Abstract
Background:
Testicular damage is one of the most hazardous effects of chemotherapy as it is frequently associated with oligozoospermia and azoospermia.
Aim of the Work:
This study aimed at evaluating the protective effect of hematopoietic stem cell mobilization by granulocyte colony-stimulating factor (G-CSF) in a rat model of busulfan-induced testicular injury.
Materials and Methods:
Twenty-four adult albino rats were divided into four groups: group I, the control, Group II: rats received two doses of busulfan (each 15 mg/kg) intraperitoneally (IP) with 14 days interval, Group III: rats received busulfan and left untreated, and Group IV received busulfan IP then G-CSF (70 μg/kg/day) subcutaneously for 5 consecutive days. Testicular sections were stained with H and E and immunohistochemically for CD34, proliferating cell nuclear antigen (PCNA) and caspase-3, and semithin sections were stained with toluidine blue.
Results:
Groups II and III showed loss of the normal histological architecture of the testis and spermatogenic cells, with increased apoptosis confirmed by significantly increased caspase-3 and significantly decreased PCNA immunoexpression. While Group IV revealed improved testicular histology, decreased apoptosis, and increased proliferative capacity of spermatogenic cells. This was confirmed by significantly decreased caspase-3 immunoexpression and increased PCNA immunoreaction.
Conclusion:
Mobilization of stem cells with G-CSF was found to improve the testicular histology following busulfan chemotherapy in albino rats.
Keywords: Busulfan, CD34, endogenous stem cells, granulocyte colony-stimulating factor, testis
INTRODUCTION
Spermatogenesis is a complex process that includes a series of morphologic, physiologic, and biochemical changes.[1] This process may be affected by cancer chemotherapy as the drugs cannot specifically target tumor cells.[2] Persistent azoospermia and infertility are common adverse effects of chemotherapeutics depending on the drug and the dose used, and busulfan is one of these drugs that markedly affect the testes. It causes obvious increase in apoptosis and influences the spermatogenesis at least temporarily and in some cases permanently. It is used in treating chronic myeloid leukemia and has been used before bone marrow (BM) or stem cell transplant for other types of cancer for nearly 20 years. It is an alkylating agent that inhibits cell division by sticking to one of the DNA strands. Therefore, organs and cells with high division activities, as testes and germ cells, are more susceptible to its side effects.[3] Damaged testis is not receptive to spermatogonial stem cell transplantation; thereby, it is not a solution to treat azoospermia.[4] That is why particular attention has been focused on stem cell mobilization therapy, and granulocyte colony-stimulating factor (G-CSF) is one of the most commonly used mobilizing agents.[5]
Homing factors can be beneficial to damaged tissue as they stimulate the migration of both hematopoietic and mesenchymal adult stem cells. Among homing factors, G-CSF that mobilizes hematopoietic stem cells (HSCs) from the BM to the peripheral circulation.[6] It regulates the growth, differentiation, and migration of many hematopoietic cells in BM, blood, and sites of inflammation.[7] Consequently, this study was carried out to evaluate the protective effect of HSCs mobilization by G-CSF in a rat model of busulfan-induced testicular injury. The effectiveness of G-CSF therapy was assessed by histological, immunohistochemical, and morphometric methods.
MATERIALS AND METHODS
Materials
Drugs
Busulfan (trade name Myleran)
Busulfan purchased in the form of 2 mg tablets (GlaxoSmithKline, Otsu Pharmaceutical Inc.). Tablets were crushed and dissolved in phosphate-buffered saline (PBS) solution.
Granulocyte colony-stimulating factor (trade name Neupogen)
A prefilled syringe of 300 μg of filgrastim in 0.5 mL solution for injection (recombinant-methionyl human G-CSF, r-metHuG-CSF, from Escherichia coli K12) (F. Hoffmann-La Roche Ltd, Basel. Kirin-Amgen Inc., Switzerland).
Animals
This study included 24 adult male albino rats about 12 weeks old, with average body weight 180–200 g. They were provided with veterinary care by the Animal House of Faculty of Medicine, Cairo University, according to the guidelines for animal research approved by the Animal Ethics Committee, Faculty of Medicine, Cairo University. The rats were caged in a conventional clean facility at 22°C–26°C and allowed food and water ad libitum. They were equally divided into four groups, 6 rats each.
Group I (Control group): rats were subdivided into:
Subgroup IA: 3 rats received 0.5 mL PBS (solvent of busulfan) by intraperitoneal (IP) injection
Subgroup IB: 3 rats received 0.5 mL PBS by IP injection and then subcutaneously received 0.5 mL glucose 5% (solvent of G-CSF) daily for 5 consecutive days.
One rat from each subgroup was sacrificed after 5 days, 4 and 6 weeks.
Group II (Busulfan group): rats received two doses of busulfan by (IP) injection with 14 days interval. Each dose was 15 mg/kg dissolved in 0.5 mL PBS. Rats were sacrificed after 4 weeks from the beginning of the experiment.[8]
Group III (Recovery group): rats received busulfan by the same route and dose as in Group II and left untreated for 4 weeks, i.e., rats were sacrificed after 6 weeks from the beginning of the experiment (to assess spontaneous recovery).
