAS scores among these eight patients were 8.7 (SD 1.1) at baseline and 2.7 (SD 2.1) post-injection.
DISCUSSION
The application of PRP in the management of musculoskeletal conditions has become more prevalent in recent years. The use of PRP in treating tendinopathies has been widely investigated including testing cultures of equine and human tendon cells which show an increase in the types of expression of collagen genes in tendon cell cultures when mixed with PRP [37–39]. Multiple reviews have been reported summarizing the available evidence and to compare the outcomes of PRP injections with other therapeutic modalities. Arirachakaran et al. [40] performed a systematic review and meta-analysis of randomized controlled trials in order to compare relevant clinical outcomes between the use of PRP, autologous blood and corticosteroids injection in treatment of lateral epicondylitis. They concluded that PRP injection can improve pain and has a lower risk of complications. Considering the application of PRP in the treatment of patellar and Achilles tendinopathy, a systematic review of the literature was performed by Di Matteo et al. in 2015 [41]. Twenty-two studies were included and analysed. All the papers concerning patellar tendinopathy reported positive outcome for PRP, which proved to be superior to other traditional approaches such as shock-wave therapy and dry needling. In the case of Achilles tendinopathy, despite the only RCT available showing no significant clinical difference between PRP and saline solution, there were encouraging findings reported by case series.
Our review illustrates a growing interest in the use of PRP in MSK conditions over the past few years. We identified a small number of RCTs and non-comparative studies reporting the use of PRP in GTPS. This review highlights the interesting conclusions from the included articles to shed light on whether this is an efficacious method of treating this condition. All of the full-text article studies used US guidance for their injections but all used differing PRP systems with differing injection volumes, spinning protocols and reported compositions of PRP. Three studies reported using leukocyte-rich PRP [28, 30, 32].
The different PRP preparations and the possibility of exerting different therapeutic effects have been previously investigated in vitro. However, the role of leukocytes in PRP has not yet been defined under tendinopathy conditions in vivo [42]. Yan et al. [42] compared the effects of the intratendinous injection of leukocyte-poor PRP (Lp-PRP) versus leukocyte-rich PRP (Lr-PRP) in a rabbit chronic tendinopathy model in vivo. They concluded that Lp-PRP is superior to Lr-PRP as it improves tendon healing and is a preferable option for the clinical treatment of tendinopathy. Another study by Zhou et al. [43] found that while both Lr-PRP and Lp-PRP appear to be safe in inducing the differentiation of tendon stem cells into active tenocytes, Lr-PRP may be disadvantageous to the healing of injured tendons because it produces catabolic and inflammatory effects on tendon cells. Conversely, using Lp-PRP to heal acute tendon injuries, may give rise to excessive scar tissue due to the strong potential of Lp-PRP to cause disproportionate cellular anabolic effects.
Summarizing the full published papers in our review; two of the RCTs concluded that patients with chronic gluteal tendinopathy achieve better clinical outcomes when treated with PRP injection compared with corticosteroid [28, 30] whereas the other RCT found no significant differences [29]. Fitzpatrick et al.’s study was deemed as high quality with the JADDAD score of 5. It was blinded, with a relatively large sample size and reporting of their trial design methodology was robust. At 12 weeks 39 out of 40 in the PRP group were available for analysis. However, the follow-up period was limited at 12 weeks so long-term results are unclear. Jacobsen et al.’s study [30] was a poor quality randomized trial based on a JADAD score of 1. Compared with Ribeiro et al.’s study [29], slightly more (30 patients) were randomized by Jacobson et al. to compare PRP injection with fenestration. Although the study showed a comparable improvement in the two groups, the authors only measured pain scores and there were no functional outcomes recorded. Furthermore, the study did not demonstrate the longevity of treatment as mean follow-up was 3 months. The Ribeiro study rated as a high-quality randomized trial based on JADAD score of 5. Although the study was randomized and double blinded, the limitations of this study were the small sample size (20 patients) and short duration of follow-up (2 months).
