Abstract
Background & objectives:
The effect of vitamin D supplementation on response to antiviral therapy in hepatitis C virus (HCV) genotype 1 and 4 infection still remains unclear, with studies yielding inconsistent results. The aim of the present study was to assess the effect of vitamin D supplementation on treatment outcome in patients with genotype 1/4 chronic hepatitis C (CHC) infection.
Methods:
Sixty consecutive, treatment-naïve, genotype 1 and 4 chronic HCV patients were included in the study. The patients were randomized into two groups: Vitamin D supplemented group received pegylated (PEG)-interferon α-2a 180 μg per week plus ribavirin (RBV) (1000-1200 mg/d) together with vitamin D3 (2000 IU/d) and control group received identical therapy without vitamin D (32 patients).
Results:
There were no significant differences between the two groups in terms of age, sex, body mass index and baseline laboratory values. Lower vitamin D levels were associated with higher grades of fibrosis in liver histology (vitamin D >20 ng/ml - 70% vs vitamin D <20 ng/ml - 37%, P<0.05). Vitamin D supplemented group had similar rapid viral response (40 vs 28%, P=0.36), complete early viral response (53.2 vs 40%, P=0.34), end of treatment response (64 vs 46%, P=0.17) and sustained virological response (SVR) (60 vs 44%, P=0.19) as compared to control group. Interleukin 28B polymorphism [odds ratio (OR)-15.37, 95% confidence interval (CI)-2.32-101.76, P=0.04] and baseline serum vitamin D levels (OR-6.36, 95% CI-1.36-29.61 P=0.02) were independent predictors of SVR in genotype 1/4 CHC. Vitamin D supplementation was not found to be predictor of response in genotype 1/4 CHC on multivariate analysis (OR-2.79, 95% CI- 0.63-12.34, P=0.74).
Interpretation & conclusions:
The present study showed that addition of vitamin D to PEG/RBV combination therapy in treatment-naïve patients who were infected with HCV genotype 1/4 had no effect on the rates of rapid, early and sustained viral responses.
Keywords: Genotype, hepatitis C interleukin 28B polymorphism, liver fibrosis, sustained virological response, vitamin
Chronic hepatitis C (CHC) owes a serious threat to global health by affecting 170 million people worldwide1. It often causes severe liver inflammation and fibrosis, bearing an increased risk of liver cirrhosis and hepatocellular carcinoma (HCC). Hepatitis C virus (HCV) accounts for 28 per cent cases of cirrhosis and 26 per cent cases of liver cancer globally2. Approximately 12-18 million people are infected with HCV in India, a significant share of the global HCV prevalence3. In the northern part of India, HCV accounts for 20 per cent of the total chronic hepatitis patients4.
Combination therapy with pegylated interferon (PEG-IFN) and ribavirin (RBV) is the current standard of care in India5. The aim of the therapy is sustained virological response (SVR), defined as undetectable HCV RNA level 24 wk after cessation of therapy. However, the treatment with PEG-IFN + RBV produces SVR in only about 40 to 60 per cent of patients with HCV genotype 1 and 46. Efforts to improve treatment outcomes have focused on improving host-related factors and adding new antiviral therapies specifically targeted to HCV.
Serum vitamin D concentration appears to be a host-related modifiable factor predicting response to antiviral therapy in CHC. Vitamin D has also been shown to have immunomodulatory and antiproliferative actions based on several recent reports7,8. Vitamin D improves insulin sensitivity and has a favourable effect on inflammatory cytokine profile by suppressing pro-inflammatory cytokines, increasing anti-inflammatory cytokines and improving CD4 T-cell hyperresponsiveness9,10,11. It has been also reported that vitamin D deficiency is common (92%) among patients with chronic liver disease12,13. Serum 25-OH vitamin D concentration was an independent predictor of SVR in patients treated with PEG-IFN plus RBV14.
Addition of vitamin D to standard PEG-IFN/RBV therapy has been found in a study from Israel to significantly improve the viral response in genotype 1/4 treatment-naïve chronic HCV patients9. The aim of the present study was to assess prospectively the influence of vitamin D supplementation on treatment outcome in patients with genotype 1/4 CHC infection.