Group IV (G-CSF group): rats received busulfan by the same route and dose as in the previous two groups followed by G-CSF. It was subcutaneously injected at a dose of 70 μg/kg/day diluted in 0.5 mL glucose 5% daily for 5 consecutive days.[9]
Two rats from each of Group I, II, and IV were sacrificed after 5 days from the beginning of the experiment by IP injection of thiopental sodium (50 mg/kg).[10] While the remaining rats were sacrificed in the same way 4 weeks from the beginning of the experiment as follow:
Four rats from Group I (two rats from each subgroup)
Four rats from Group II (Busulfan group)
Four rats from Group IV (G-CSF group)
Eight rats were sacrificed after another 2 weeks as follow:
Two rats from Group I (one rat from each subgroup)
Six rats from Group III (Recovery group).
Methods
Histological examination
Right testis specimens were fixed in Bouin's solution and embedded in paraffin. Serial sections of 5–7 μm thickness were cut and subjected to the following:
Anti-CD34 antibody for detection of BM-derived HSCs. It is a ready-to-use mouse monoclonal antibody (Lab Vision Corporation laboratories, CA 94539, USA, catalog number MS-363-R7)
Antiproliferating cell nuclear antigen (PCNA) antibody, proliferating cell nuclear antigen, is an auxiliary protein of DNA-polymerase enzymes necessary for DNA synthesis and is used as a standard marker in proliferating cells.[13] It is a ready-to-use mouse monoclonal antibody (Lab. Vision Corporation Laboratories, CA, USA, catalogue number: MS106P)
Anti-caspase-3 antibody to demonstrate apoptosis. It is a ready-to-use rabbit polyclonal antibody (NEO markers, Thermo scientific Laboratories, USA, catalogue number RB-1197-R7).
Steps of immunohistochemical staining
Sections were incubated in H2O2 for 15 min then rinsed in PBS (Sigma Chemical CO. P. 3813 USA). They were boiled in 10 Mm citrate buffer pH 6 (Lab Vision Corporation Laboratories, cat no AP 9003) for 10 min for antigen retrieval and left to cool in room temperature then rinsed in PBS. To eliminate nonspecific background, sections were incubated with two drops of Ultra V Block for 5 min and then incubated for 60 min with two drops of the primary antibody (this was omitted in the negative control). Afterward, slides were incubated for 10 min with two drops of Biotinylated Goat Anti-Polyvalent secondary antibody at room temperature then rinsed well with PBS. Followed by incubation with two drops of streptavidin-peroxidase for 10 min at room temperature then washed in PBS. One drop of DAB Plus Chromogen was mixed with 2 ml of DAB Plus substrate then applied to the slides and incubated for 10 min at room temperature then rinsed well with distilled water. Slides were then counterstained with Mayer's hematoxylin (Lab Vision Corporation Laboratories, cat no TA-060-MH), dehydrated, and mounted.
Left testis specimens (about 1 mm) were fixed in 2.5% phosphate-buffered glutaraldehyde (pH 7.3) for 3 h, then postfixed in 1% osmium tetroxide and processed for embedding in epoxy resin. Semithin sections were cut at 1 μm thickness and stained with toluidine blue, then examined by light microscope.[14]
Morphometric study
Data were obtained using “Leica Qwin 500 C” image analyzer computer system (Cambridge, England). All measurements were done using ×400 magnification and within 10 nonoverlapping fields for each specimen. The following were measured: mean height of spermatogenic epithelium and mean number of CD34, PCNA, and caspase-3 immunopositive cells.
Statistical analysis
The measurements obtained were analyzed using SPSS software version 16 (SPSS, Chicago, USA). Comparison between different groups was made using analysis of variance followed by post hoc Tukey test. The results were expressed as means ± standard deviation. The differences were considered statistically significant when “P” < 0.05.
RESULTS
No gross pathology or congenital malformations was detected in the examined testes from all groups.
Histological results
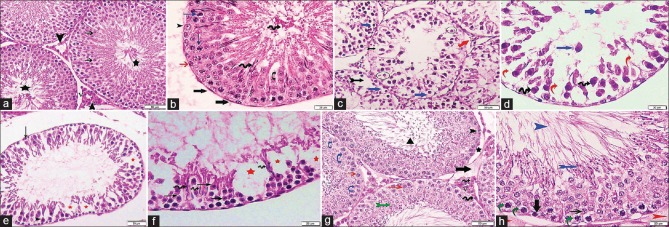
Sections from control rats (Subgroups IA and IB) showed seminiferous tubules (ST) surrounded by thin basal lamina with flat myoid cells having flattened nuclei. Each tubule was lined with stratified epithelium formed of Sertoli and spermatogenic cells. Resting on the basal lamina, there were the spermatogonia with their small dark nuclei. Inner to spermatogonia, there were the primary spermatocytes with their relative large size and large rounded characteristic nuclei. Spermatids were detected near the lumen and appeared rounded with central rounded nuclei. Spermatozoa were seen within the lumen. Sertoli cells appeared with irregular poorly defined outline among the spermatogenic cells. Their cytoplasm was pale and contained basal oval pale nuclei with prominent nucleoli. The interstitial tissue surrounding the ST-contained polyhedral cells with acidophilic cytoplasm and pale rounded nuclei (interstitial cells of Leydig) around blood vessels [Figures 1a, b and 2a].
