Abstract
Background
Parathyroid hormone (PTH) is required for the maintenance of normal bone physiology. This study describes the properties of a sustained-release formulation of recombinant human PTH (rhPTH) using chitosan and silk fibroin microparticles as carriers for drug delivery, developed using a spray-drying method.
Material/Methods
Chitosan, silk fibroin, and chitosan/silk fibroin microparticles loaded with rhPTH were studied with scanning electron microscopy (SEM) to estimate the particle size and surface morphology. The in vitro release of rhPTH was used to assess the developed formulation. The effect of the spray-drying process was assessed by powder X-ray diffraction (PXRD) of the microparticles. Quantification of the released rhPTH was performed by enzyme-linked immune sorbent assay (ELISA). Fourier-transform infrared spectroscopy (FTIR) was used to determine the differences in the absorption frequency of samples.
Results
Surface morphology of the final formulation showed the absence of pure crystals of chitosan and silk fibroin in the final formulation and FTIR demonstrated electrostatic interactions between chitosan and silk fibroin, which was supported by PXRD. The chitosan/silk fibroin microparticles loaded with rhPTH showed an entrapment efficiency (EE) that ranged from 60.36–72.99% with a 50% rhPTH release profile at pH 7.5 in 24 hours. There was no particle aggregation in blood and little hemolysis, indicating stability of the rhPTH-loaded microparticles.
Conclusions
A silk fibroin/chitosan microparticle formulation loaded with rhPTH was shown to be stable and to provide sustained-release of rhPTH, supporting a potential role of this formulation in the treatment of bone diseases including osteoporosis and bone fracture.
MeSH Keywords: Chitosan, Parathyroid Hormone, Recombinant Proteins
Background
Bone contains large amounts of mineral, including hydroxyapatite and calcium, keratin sulfate, chondroitin sulfate, collagen, lipids, and water [1]. The proportions of the components of bone depend on the several factors, including age, gender, site and the presence of disease, particularly metabolic disease, osteoporosis, and bone trauma, which can lead to bone loss or bone fracture [2].
Recombinant human parathyroid hormone (rhPTH) is a genetically engineered peptide (34 amino acids), approved by the US Food and Drug Administration (FDA) as teriparatide (Forteo or PTH 1–34) for the treatment of bone diseases, which increases bone turnover by stimulating osteoblasts [2]. The sequence and structure of rhPTH are similar to the naturally secreted human parathyroid hormone (PTH). However, the main problem with rhPTH therapy is the long duration of treatment (up to two years), rapid drug clearance, and short half-life, which can be associated with lack of treatment compliance by patients [3]. The half-life of teriparatide rhPTH in serum is five minutes when administered intravenously, and approximately one hour when administered by subcutaneous injection [3]. There have been some studies conducted to develop improved delivery systems for the rhPTH formulation, including dry powder inhalation [4], and the use of chitosan nanoparticles [5]. However, there still remains a need to develop an effective and noninvasive delivery system with the sustained release of rhPTH to improve patient treatment compliance.
In recent decades, the use of natural polymers in the treatment of bone defects has been increasing due to their availability and advantages, which include versatility, biodegradability, and biocompatibility. Among the most commonly used polymers for the treatment of bone defects are hyaluronic acid [6,7], alginates [8], chitosan [9], polylactic acid [10], polycyanoacrylate [11,12], collagen [13], silk fibroin [14], and polycaprolactone [15].
The natural polymers selected for the delivery of rhPTH-loaded microparticles are chitosan, an amino polysaccharide and deacetylated form of chitin, and silk fibroin. Chitosan is a naturally occurring linear biodegradable polysaccharide, containing N-acetyl-D-glucosamine and D-glucosamine moieties in its chemical structure. Recently, chitosan has been extensively studied as a potential material for the delivery of therapeutic biomolecules, proteins, and gene vectors [16,17]. At neutral pH, protein and genes carry negative charge undergo electrostatic interactions with large numbers of -NH2 groups at the deacetylated backbone of glucosamine units in chitosan [18,19].
Silk fibroin is a fibrous protein obtained from the domesticated silkworm, Bombyx mori, contains glycine and alanine as the main amino acid residues, and is widely used as a suture material. Several studies have been undertaken on silk fibroin to determine its mechanical and structural properties, biocompatibility, and biodegradable nature. Silk fibroin is formed from β-sheet structures resulting from protein self-assembly, which leads to stability and the improved mechanical properties. Silk fibroin exists in three molecular conformations, Silk I, Silk II, Silk III, which are soluble in water and are characterized by a mixture of random coil structures, the α-helix, and β-turn. The predominance of β-sheets leads to a stable and water-insoluble fibroin characterized by α-helix. Tetracycline-loaded silk fibroin/chitosan microparticles have previously been formulated using a spray-drying method to prolong the release of tetracycline [20,21]. In 2009, Yang et al. investigated silk fibroin and silk fibroin/chitosan microparticles as cardiac patches and their effects on rat mesenchymal stem cells in vitro [21].