Material & Methods
This was a prospective, randomized, open label study conducted on 60 consecutive patients with chronic HCV genotype 1/4 infection attending department of Gastroenterology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, from September 1, 2012 to January 31, 2015. Study inclusion criteria were age 18-65 yr, chronic HCV genotype 1/4 infection with detectable HCV RNA for six months and no previous treatment for hepatitis C.
Patients with advanced cirrhosis (Child-Pugh B or C), presence of HCC, human immunodeficiency and hepatitis B co-infection, autoimmune liver disease, Wilson disease, haemochromatosis, α1-antitrypsin deficiency, concomitant use of medications known to affect serum vitamin D metabolism and patients with active intravenous drug addiction were excluded from the study.
Study design: The study was approved by the Institutional Review Board. Informed written consent was obtained from all the participants. The computer-generated block randomization schedule was prepared using random number generator to create a list of random numbers. Stat Trek programme (https://stattrek.com) was used to derive the randomization list. The patients were randomized into two groups: Vitamin D supplemented group received PEG-IFN/RBV + vitamin D3 (28 patients), and control group received only PEG-IFN/RBV therapy (32 patients) (Figure). Patients received PEG-IFN alfa-2a (Exxura, Roche, Switzerland) at a dosage of 180 μg per week, and RBV was administered according to body weight (1000 mg/day for patients weighing <75 kg, 1200 mg/day for patients weighing >75 kg) for 48 wk. Vitamin D3 was given at a dose of 2000 IU/day from day 0 and continued whole course of treatment duration in vitamin D supplementation group only. The study was registered in clinical trial registry in India (CTRI number CTRI/2015/07/005992).
Figure.
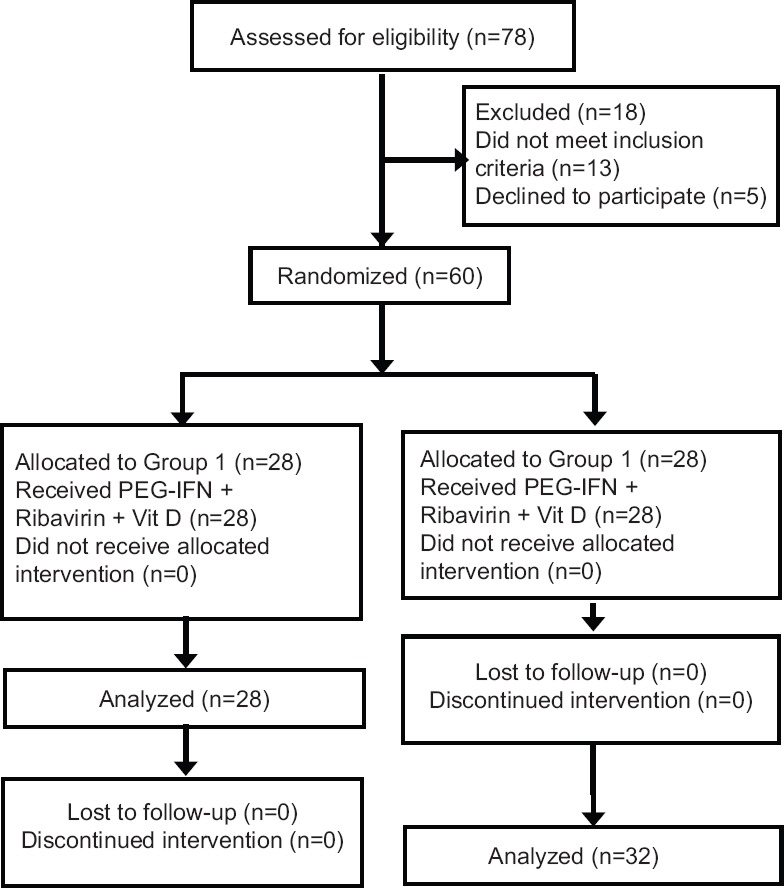
Flow chart showing the study design. PEG-IFN, pegylated interferon alpha.