Figure 1.
Photomicrographs of testicular sections stained with H and E. (a) Group I shows seminiferous tubules lined by several layers of rounded spermatogenic cells with central rounded nuclei. The spermatids are near the lumen (thin arrow) and the spermatozoa are in the lumen (stars). The interstitial Leydig cells (arrowheads) appear acidophilic with pale rounded nuclei and surround blood vessels (V) ×200. (b) A higher magnification of a seminiferous tubule surrounded by basal lamina and myoid cells (thick arrows). It displays spermatogonia (red arrow), primary spermatocytes (blue arrows), and spermatids (curved arrow) appear near to the lumen that contains spermatozoa (spiral arrows). Sertoli cell (arrowhead) with its vesicular nucleus can be seen ×400. (c) Group II reveals partial separation of the basement membrane (red arrow) with a lot of empty spaces (bifid black arrow). Some spermatocytes and spermatids have fragmented nuclei (circles) and others have deeply stained ones with vacuolated cytoplasm (blue arrows) ×200. (d) A higher magnification presenting loss of the normal architecture of spermatogenic cells, with the presence of empty spaces in between (red arrows). Some cells appear with deeply stained nuclei (spiral arrows) and some are shed off in the lumen (blue arrows) ×400. (e) Group III shows few layers of spermatogenic epithelium. Both spermatogonia (black arrow) and primary spermatocytes (arrowhead) have deeply stained nuclei. Empty spaces are still found within the tubule (red stars) ×200. (f) A higher magnification revealing spermatogenic cells with dark nuclei and deep acidophilic cytoplasm (black arrows). Loss of cellular connection between cells is apparent and there are empty spaces devoid of spermatogenic cells (red stars). Early sperms start to appear near the lumen (spiral arrows) ×400. (g) Group IV displays multiple layers of spermatogenic cells; spermatogonia (arrowhead) rest on basement membrane that is partially separated at some areas (star). There are primary spermatocytes (red arrows), spermatids near the lumen (bifid green arrow), spermatozoa inside it (►) and Sertoli cells (blue curved arrows). In interstitial tissue, Leydig cells with vesicular nucleus and prominent nucleolus (spiral arrow) surround blood vessels (V), with the presence of minimal exudate (thick black arrow) ×200. (h) A higher magnification displaying spermatogonia (thick arrow), primary spermatocytes (thin arrow), early sperms (bifid blue arrow), and mature sperms (blue arrowhead) within the lumen. Some cells are vacuolated (curved green arrows). There is slight exudate outside the tubule with separation of the basement membrane (red arrowheads) ×400
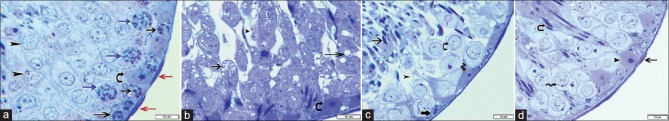
Figure 2.
Photomicrographs of testicular semithin sections stained with toluidine blue ×1000. (a) Group I reveals part of a seminiferous tubule surrounded by basal lamina and flat myoid cells with flattened nuclei (red arrows). Spermatogonia (black arrows) rest on the basement membrane, primary spermatocytes with their characteristic nuclei (blue arrows) and spermatids near the lumen (arrowheads) are seen. Sertoli cell (curved arrow) appears among the spermatogenic cells, with irregular poorly defined outline and basal oval pale nucleus with prominent nucleolus. (b) Group II loses the normal appearance of spermatogenic cells; separated in most areas (arrowhead), there is extensive vacuolation in their cytoplasm with loss of their nuclei (arrows). Sertoli cell (curved arrow) appears with its basal pale nucleus and prominent nucleolus. (c) Group III has reduced layers of spermatogenic epithelium with loss of cellular connection and a lot of empty spaces among them (arrowhead). Spermatogonia (thick arrow) and Sertoli cells (spiral arrow) rest on the basement membrane. Spermatocytes with their large nuclei (curved arrows) and sperms appear near the lumen (thin arrow) are seen. (d) Group IV shows seminiferous tubule surrounded with basal lamina and myoid cells (thin arrow). Primary spermatocytes (spiral arrow), early sperms near the lumen (curved arrow), and Sertoli cell are present (arrowhead)
Testicular sections from Group II (Busulfan group) revealed loss of the normal architecture of spermatogenic cells with partial separation from the basement membrane and appearance of a lot of empty spaces. There was extensive vacuolation inside the cytoplasm of spermatogenic cells; separation between most of them and some was shed off in the lumen. Furthermore, some of these cells showed fragmentation of their nuclei, some had deeply stained ones, while others lost theirs. Sertoli cells were seen among the spermatogenic cells containing basal pale nuclei with prominent nucleoli [Figures 1c, d, and 2b].