Given the findings of these previous studies, the primary aim of this study was to prepare a sustained release rhPTH-loaded chitosan and silk fibroin microparticles for the treatment of bone disease. The developed delivery system, physical properties of the microparticles, including in vitro rhPTH drug release, compatibility with the presence in blood, and drug stability studies were also assessed.
Material and Methods
Materials
Chitosan, 80% deacetylated (100–150 KDa) was purchased from Shin Dar Biotechnology Company (Taipei, China). Recombinant human parathyroid hormone (PTH) (rhPTH) pellets (4.1 KDa) were obtained from Shanghai Celgen Pharmaceutical Co. Ltd. Silkworm cocoons were kindly supplied by the Department of Engineering Mechanics, Tsinghua University, China, to allow for the extraction of silk fibroin.
Study design
To examine the potential of chitosan and silk fibroin microparticles, particles were characterized and evaluated in vitro. Particle size estimation and surface morphology characterization were performed using scanning electron microscopy (SEM). The in vitro release of rhPTH, was used to assess the release pattern of the developed formulation. Compatibility of the formulation in the blood and stability studies of the microparticles in blood were also performed.
Silk fibroin extraction
Fibroin was extracted according to the methods previously described in the literature, with some modifications [22]. Briefly, obtained silk fibroin was boiled and then minced into small pieces followed by boiling in 0.2% Na2CO3 solution for 30 min. The resulting silk fibroin mesh was washed with distilled water multiple times and dried for next 12 hours, and then dissolved in lithium bromide (LiBr) 9M at 60°C for three hours. The mixture was dialyzed using a dialysis membrane with a 12 kDa molecular weight-cutoff against phosphate buffer 10 mM (pH 7.0) for 24 hours, with four replacements with fresh buffer. The final concentration of the resulting silk fibroin solution was adjusted to 5% with phosphate buffer and stored at 4°C until further use.
Microsphere preparation by spray-drying
To prepare the microparticles, chitosan solution (1% w/v) in acetic acid, small amounts of polyethylene glycol (<0.01%), and increasing concentrations of sodium tripolyphosphate were added to the silk fibroin solution (2.5%, w/v), followed by mixing for one hour to obtain particles used for the spray-drying technique. The developed microparticles were then immersed in 95% alcohol for 24 hours and then stored until further use. Table 1 shows the optimized process parameters for the manufacture of the chitosan and silk fibroin microparticles.
Table 1.
Spray drying process optimized parameters for the preparation of chitosan-silk fibroin microparticles of recombinant human parathyroid hormone peptide.
| Parameters | Values |
|---|---|
| Flow rate | 40 ml/min |
| Nozzle size | 0.7 mm |
| Atomization | 40 kPa |
| Airflow rate | 0.65 m3/min |
| Inlet temperature | 120°C |
| Outlet temperature | 100°C |
Scanning electron microscopy (SEM) of the surface morphology of the microparticles
Images of the scanning electron microscopy (SEM) of the microparticles were taken to determine their size and surface morphology. Before the SEM examination, the samples were placed onto adhesive tape and adhered to an aluminum disc. To enhance the conductivity, a thin layer of gold coating was applied by spraying and the images were observed in a 10 Torr vacuum by the scanning electron microscope (JEOL, JFC-1100 fine coat ion sputter, Tokyo, Japan). An electron beam with an acceleration potential of 1.2 kV was used to scan the specimens, and the images were collected using the secondary electron mode.
Powder X-ray diffraction (PXRD) of the microparticles
To evaluate the effect of the spray-drying process, microparticles were subjected to powder X-ray diffraction (PXRD) (D8 Advance, Bruker AXS GmbH, Karlsruhe, Germany) using Cu(Kα) radiation (λ=0.1542 nm) to obtain the diffraction patterns of microparticles at room temperature on 2θ scale from 2–50° in continuous mode.