Laboratory measurement: An overnight fasting blood sample (10 ml) was drawn to determine baseline blood tests, including HCV RNA quantification using real-time polymerase chain reaction (PCR) (COBAS TaqMan, Roche) and HCV genotype using the Inno-Lipa genotyping kit (Innogenetics, Zwijndrecht, Belgium).
Vitamin D assay and homeostasis model assessment (HOMA) score: Serum samples of all the patients included in the study, were collected and stored at −80°C until used. These samples were subjected to measure pre-treatment serum 25OH vitamin D levels using a chemiluminescent immunoassay (Architect, Abbott, USA). The data were expressed as nanograms per millilitre. The same serum samples were also used to measure glucose and insulin levels. The insulin level was determined using an electrochemiluminescence immunoassay (Immulite 1000, Siemens, Gwynedd, UK). The HOMA score was calculated as described15 using fasting glucose and insulin levels.
Molecular biology: Genotyping for the interleukin (IL) 28B rs12979860 C/T was done by PCR-based restriction fragment length polymorphism (RFLP) assay. The genomic DNA was isolated from whole blood by using commercial kit (Roche Diagnostics GmbH, Mannheim) as per the manufacturer's instruction. The isolated DNA was electrophoresed on one per cent agarose gel for quality check and also quantified using nanodrop for purity. The PCR was carried out using the following primer pairs: 5’-GCGGAAGGAGCAGTTGCGCT-3’ and 5’-GGGGCTTTGCTGGGGGAGTG-3’. (Taqman probes, Thermo Fisher Scientific, USA) Each reaction included 100-150 ng genomic DNA as template in a total volume 25 μl having 1x PCR reaction buffer. Forward and reverse primers were added to a final concentration of 10 pmol/reaction. Taq DNA polymerase (Fermentas, Thermo Fisher Scientific, USA) was used for DNA amplification with 125 μmol each of deoxynucleotide triphosphates. The reaction mixture was subjected to PCR with the following condtions: initial denaturation at 94°C for five minutes, followed by 35 cycles denaturation at 94°C for 30 sec, annealing at 65°C for 30 sec and elongation at 72°C for 30 sec, and lastly final elongation at 72°C for five minutes by using veriti 96-well thermal cycler (Applied Biosystem, Life technologies, USA). For RFLP analysis, 10 μl of amplified product was digested with 5 U of Bst U1 restriction endonuclease (New England Biolabs, UK) in a total volume of 20 μl at 60°C for overnight. The restriction digested DNA was electrophoresed on 3 per cent agarose gel along with 100 bp ladder and visualized by gel doc unit (BioRad, USA). The digested fragments were 196 and 45 bp for CC genotype, 241, 196 and 45 bp for CT and 241 bp for TT genotype.
Histology: Thirty six of the 60 patients (60%) underwent a liver biopsy before starting therapy. The biopsy specimens were fixed in 10 per cent formalin and stained with haematoxylin and eosin and reticulin stains. Slides were coded and read by one pathologist blinded to patient's identity. Minimum length of 15 mm of biopsy specimen or presence of at least 10 portal tracts was required. Stage of fibrosis was scored according to the metavir scoring system16. Fibroscan was not done in our study.
Efficacy assessment: Rapid viral response (RVR) was defined as an undetectable serum HCV RNA level (<50 IU/ml) four weeks after starting therapy. Complete early viral response (cEVR) was defined as an undetectable serum HCV RNA level 12 wk after starting therapy. The end of treatment response (EOT) was defined as an undetectable serum HCV RNA level after completing the treatment schedule. Sustained viral response (SVR) was defined as an undetectable serum HCV RNA level at 24 wk after stopping antiviral therapy17.