Sections from rats from Group III (Recovery group) displayed degeneration of spermatogenic epithelium with a lot of empty spaces in between. Spermatogonia resting on the basement membrane and primary spermatocytes with their large nuclei with deeply stained nuclei were seen. Sperms started to appear near the lumen. Loss of cellular connection between most of the cells was also present [Figures 1e, f, and 2c].
As regards testicular sections from Group IV (G-CSF group), ST was lined by almost the normal stratified epithelium (Sertoli and spermatogenic cells). The tubules are surrounded by basal lamina with myoid cells, but the laminae were separated at few areas. The spermatogonia appeared resting on the basal lamina; then primary spermatocytes with central round characteristic nuclei were seen. Spermatids were detected near the lumen and sperms inside it. Sertoli cells were also apparent. Few spermatogenic cells showed vacuolated cytoplasm. The interstitial tissue contained Leydig cells with vesicular nuclei and prominent nucleoli around blood vessels, with the presence of minimal exudate [Figures 1g, 1h, and 2d].
Immunohistochemical results
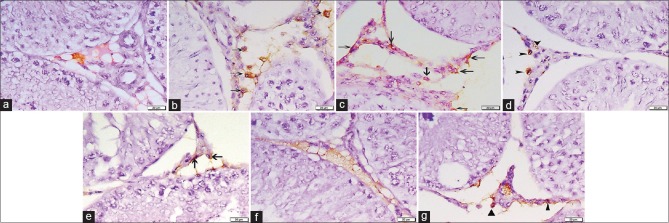
CD34 immunostained sections
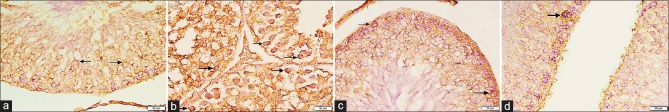
On day 5: No CD34 immunopositive HSCs could be detected in both control subgroups (IA and IB) [Figure 3a]. Concerning Group II, some CD34 positive HSCs were seen within the interstitial tissue [Figure 3b]. While in Group IV, the interstitial tissue contained many cells with positive CD34 immunoreactivity [Figure 3c]. The immunopositive cells appeared irregular in shape with dark nuclei and brown cytoplasmic reaction
After 4 weeks: Sections from the control showed positive immunoreactivity for CD34 in the walls of blood vessels in the interstitial tissue [Figure 3d]. Regarding sections from Group II, there were few CD34 positive cells in the interstitial tissue [Figure 3e]. Moreover, in Group IV, no immunopositive HSCs could be detected [Figure 3f]
After 6 weeks: Group III (recovery group) exhibited few CD34 immunopositive HSCs within the interstitial tissue [Figure 3g].
Figure 3.
Photomicrographs of testicular sections stained with CD34 immunohistochemical stain ×400. (a) Group I on day 5 displays no CD34 +ve HSCs. (b) Group II on day 5 shows some +ve CD34 cells with brown cytoplasmic reaction in the interstitial cells (arrows). (c) Group IV on day 5 reveals many CD34 +ve cells within the interstitial tissue (arrows). (d) Group I after 4 weeks shows positive immunoreactivity for CD34 in the walls of blood vessels in the interstitial tissue (arrowheads). No CD34 +ve cells can be detected. (e) Group II after 4 weeks presents few CD34 immunopositive HSCs in the interstitial tissue (arrows). (f) Group IV after 4 weeks has no CD34 immunopositive cells. (g) Group III after 6 weeks reveals few +ve HSCs (▲)
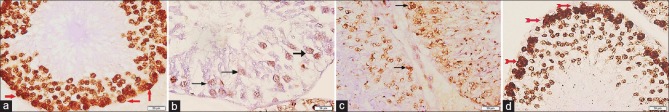
Proliferating cell nuclear antigen immunostained sections
Testicular sections from both control subgroups revealed widespread brown PCNA immunoreactivity in the nuclei of spermatogenic cells, especially in spermatogonia [Figure 4a]. Sections from Group II revealed mild immunoreaction for PCNA in the remaining spermatogenic cells [Figure 4b]. As for sections from Group III, there was moderate PCNA immunoreactivity in spermatogenic cells [Figure 4c]. While in Group IV, there was widespread immunoreaction for PCNA in the spermatogenic cells especially the spermatogonia [Figure 4d].
Figure 4.
Photomicrographs of testicular sections stained with proliferating cell nuclear antigen immunohistochemical stain ×400. (a) Group I shows widespread proliferating cell nuclear antigen immunoreaction in the nuclei of spermatogenic cells, especially the spermatogonia (red arrows). (b) Group II displays few proliferating cell nuclear antigen immunopositive cells (arrows). (c) Group III reveals moderate proliferating cell nuclear antigen immunoreaction (arrows). (d) Group IV show increased number of proliferating cell nuclear antigen +ve cells (red bifid arrows)
Caspase-3 immunostained sections
Sections from the control subgroups (IA and IB) displayed scarce positive caspase-3 immunoreactivity in the cytoplasm of spermatogenic cells [Figure 5a]. Testicular sections from Group II showed widespread immunoreaction for caspase-3 in the ST [Figure 5b]. Concerning Group III, some spermatogenic cells were immunopositive for caspase-3 [Figure 5c]. While sections from Group IV revealed very few spermatogenic cells with brown cytoplasmic reaction [Figure 5d].