The two case series reported improvements in treating gluteal tendinopathy [31, 32]. Lee et al.’s case series [32] was a high-quality study based on MINORS score. The study showed statistically significant improvement with values that exceeded the MCID. The criticisms of this study were the small sample size, no comparative group and the non-consistency of the post-injection therapy programme among patients. There was little information on the PRP composition. Furthermore, patients had both needle tenotomy and PRP injection, hence the relative effects could not be distinguished. Conversely Mautner et al.’s [31] case series was a poor-quality study based on MINORS score. Although the study was multi-centred, it was retrospective, over-ridden with heterogeneity and the PRP injection methodology was non-uniform. Furthermore, they did not present specific outcome measure values with significance for gluteus medius tendinopathy, forcing us to draw conclusions based on a percentage.
Considering the published abstracts, there is a lack of valuable study information compared with the full published articles therefore the conclusions provided are of low quality and clinical value as the experiments cannot be replicated. All of the abstracts reported good outcomes in treating GTPS with PRP [33–36]. The randomized study demonstrated PRP to be superior compared with corticosteroid [34]. Although the study was randomized with a modest sample size compared with other reviewed articles, without a full-text article the randomization process and study design could not be evaluated. The main limitation shared by the non-randomized studies outside of being non-peer reviewed were a lack of a control group [33, 35, 36]. Blucher et al.’s [33] study strengths include a large sample size and prospectively collected data. Although the study reported promising results and good outcomes in both subjective and objective scoring, it did not report the follow-up period. Rajeev et al.’s prospective case series did not report the significance of their results or intra-group comparisons [35]. Furthermore, as GTPS may have been secondary to surgery or distortion of biomechanics, the judgement on the efficacy in this study might not be accurate, therefore conclusions are difficult to draw from this article. Lasalle et al.’s study was limited by a small sample size of 10 patients [36].
CONCLUSION
Our review highlights the lack of adequately powered studies providing high-quality evidence, especially when the global pathology of GTPS is considered. Quite often the pathology may be in the gluteus medius and minimus tendon and not exclusively the bursa, therefore the site of injection needs to be considered. In most of the studies improvements were observed during the first 3 months after injection [28–36]. Significant improvements were reported when patients were followed up to 12 months post treatment [31, 32]. There are, however, conflicting results between the randomized studies as to whether PRP is superior to corticosteroid. Furthermore, the use of different PRP systems, concentrations and volumes provides heterogeneity when trying to provide comparisons. Varying outcome measures were used to assess pain and functional outcomes with short follow-up and small sample sizes. Considering these factors, PRP seems a viable alternative treatment with the current evidence in patients with GTPS refractory to conservative measures. However due to the limitations in these studies, the definitive role for PRP in managing GTPS is open for debate. We therefore recommend further large-sample and high-quality randomized clinical trials in the future to provide evidence of the efficacy for PRP as a treatment in GTPS.