Follow up of patients and safety assessment: Clinical evaluation of patients and complete blood count was performed weekly for the first four weeks of therapy and monthly thereafter. Quantitative HCV RNA and serum alanine transaminase (ALT) levels were checked at 4, 12, 48 and 72 wk after the start of antiviral therapy. An autoimmune profile and thyroid function tests were checked every three months. Serum vitamin D level was measured at the end of the therapy. PEG-IFN alpha 2a was reduced stepwise from 180 to 135, 90 μg/dl in patients with an absolute neutrophil count <750/μl and withdrawn temporarily in patients with an absolute neutrophil count <500/μl. Treatment was suspended until absolute neutrophil count (ANC) values returned to more than 1000 cells/μl. The same dose reduction was recommended if platelet levels fell under 50,000 cells/μl, with PEG-IFN being discontinued when the 25,000 cells/μl threshold was reached. RBV dose was tapered by 200 mg/day in patients with a haemoglobin level <10 g/dl and discontinued altogether in patients with a Hb level <8.0 g/dl in both treatment groups.
Compliance was assessed at each follow up and patients were counselled to take the allocated treatments. The patients were asked about the number of prescribed doses of medication taken by them during the last 90 days. Good compliance was defined as the completion of 80 per cent of the prescribed treatment doses18. Three patients in the vitamin D + PEG-IFN/RBV group and four in control group had platelet count under 50,000 cells/μl for which dose was reduced to 135 and 90 μg/dl. Another patient in vitamin D + PEG IFN/RBV group and three in control group had ANC <1500 cells/μl requiring dose reduction. None of the patients had stopped treatment due to adverse events.
Statistical analysis: Statistical analysis of the data was performed using the SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were presented as the mean±standard deviation, and categorical variables were presented as frequencies (%). The associations between categorical variables were evaluated using Chi-square test, and Student's t test was used for continuous variables. Wilcoxon's rank sum test was applied for non-parametric tests. Stepwise logistic regression analysis with a forward approach was performed to identify independent predictors of SVR.
Results
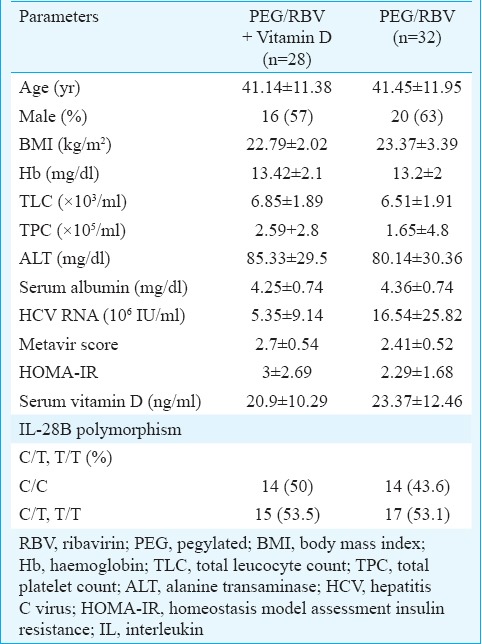
Of the 60 chronic HCV patients, 55 were infected with genotype 1 and five patients with genotype 4. There were no significant differences between the vitamin D + PEG IFN/RBV group and control groups in terms of age, sex, body mass index and baseline laboratory values (Table I). There were no differences in HCV RNA, stage of liver fibrosis, insulin resistance as measured by homeostasis model assessment insulin resistance and baseline vitamin D levels in both groups. In our study, 25 per cent of vitamin D + PEG-IFN/RBV group and 28 per cent of control group had severe vitamin D deficiency (vitamin D level <13 ng/ml). In the vitamin D + PEG-IFN/RBV group, serum vitamin D levels were significantly lower at baseline (20.9±10.29 ng/ml) and increased after 48 wk of vitamin D treatment to a mean level of 51.25±26.54 ng/ml (P<0.01). The SVR rate was not significantly different between patients with HCV genotype 1/4 in the vitamin D + PEG IFN/RBV group versus those in the control group (48.6/35% vs 50/33%).
Table I.