Figure 5.
Photomicrographs of testicular sections stained with caspase-3 immunohistochemical stain ×400. (a) Group I presents scarce cytoplasmic reaction for caspase-3 in ST (arrows). (b) Group II shows widespread +ve caspase-3 immunoreactivity in the spermatogenic cells (arrows). (c) Group III appears with some caspase-3 +ve cells (arrows). (d) Group IV has very few spermatogenic cells exhibiting immunoreaction for caspase-3 (arrow)
Morphometric results
Morphometric results are illustrated in Table 1.
Table 1.
Morphometric results of the experimental groups±standard deviation
| Group I (control) | Group II (busulfan) | Group III (recovery) | Group IV (G-CSF) | |
|---|---|---|---|---|
| Mean height of spermatogenic epithelium | 73.8±3.4 | 22.4±2.7* | 29.9±2.1* | 61.4±2.7** |
| Mean number of of positive CD34 cells on day 5 | 0.00±0.00 | 2.3±1.3* | - | 15.7±2.1# |
| Mean number of of positive CD34 cells after 4 weeks | 0.000.00 | 2.00±0.8* | - | 0.00±0.00† |
| Mean number of positive CD34 cells after 6 weeks | - | - | 1.4±0.3∞ | - |
| Mean number of positive PCNA cells | 48.9±2.6 | 7.2±1.3∞ | 16.5±2.1* | 39.2±2.6‡ |
| Mean number of positive caspase-3 cells | 4.4±12 | 27.00±1.8∞ | 21.6±2.2* | 7.1±1.9** |
*Significant compared to Group I, **Significant compared to both Groups II and III but nonsignificant compared to Group I, #Significant compared to both Groups I, II, †Significant compared to Group II and IV on day 5, ∞Significant compared to Group I and Group IV, ‡Significant compared to Group II. PCNA: Proliferating cell nuclear antigen, G-CSF: Granulocyte colony-stimulating factors
DISCUSSION
In the present study, two doses of busulfan (each 15 mg/kg) were given by IP injection with 14 days interval;[8] as the required dose for sterilizing rats is usually two injections of 10–15 mg/kg.[15] Researchers, who used busulfan by the same dose and route, found testicular atrophy with spermatocytic arrest.[16] Others reported thinning of the walls of the majority of ST and depletion of spermatids and spermatozoa in the innermost layers, which is a typical feature of “partial spermatogenesis.” Moreover, in rats treated with higher doses of busulfan (20 or 30 mg/kg), even thinner walls with only one layer of spermatogonia were found in most of the ST.[15] Even when some scientists used single dose of busulfan 40 mg/kg, they found degenerations of germinal epithelium in most of the tubules that showed wide lumens: as the atrophic epithelium covered the peripheral zones of ST-forming thin bands.[17] This could be attributed to incomplete or impaired spermatogenesis based on previous studies on anticancer drugs that had shown that these drugs reduced the number of germ cells by increasing the apoptosis.[18]
Sections from Group II (busulfan group) showed marked histological alterations within the ST and significant apoptosis and loss of spermatogenic cells, so the sperms appeared lower than their original position. This is consistent with other studies that mentioned that number of spermatogonia and primary spermatocytes were decreased significantly and showed pyknotic nuclei after busulfan administration, even if they had might have normal size.[8,17] Furthermore, spermatogonial cell death and cessation of spermatogenesis after busulfan administration was reported and it was explained by the inhibition of spermatogonial stem cells proliferation that would lead to infertility.[19,20] Furthermore, increased apoptosis could be attributed to oxidative stress that causes impairment in mitochondrial functions, cytochrome c release, and subsequent caspase activation.[21] Group II specimens also revealed extensive vacuolation within the cytoplasm of spermatogenic cells. This is in agreement with previous studies, where these vacuoles were considered as an apoptotic sign due to cell loss and detachment.[22] Increased cell death could be explained by the fact that busulfan is a DNA alkylating agent that could deplete germ cells with the disruption of cellular junctions.[15] Going parallel, Group II revealed significant decrease in PCNA immunoreaction, indicating a very weak proliferation. This was proved by other scientists, reporting low levels of PCNA expression along with other spermatogenic markers after busulfan treatment.[8]
Testicular sections from Group III (recovery group) still showed morphological changed within the spermatogenic epithelium, especially apoptosis. Furthermore, the mean number of caspase-3 immunopositive cells was significantly increased, with concomitant decrease in PCNA immunoexpression. At the same time, the sperms started to appear near to the lumen, indicating the beginning of recovery from the toxic effect of busulfan. Concurrently, other researchers documented that partial or full recovery of spermatogenic cells was very limited in animals treated with busulfan.[15] Similarly, some reported that high-dose of busulfan (40 mg/kg) completely eliminates germ cells, sterilizes males, and causes long-term morphological damage to sperms produced by the surviving stem spermatogonia; however, after a single dose, partial recovery of spermatogenesis after two cycles, 52 days per cycle, could occur.[23]
Whereas other scientists found that ST was sporadically repopulated with spermatogonia 28 days after busulfan treatment, demonstrating that spermatogonial repopulation is dose dependent.[24] Alternatively, other ones thoroughly analyzed the testes for any spontaneous recovery of spermatogenesis, and no sign was observed for its reinitiation.[16,25]