FUNDING
None declared.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Bird PA, Oakley SP, Shnier R.. Prospective evaluation of magnetic resonance imaging and physical examination findings in patients with greater trochanteric pain syndrome. Arthritis Rheum 2001; 44: 2138–45. [DOI] [PubMed] [Google Scholar]
- 2. Visser LC, Arnoczky SP, Caballero O.. Growth factor-rich plasma increases tendon cell proliferation and matrix synthesis on a synthetic scaffold: an in vitro study. Tissue Eng Part A 2010; 16: 1021–9. [DOI] [PubMed] [Google Scholar]
- 3. Alsousou J, Thompson M, Harrison P. et al. Effect of platelet-rich plasma on healing tissues in acute ruptured Achilles tendon: a human immunohistochemistry study. Lancet 2015; 385: S19.. [DOI] [PubMed] [Google Scholar]
- 4. Fearon AM, Scarvell JM, Cook JL. et al. Does ultrasound correlate with surgical or histologic findings in greater trochanteric pain syndrome? A pilot study. Clin Orthopaedics Relat Res 2010; 468: 1838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Segal NA, Felson DT, Torner JC. et al. Greater trochanteric pain syndrome: epidemiology and associated factors. Arch Phy Med Rehabil 2007; 88: 988–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sayegh F, Potoupnis M, Kapetanos G.. Greater trochanter bursitis pain syndrome in females with chronic low back pain and sciatica. Acta Orthop Belg 2004; 70: 423–8. [PubMed] [Google Scholar]
- 7. Raman D, Haslock I.. Trochanteric bursitis – a frequent cause of ‘hip’ pain in rheumatoid arthritis. Ann Rheum Dis 1982; 41: 602–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pozzi G, Lanza E, Parra CG. et al. Incidence of greater trochanteric pain syndrome in patients suspected for femoroacetabular impingement evaluated using magnetic resonance arthrography of the hip. Radiol Med 2017; 122: 208–14. [DOI] [PubMed] [Google Scholar]
- 9. Williams BS, Cohen SP.. Greater trochanteric pain syndrome: a review of anatomy, diagnosis and treatment. Anesth Analg 2009; 108: 1662–70. [DOI] [PubMed] [Google Scholar]
- 10. Lustenberger DP, Ng VY, Best TM. et al. Efficacy of treatment of trochanteric bursitis: a systematic review. Clin J Sport Med 2011; 21: 447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Del Buono A, Papalia R, Khanduja V. et al. Management of the greater trochanteric pain syndrome: a systematic review. Br Med Bull 2012; 102: 115–31. [DOI] [PubMed] [Google Scholar]
- 12. Govaert LHM, van der Vis HM, Marti RK. et al. Trochanteric reduction osteotomy as a treatment for refractory trochanteric bursitis. J Bone Joint Br 2003; 85-B: 199–203. [DOI] [PubMed] [Google Scholar]
- 13. Craig RA, Jones DP, Oakley AP. et al. Iliotibial band Z-lengthening for refractory trochanteric bursitis (greater trochanteric pain syndrome). ANZ J Surg 2007; 77: 996–8. [DOI] [PubMed] [Google Scholar]
- 14. Castricini R, Longo UG, De Benedetto M. et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med 2011; 39: 258–65. [DOI] [PubMed] [Google Scholar]
- 15. Mei-Dan O, Carmont MR.. Autologous blood products in rota- tor cuff repair. Med Sport Sci 2012; 57: 65–75. [DOI] [PubMed] [Google Scholar]
- 16. Coleman BD, Khan KM, Maffulli N. et al. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports 2000; 10: 2–11. [DOI] [PubMed] [Google Scholar]
- 17. Liddle AD, Rodríguez-Merchán EC.. Platelet-rich plasma in the treatment of patellar tendinopathy: a systematic review. Am J Sports Med 2015; 43: 2583–90. [DOI] [PubMed] [Google Scholar]
- 18. Meheux CJ, McCulloch PC, Lintner DM. et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy 2016; 32: 495–505. [DOI] [PubMed] [Google Scholar]
- 19. Mishra AK, Skrepnik NV, Edwards SG. et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med 2014; 42: 463–71. [DOI] [PubMed] [Google Scholar]
- 20. Mei-Dan O, Carmont MR, Laver L. et al. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med 2012; 40: 534–41. [DOI] [PubMed] [Google Scholar]
- 21. Foster TE, Puskas BL, Mandelbaum BR. et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 2009; 37: 2259–72. [DOI] [PubMed] [Google Scholar]