Baseline demographic, biochemical and virological characteristics of patients
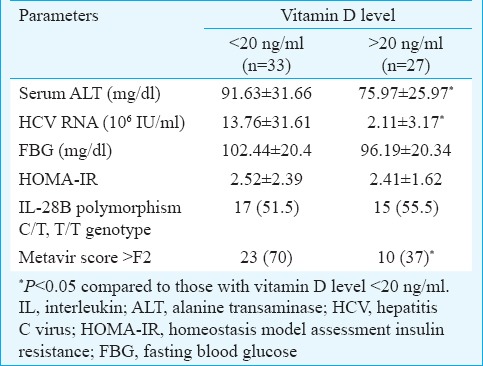
Relationship of serum vitamin D levels with clinical variables: Table II displays the possible associations between several clinical variables and serum vitamin D concentration. Serum ALT and HCV RNA levels were significantly lower in patients with baseline vitamin D level ≥20 ng/ml as compared to those with vitamin D <20 ng/ml (P<0.05). No significant association was found between vitamin D levels and IL 28B polymorphism C/T genotype. It was found that lower vitamin D levels were associated with higher grades of fibrosis in liver histology (vitamin D >20 ng/ml-70% vs. vitamin D <20 ng/ml - 37%, P<0.05).
Table II.
Relationship between baseline clinical variables and baseline serum 25-hydroxyvitamin D level
Efficacy of vitamin D supplementation: Vitamin D was supplemented in 28 of the 60 individuals. (Figure) Those who have been supplemented with vitamin D had similar RVR (40 vs 28%, P=0.36), cEVR (53.2 vs 40%, P=0.34), EOT (64 vs 46%, P=0.17) and SVR (60 vs. 44%, P=0.19) as compared with individuals, who were not supplemented with vitamin D.
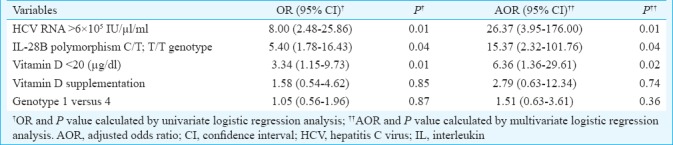
Predictors of viral response: Multivariate analysis was performed using logistic regression (Table III) which identified baseline HCV RNA [odds ratio (OR)-26.37, 95% confidence interval (CI)- 0.3.95-176, P=0.01], IL 28B polymorphism (OR-15.37, 95% CI-2.32-101.76, P=0.04) and baseline serum vitamin D levels (OR-6.36, 95% CI-1.36-29.61, P=0.02) as independent predictors of SVR in genotype 1-4 CHC. Vitamin D supplementation was not found to be predictor of response in genotype 1 and 4 CHC on multivariate analysis (OR-2.79, 95% CI-0.63-12.34, P=0.74). There was no difference in SVR rates between patients with HCV genotype 1 and 4 infection (OR-1.51, 95% CI -0.63-3.61, P=0.36).
Table III.
Logistic regression analysis for predicting absence of sustained viral response in genotypes 1/4 treatment-naïve chronic hepatitis C virus-infected patients
Discussion
Our study showed that lower vitamin D levels were associated with higher grades of fibrosis in liver histology, those individuals who were supplemented with vitamin D had similar RVR, cEVR, EOT and SVR as compared with individuals not supplemented and vitamin D supplementation was not found to be a significant predictor of response in genotype 1 and 4 CHC.
Vitamin D deficiency was found to be common in patients of CHC with unclear reasons12,19. In this study, vitamin D levels were found to influence the attainment of viral clearance after antiviral therapy in patients with genotype 1 and 4 chronic HCV infection. In particular, vitamin D deficiency was found to be a negative predictor of viral clearance in multivariate analysis. Many studies have shown a possible association between vitamin D status and outcome of antiviral therapy in patients with chronic HCV viral infection. Petta et al14 retrospectively analyzed 167 patients treated with PEG/RBV for hepatitis C and found that low vitamin D level was a significant negative predictor of viral clearance. Another prospective study by Bitetto et al20 reported a significant positive association between baseline serum vitamin D levels and the rates of viral clearance in genotype 1-infected HCV patients. Vitamin D possibly plays an important role in early HCV decline after antiviral treatment.