G-CSF, a hematopoietic growth factor, is known for its proliferative and antiapoptotic activities. It is required for cell proliferation, differentiation, and survival of hematopoietic progenitor cells and these functions are carried out through several signaling pathways including JAK/STAT, ERK, and PI3K/PDK/Akt.[8] It can minimize inflammation by downregulation of pro-inflammatory cytokines’ production and thus ameliorates the destructive inflammatory response. The JAK/STAT signaling pathway has been suggested as a mediator of the antiapoptotic effect of G-CSF. Besides, it can induce the proliferation of the protected stem cells by some of its components. Another possible mechanism is the ability of hematopoietic growth factors to increase glucose and Vitamin C uptake in testes, which results in enhanced sperm mobility and increased antioxidant defense in cells as spermatozoa.[26] Furthermore, the beneficial effect of G-CSF is mediated by mobilizing BM stem cells and recruitment to injured tissues, where the stem cells interact with the local microenvironment to accelerate endogenous repair process. It has been shown to promote HSCs mobilization by reducing stromal cell-derived factor 1(SDF-1) expression in the BM, as well as C-X-C chemokine receptor type 4 (CXCR4) expression on HSCs. SDF-1 is a cytokine that plays an important role in stem cell maintenance within the BM, and when it is upregulated, it promotes stem cell tropism through interactions with its cellular receptor CXCR4.[27]
Mobilization of endogenous HSCs was enhanced in this study by G-CSF (70 μg/kg/day) for 5 consecutive days following the injection of the first dose of busulfan. Scientists found that the outcome of G-CSF treatment regarding spermatogenesis was better when injection was given at the beginning of busulfan exposure rather than at the mid time period of receiving the cytotoxic drug.[8] In addition, daily administration of G-CSF for 4–6 days resulted in 10–30-fold increase in the number of circulating stem cells.[28] Accordingly, homing of HSCs was assessed using anti-CD34 antibody that is a standard agent for HSCs isolation. This was done on the 5th day, which is the day of maximum mobilization as previously reported.[29] Furthermore, it was assessed at the end of experiment (after 6 weeks).
In the current work, sections from Group IV (G-CSF group) displayed almost normal stratified spermatogenic epithelium within ST, significantly decreased caspase-3 immunoexpression, and significant increase in the number of PCNA immunopositive cells. Similarly, previous studies reported high levels of PCNA expression in the spermatogenic cells, especially spermatogonia in rats treated with G-CSF, indicating their rapid rate of proliferation.[8] These results could be ascribed for the antiapoptotic effect of G-CSF that was previously documented in various organs as liver, kidneys, and ovaries including testis.[26] These results are going parallel with other researches, which proved that G-CSF could prevent the loss of spermatogenesis resulted from busulfan chemotherapy through protecting the undifferentiated spermatogonia. They detected G-CSF receptor in the mouse testis and thus suggested that it might promote spermatogonial cell survival to increase the regenerative pool or might drive their proliferation later to promote regeneration. It is possible that G-CSF may play a role in supporting the ongoing spermatogenesis as in hematopoiesis, it has been known as a mitogen that drives granulopoiesis and a modulator of HSCs function.[2]
It was proved that G-CSF is the most effective mobilizing agent used. Its repetitive stimulations favor accelerated myeloid hematopoiesis and indirectly enhance HSCs motility and recruitment to the circulation. Several mechanisms were reported to enhance this mobilization capability; it was found that G-CSF induced accumulation of neutrophils in the BM with the release of neutrophil elastase that selectively cleaves and inactivates adhesion molecules necessary for the retention of HSCs within the BM. Furthermore, G-CSF mobilizes stem cells by triggering the enzymatic cleavage of membrane-bound stem cell factor (SCF) that is produced by BM endothelial cells, perivascular stromal cells, and other stromal cells and this SCF binds the HSCs to the surface of these marrow cells.[30] Regarding the antiapoptotic capability of G-CSF, previous studies denoted that GCSF profoundly decreases the damage-induced cell death, increase in caspase-3 activity, and apoptosis and it achieved a remarkable promotion in cell proliferation may be through influencing the expression of cell cycle proteins such as cyclinB, cyclinD, and cyclinE and bloching p53 activity, so subsequently speeding up cell cycle process.[31]
An important character of stem cells is their capability to home to sites of inflammation or tissue damage, so immunohistochemical staining using anti-CD34 antibody was used to detect homing of CD34+ve HSCs.[32] On day 5, examination of sections from busulfan group revealed some CD34 immunopositive HSCs that were spontaneously mobilized to the site of injury. In contrast, sections from G-CSF group displayed significant increase in the mean number of CD34 immunopositive HSCs when compared to Group II and the control. Similar results were reported in previous studies carried on the kidneys of rats after receiving G-CSF.[6,29] Nevertheless, after 4 weeks, no immunopositive HSCs could be detected in Group IV, suggesting their complete differentiation. This was confirmed statistically by significant decrease in the number of CD34 immunopositive HSCs in this group as compared to both Groups II and IV on day 5. This suggestion is based on the fact that, when HSCs differentiate, they lose the CD34 marker and acquire other biochemical markers.[33] Whereas sections from both Groups II and III (after 6 weeks) had few CD34 immunopositive cells in the interstitial tissue that was significantly increased as compared to control, suggesting some differentiation of HSCs but less than Group IV.