- 22. Lee JW, Kwon OH, Kim TK. et al. Platelet-rich plasma: quantitative assessment of growth factor levels and comparative analysis of activated and inactivated groups. Arch Plast Surg 2013; 40: 530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kakudo N, Morimoto N, Kushida S. et al. Platelet-rich plasma releasate promotes angiogenesis in vitro and in vivo. Med Mol Morphol 2014; 47: 83–9. [DOI] [PubMed] [Google Scholar]
- 24. Kajikawa Y, Morihara T, Sakamoto H. et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cell Physiol 2008; 215: 837–45. [DOI] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8: 336–41. [DOI] [PubMed] [Google Scholar]
- 26. Jadad AR, Moore RA, Carroll D. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 27. Slim K, Nini E, Forestier D. et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003; 73: 712–6. [DOI] [PubMed] [Google Scholar]
- 28. Fitzpatrick J, Bulsara MK, O’Donnell J. et al. The effectiveness of platelet-rich plasma injections in gluteal tendinopathy: a randomized, double-blind controlled trial comparing a single platelet-rich plasma injection with a single corticosteroid injection. Am J Sports Med 2018; 46: 933–9. [DOI] [PubMed] [Google Scholar]
- 29. Ribeiro A. d G, Ricioli W, Silva ARNSE. et al. PRP in the treatment of trochanteric syndrome: a pilot study. Acta Ortop Bras 2016; 24: 208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jacobson JA, Yablon CM, Henning PT. et al. Greater trochanteric pain syndrome: percutaneous tendon fenestration versus platelet-rich plasma injection for treatment of gluteal tendinosis. J Ultrasound Med 2016; 35: 2413–20. [DOI] [PubMed] [Google Scholar]
- 31. Mautner K, Colberg RE, Malanga G. et al. Outcomes after ultrasound-guided platelet-rich plasma injections for chronic tendinopathy: a multicenter, retrospective review. PM R 2013; 5: 169–75. [DOI] [PubMed] [Google Scholar]
- 32. Lee JJ, Harrison JR, Boachie-Adjei K. et al. Platelet-rich plasma injections with needle tenotomy for gluteus medius tendinopathy: a registry study with prospective follow-up. Orthopaedic J Sports Med 2016; 4: 232596711667169.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blucher N, Nahas S, Bonatsos V. et al. Platelet-rich-plasma injections for the treatment of resistant trochanteric pain. Int J Surg 2016; 36: S32. [Google Scholar]
- 34. Abstracts from the 11th Congress of the European Hip Society. Hip Int 2014; 24: 491–553. [Google Scholar]
- 35. Abstracts from the 12th Congress of the European Hip Society. Hip Int 2016; 26: 3–92. [DOI] [PubMed] [Google Scholar]
- 36. Massimi S, LaSalle E, Vongvorachoti J. et al. Ultrasound-guided platelet rich plasma (PRP) injections for greater trochanteric pain syndrome (GTPS): a retrospective case series. PM&R 2013; 5: S206–7. [Google Scholar]
- 37. Mishra A, Woodall J Jr, Vieira A.. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med 2009; 28: 113–25. [DOI] [PubMed] [Google Scholar]
- 38. Silva R, Heidrich F.. Therapy with use of platelet-rich plasma in orthopedics and sports traumatology: literature review, evidence and personal experience In: Lana JF. (ed.). Platelet-Rich Plasma Regenerative Medicine: Sports Medicine, Orthopedic, and Recovery of Musculoskeletal Injuries. Berlin, Heidelberg: Springer, 2014, pp. 153–70. [Google Scholar]
- 39. Schnabel LV, Mohammed HO, Miller BJ. et al. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res 2007; 25: 230–40. [DOI] [PubMed] [Google Scholar]
- 40. Arirachakaran A, Sukthuayat A, Sisayanarane T. et al. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol 2016; 17: 101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Di Matteo B, Filardo G, Kon E. et al. Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy – a systematic review. Musculoskelet Surg 2015; 99: 1–9. [DOI] [PubMed] [Google Scholar]
- 42. Yan R, Gu Y, Ran J. et al. Intratendon delivery of leukocyte-poor platelet-rich plasma improves healing compared with leukocyte-rich platelet-rich plasma in a rabbit achilles tendinopathy model. Am J Sports Med 2017; 45: 1909–20. [DOI] [PubMed] [Google Scholar]
- 43. Zhou Y, Zhang J, Wu H. et al. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells - implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther 2015; 6: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]