In the present study, majority of patients had advanced fibrosis in histology (metavir stage>F2). Patients with vitamin D deficiency (vitamin D <20 ng/ml) had moderate to advanced fibrosis. Petta et al14 reported an inverse relationship between vitamin D level and staging of liver fibrosis. Vitamin D deficiency was more prevalent in those with high histologic activity, but no association was found between vitamin D level and viral clearance in multivariate analysis, as reported by Kitson et al21. However, Bitetto et al20 found no significant association between vitamin D level and fibrosis staging.
The results of our study suggested that the addition of vitamin D to current standard therapy had no significant effect on the rate of RVR, cEVR, EOT and SVR in patients with HCV genotype 1 and 4 as compared to standard therapy alone. However, the rate of SVR and EOT was higher in vitamin D + PEG-IFN/RBV group than the control group, but it was not significant. Esmat et al22 in a prospective randomized study did not find a significant impact of vitamin D supplementation on SVR in genotype 4 patients. Ladero et al23 in a study from Spain found that vitamin D had no immediate effect on HCV RNA levels. Contrary to our observation, Abu-Mouch et al9 found that adding vitamin D to standard therapy significantly improved the viral response in treatment-naïve patients with chronic HCV genotype 1 infection. Another randomized controlled study by Yokoyama et al24 reported similar rate of SVR in vitamin D and control groups. However, vitamin D supplementation significantly contributed to viral clearance at 24 wk in multivariate analysis suggesting a possible effect of vitamin D in enhancing the effect of PEG-IFN/RBV in HCV genotype 1 b-infected patients24, in contrary to our results.
IL 28B rs12979860 C/T polymorphism has been found to be a powerful predictor of viral clearance to IFN-based HCV therapy25. Combination of low vitamin D levels and unfavourable genotype of IL 28B genotype has the lowest chance of attaining viral clearance26. We did not find any association between vitamin D levels and IL 28B rs12979860 C/T polymorphism in the current study. The IL 28B rs12979860 C/T polymorphism played an important role in predicting the viral clearance independent of the other predictors of SVR achievement. Bitteto et al20 also confirmed the role of IL 28B rs12979860 C/T polymorphism as an independent predictor of SVR. In addition, the rs12979860 C/C genotype was found to be the strongest predictor of SVR, independent of the other accepted predictors of SVR attainment in multiple studies26,27.
The lack of response to vitamin D supplementation may be attributable to several factors. First, most of the patients in our study had higher stages of fibrosis as compared to other published studies. Second, vitamin D was supplemented four weeks before PEG-IFN/RBV therapy in other studies and continued till the end of therapy, but we started administration of vitamin D from week 09. The main limitation of our study was the small patient number, which reduced the impact of significance tests. Another limitation of the study was lack of data on vitamin D receptor polymorphism.
In conclusion, our results showed a significant association between serum vitamin D levels and stage of fibrosis; a higher stage of fibrosis was seen in patients with vitamin D deficiency. Addition of vitamin D to PEG/RBV combination therapy in HCV genotype 1and 4 treatment-naïve patients had no effect on rate of sustained viral response.