CONCLUSION
The current work provides evidence that mobilization of HSCs using G-CSF at the beginning of busulfan treatment resulted in maintenance of testicular histology, decreased apoptosis, and increased proliferation of spermatogenic cells. Moreover, this needs further investigations, so G-CSF could be used in male patients, especially prepubertal, who have to receive gonadotoxic therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Luo Z, Liu Y, Chen L, Ellis M, Li M, Wang J, et al. MicroRNA profiling in three main stages during porcine spermatogenesis. J Assist Reprod Genet. 2015;32:451–60. doi: 10.1007/s10815-014-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benavides-Garcia R, Joachim R, Pina NA, Mutoji KN, Reilly MA, Hermann BP, et al. Granulocyte colony-stimulating factor prevents loss of spermatogenesis after sterilizing busulfan chemotherapy. Fertil Steril. 2015;103:270–80.e8. doi: 10.1016/j.fertnstert.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panahi M, Keshavarz S, Rahmanifar F, Tamadon A, Mehrabani D, Karimaghai N, et al. Busulfan induced azoospermia: Stereological evaluation of testes in rat. Vet Res Forum. 2015;6:273–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Easley CA, 4th, Simerly CR, Schatten G. Stem cell therapeutic possibilities: Future therapeutic options for male-factor and female-factor infertility? Reprod Biomed Online. 2013;27:75–80. doi: 10.1016/j.rbmo.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C, Zhang X, Chen XH. Granulocyte-colony stimulating factor-mobilized mesenchymal stem cells: A new resource for rapid engraftment in hematopoietic stem cell transplantation. Med Hypotheses. 2011;76:241–3. doi: 10.1016/j.mehy.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Modelli de Andrade LG, Viero RM, Cordeiro de Carvalho MF. Treatment of adriamycin-induced nephropathy with erythropoietin and G-CSF. J Nephrol. 2013;26:534–9. doi: 10.5301/jn.5000200. [DOI] [PubMed] [Google Scholar]
- 7.Broughton SE, Nero TL, Dhagat U, Kan WL, Hercus TR, Tvorogov D. The βc receptor family – Structural insights and their functional implications. Cytokine. 2015;74:247–58. doi: 10.1016/j.cyto.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Khanlarkhani N, Pasbakhsh P, Mortezaee K, Naji M, Amidi F, Najafi A, et al. Effect of human recombinant granulocyte colony-stimulating factor on rat busulfan-induced testis injury. J Mol Histol. 2016;47:59–67. doi: 10.1007/s10735-015-9647-y. [DOI] [PubMed] [Google Scholar]
- 9.Prakash A, Chopra K, Medhi B. Granulocyte-colony stimulating factor improves Parkinson's disease associated with co-morbid depression: An experimental exploratory study. Indian J Pharmacol. 2013;45:612–5. doi: 10.4103/0253-7613.121374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rafsanjani FN, Adeli S, Ardakani ZV, Ardakani JV, Ghotbi P. Effects of diabetes mellitus on gastric motility in rats. Pak J Physiol. 2009;5:20–4. [Google Scholar]
- 11.Bancroft J, Gamble M. 7th ed. Edinburgh, London, Madrid, Melbourne, New York, Tokyo: Churchill Livingstone; 2008. Staining methods. Theory and Practice of Histological Techniques; pp. 121–35. 263-325. [Google Scholar]
- 12.Suvarna SK, Layton C, Bancroft JD. 7th ed. New York, USA: Elsevier Health Sciences, Churchill Livingstone; 2012. Bancroft's Theory and Practice of Histological Techniques; pp. 215–39. [Google Scholar]
- 13.Kerr JB, Duckett R, Myers M, Britt KL, Mladenovska T, Findlay JK, et al. Quantification of healthy follicles in the neonatal and adult mouse ovary: Evidence for maintenance of primordial follicle supply. Reproduction. 2006;132:95–109. doi: 10.1530/rep.1.01128. [DOI] [PubMed] [Google Scholar]
- 14.Nisticò R, Florenzano F, Mango D, Ferraina C, Grilli M, Di Prisco S, et al. Presynaptic c-Jun N-terminal kinase 2 regulates NMDA receptor-dependent glutamate release. Sci Rep. 2015;5:9035. doi: 10.1038/srep09035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang DZ, Zhou XH, Yuan YL, Zheng XM. Optimal dose of busulfan for depleting testicular germ cells of recipient mice before spermatogonial transplantation. Asian J Androl. 2010;12:263–70. doi: 10.1038/aja.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cakici C, Buyrukcu B, Duruksu G, Haliloglu AH, Aksoy A, Isık A, et al. Recovery of fertility in azoospermia rats after injection of adipose-tissue-derived mesenchymal stem cells: The sperm generation. Biomed Res Int. 2013;2013:529589. doi: 10.1155/2013/529589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monsefi M, Fereydouni B, Rohani L, Talaei T. Mesenchymal stem cells repair germinal cells of seminiferous tubules of sterile rats. Iran J Reprod Med. 2013;11:537–44. [PMC free article] [PubMed] [Google Scholar]