Acknowledgment:
Authors thank the patients for their consent and cooperation to conduct this study.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Chung RT, Baumert TF. Curing chronic hepatitis C - The arc of a medical triumph. N Engl J Med. 2014;370:1576–8. doi: 10.1056/NEJMp1400986. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhiman RK. Future of therapy for hepatitis C in India: A matter of accessibility and affordability? J Clin Exp Hepatol. 2014;4:85–6. doi: 10.1016/j.jceh.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixit VK, Ghosh JK, Lamtha SC, Kaushik P, Goyal SK, Behera MK, et al. Clinical profile and response to treatment with pegylated interferon α 2b and ribavirin in chronic hepatitis C - A reappraisal from a tertiary care center in Northern India. J Clin Exp Hepatol. 2014;4:101–5. doi: 10.1016/j.jceh.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadigan C, Kottilil S. Hepatitis C virus infection and coinfection with human immunodeficiency virus: Challenges and advancements in management. JAMA. 2011;306:294–301. doi: 10.1001/jama.2011.975. [DOI] [PubMed] [Google Scholar]
- 6.Jiménez-Sousa MA, Fernández-Rodríguez A, Guzmán-Fulgencio M, García-Álvarez M, Resino S. Meta-analysis: Implications of interleukin-28B polymorphisms in spontaneous and treatment-related clearance for patients with hepatitis C. BMC Med. 2013;11:6. doi: 10.1186/1741-7015-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLuca HF. Overview of general physiologic features and functions of Vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 8.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Mouch S, Fireman Z, Jarchovsky J, Zeina AR, Assy N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J Gastroenterol. 2011;17:5184–90. doi: 10.3748/wjg.v17.i47.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez JA, Ashraf A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010;2010:351385. doi: 10.1155/2010/351385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–32. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 12.Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55:2624–8. doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 13.Nimer A, Mouch A. Vitamin D improves viral response in hepatitis C genotype 2-3 naïve patients. World J Gastroenterol. 2012;18:800–5. doi: 10.3748/wjg.v18.i8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petta S, Cammà C, Scazzone C, Tripodo C, Di Marco V, Bono A, et al. Low Vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology. 2010;51:1158–67. doi: 10.1002/hep.23489. [DOI] [PubMed] [Google Scholar]
- 15.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 16.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J Hepatol. 2011;55:245–64. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 18.McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–9. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 19.Fisher L, Fisher A. Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin Gastroenterol Hepatol. 2007;5:513–20. doi: 10.1016/j.cgh.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Bitetto D, Fattovich G, Fabris C, Ceriani E, Falleti E, Fornasiere E, et al. Complementary role of vitamin D deficiency and the interleukin-28B rs12979860 C/T polymorphism in predicting antiviral response in chronic hepatitis C. Hepatology. 2011;53:1118–26. doi: 10.1002/hep.24201. [DOI] [PubMed] [Google Scholar]
- 21.Kitson MT, Dore GJ, George J, Button P, McCaughan GW, Crawford DH, et al. Vitamin D status does not predict sustained virologic response or fibrosis stage in chronic hepatitis C genotype 1 infection. J Hepatol. 2013;58:467–72. doi: 10.1016/j.jhep.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Esmat G, El Raziky M, Elsharkawy A, Sabry D, Hassany M, Ahmed A, et al. Impact of vitamin D supplementation on sustained virological response in chronic hepatitis C genotype 4 patients treated by pegylated interferon/ribavirin. J Interferon Cytokine Res. 2015;35:49–54. doi: 10.1089/jir.2014.0060. [DOI] [PubMed] [Google Scholar]
- 23.Ladero JM, Torrejón MJ, Sánchez-Pobre P, Suárez A, Cuenca F, de la Orden V, et al. Vitamin D deficiency and vitamin D therapy in chronic hepatitis C. Ann Hepatol. 2013;12:199–204. [PubMed] [Google Scholar]
- 24.Yokoyama S, Takahashi S, Kawakami Y, Hayes CN, Kohno H, Kohno H, et al. Effect of vitamin D supplementation on pegylated interferon/ribavirin therapy for chronic hepatitis C genotype 1b: A randomized controlled trial. J Viral Hepat. 2014;21:348–56. doi: 10.1111/jvh.12146. [DOI] [PubMed] [Google Scholar]
- 25.Ladero JM, Martin EG, Fernández C, Carballo M, Devesa MJ, Martínez C, et al. Predicting response to therapy in chronic hepatitis C: An approach combining interleukin-28B gene polymorphisms and clinical data. J Gastroenterol Hepatol. 2012;27:279–85. doi: 10.1111/j.1440-1746.2011.06834.x. [DOI] [PubMed] [Google Scholar]
- 26.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 27.Tillmann HL, Thompson AJ, Patel K, Wiese M, Tenckhoff H, Nischalke HD, et al. A polymorphism near IL28B is associated with spontaneous clearance of acute hepatitis C virus and jaundice. Gastroenterology. 2010;139:1586–92. doi: 10.1053/j.gastro.2010.07.005. 1592.e1. [DOI] [PubMed] [Google Scholar]