- 18.Ismail DI. Histological study on doxorubicin-induced testicular toxicity and the protective role of sesamol in rats. Egypt J Histol. 2016;39:38–49. [Google Scholar]
- 19.Puri P, Phillips BT, Suzuki H, Orwig KE, Rajkovic A, Lapinski PE, et al. The transition from stem cell to progenitor spermatogonia and male fertility requires the SHP2 protein tyrosine phosphatase. Stem Cells. 2014;32:741–53. doi: 10.1002/stem.1572. [DOI] [PubMed] [Google Scholar]
- 20.Cai Y, Liu T, Fang F, Shen S, Xiong C. Involvement of ICAM-1 in impaired spermatogenesis after busulfan treatment in mice. Andrologia. 2016;48:37–44. doi: 10.1111/and.12414. [DOI] [PubMed] [Google Scholar]
- 21.Yuluğ E, Türedi S, Alver A, Türedi S, Kahraman C. Effects of resveratrol on methotrexate-induced testicular damage in rats. ScientificWorldJournal. 2013;2013:489659. doi: 10.1155/2013/489659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirhoseini M, Saki G, Hemadi M, Khodadadi A, Mohammadi Asl J. Melatonin and testicular damage in busulfan treated mice. Iran Red Crescent Med J. 2014;16:e14463. doi: 10.5812/ircmj.14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez-Crespo M, Pericuesta E, Pérez-Cerezales S, Arenas MI, Lobo MV, Díaz-Gil JJ, et al. Effect of liver growth factor on both testicular regeneration and recovery of spermatogenesis in busulfan-treated mice. Reprod Biol Endocrinol. 2011;9:21. doi: 10.1186/1477-7827-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westernströer B, Terwort N, Ehmcke J, Wistuba J, Schlatt S, Neuhaus N, et al. Profiling of cxcl12 receptors, cxcr4 and cxcr7 in murine testis development and a spermatogenic depletion model indicates a role for cxcr7 in controlling cxcl12 activity. PLoS One. 2014;9:e112598. doi: 10.1371/journal.pone.0112598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehrabani D, Hassanshahi MA, Tamadon A, Zare S, Keshavarz S, Rahmanifar F, et al. Adipose tissue-derived mesenchymal stem cells repair germinal cells of seminiferous tubules of busulfan-induced azoospermic rats. J Hum Reprod Sci. 2015;8:103–10. doi: 10.4103/0974-1208.158618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Lee S, Jeon B, Jang W, Moon C, Kim S, et al. Protection of spermatogenesis against gamma ray-induced damage by granulocyte colony-stimulating factor in mice. Andrologia. 2011;43:87–93. doi: 10.1111/j.1439-0272.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- 27.Rennert RC, Sorkin M, Garg RK, Gurtner GC. Stem cell recruitment after injury: Lessons for regenerative medicine. Regen Med. 2012;7:833–50. doi: 10.2217/rme.12.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin P, Wang E, Ren J, Childs R, Shin JW, Khuu H, et al. Differentiation of two types of mobilized peripheral blood stem cells by microRNA and cDNA expression analysis. J Transl Med. 2008;6:39. doi: 10.1186/1479-5876-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadek EM, Salama NM, Ismail DI, Elshafei AA. Histological study on the protective effect of endogenous stem-cell mobilization in Adriamycin-induced chronic nephropathy in rats. J Microsc Ultrastruct. 2016;4:133–42. doi: 10.1016/j.jmau.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lévesque JP, Winkler IG, Larsen SR, Rasko JE. Bone Marrow-Derived Progenitors. Handbook of Experimental Pharmacology. In: Kauser K, Zeiher AM, editors. Mobilization of bone marrow-derived progenitors. New York: Springer, Berlin, Heidelberg; 2007. p. 180. [DOI] [PubMed] [Google Scholar]
- 31.Su J, Zhou H, Tao Y, Guo J, Guo Z, Zhang S, et al. G-CSF protects human brain vascular endothelial cells injury induced by high glucose, free fatty acids and hypoxia through MAPK and Akt signaling. PLoS One. 2015;10:e0120707. doi: 10.1371/journal.pone.0120707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alison MR, Brittan M, Lovell MJ, Wright NA. Markers of adult tissue-based stem cells. Handb Exp Pharmacol. 2006;174:185–227. [PubMed] [Google Scholar]
- 33.Devine H, Tierney K, Schmit-Pokorny K, McDermott K. Mobilization of hematopoietic stem cells for use in autologous transplantation. Clin J Oncol Nurs. 2010;14:212–22. doi: 10.1188/10.CJON.212-222. [DOI] [PubMed] [Google Scholar]