Determination of the entrapment efficiency (EE) and in vitro release studies
Following generation of the microparticle formulation, non-entrapped rhPTH was separated from the particles by ultra-centrifugation at 10,000 rpm at 4°C for 30 minutes. To determine the entrapment efficiency of rhPTH within the particles, the supernatant was collected. A rhPTH enzyme-linked immunosorbent assay (ELISA) kit was employed to quantify the peptide. The entrapment efficiency percentage was defined as the ratio of the amount of drug present in the particles to the amount of drug used in the loading process, according to the equation given below:
| (1) |
To examine the release profile of rhPTH from the chitosan/silk fibroin particles, in vitro release studies were performed at pH 7.4 for a 96-hour timespan. Then, the microparticles in suspension were centrifuged at 20,000 rpm for 45 min and at a final concentration of 2×10−3 mg/mL, and the obtained pellet was redispersed in 10 mL of phosphate-buffered saline solution (pH 7.4). The total volume was divided equally into the required number of Eppendorf tubes for a time-dependant release study at the following time points: 0, 0.5, 1, 3, 6, 9, 12, 24, 48, 72, and 96 hours. The Eppendorf tubes were incubated at 37°C with gentle shaking. At the set time points, the Eppendorf tube contents were centrifuged and the rhPTH released from the rhPTH and chitosan/silk fibroin particles were quantified using the ELISA kit. The percentage of peptide released was calculated based on the ratio of the amount of peptide released at a given time to the amount loaded in the particles, which followed the equation given below:
| (2) |
Where rhPTH-t is the amount of rhPTH released at the time ‘t’ and rhPTH-L is the amount of rhPTH loaded in the microparticle. At the end of the studies, a cumulative percentage of the rhPTH released profile versus each time point was plotted.
Blood compatibility studies
The chitosan and silk fibroin microparticles underwent blood compatibility using a hemolysis assay. Briefly, freshly taken, anticoagulant-treated (acid citrate dextrose) human blood was used. To detect the color change in the plasma due to hemolysis, the chitosan and silk fibroin particles (100 μL) were added to 1 mL blood sample then kept at 37°C in an incubator shaker for 2 h with continuous shaking. To obtain the plasma, the samples were spun down at 5,000 rpm for 20 min at 10°C, and the color of the plasma was observed to determine whether hemolysis had taken place. Then, 100 μL of the plasma was added to 1 mL of 0.01% Na2CO3 and the absorbance was measured at 380, 415, and 450 nm. The plasma hemoglobin can be determined according to the following equation:
| (3) |
where A415, A380, and A480 were the absorbances at respective wavelengths. The obtained sample values were compared with that of the control (blood + saline) [23].
Once the plasma hemoglobin content was estimated the percentage of hemolysis was calculated using the following formula:
| (4) |
Stability of the chitosan and silk fibroin microparticles in the blood
Stability studies of the chitosan and silk fibroin microparticles in the plasma were also performed. Freshly taken blood samples were taken in acid citrate dextrose (anticoagulant), followed by centrifugation at 5,000 rpm for 20 min at 10°C. The equal volumes of chitosan and silk fibroin microparticles (0.1 mg/mL chitosan concentration) were mixed with 1 mL of plasma in an incubator shaker at 37°C, at 150 rpm for 2 h with continuous stirring. Then, centrifugation at 5,000 rpm for 20 min at 10°C was performed to obtain microparticles that could be examined by SEM.
Statistical analysis
One-way analysis of variance (ANOVA) and the Student-Newman-Keuls multiple comparisons test were performed to obtain the significant difference (p<0.05). All experiments were performed in triplicate and presented as the mean ± standard deviation (SD).
Results
Functional group estimation
Fourier-transform infrared spectroscopy (FTIR) was used to determine the differences in the absorption frequency of samples of silk fibroin and chitosan and these formulations are shown in Figure 1. Various peaks of silk fibroin (Figure 1(A)) were seen at 1627 cm−1, 1516 cm−1, and 1239 cm−1, which confirmed the presence of a -CONH2 bond in its structure. However, a slight change in the absorption frequency of silk fibroin at 1655 cm−1, 1530 cm−1, and 1237 cm−1 was observed (Figure 1(B)). Figure 1(B) shows the characteristic peaks of chitosan at 1662 cm−1, 1605 cm−1, and 1393 cm−1, which corresponds to amide I, -NH2 bending and amide III, respectively. Based on these results, it could be concluded that some weak physical interactions between silk fibroin and chitosan occurred following the spray-drying process.
Figure 1.
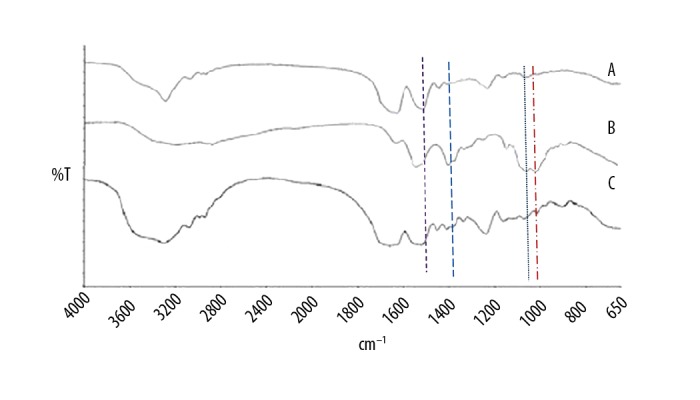
The Fourier-transform infrared spectroscopy (FTIR) spectra of silk fibroin (SF), chitosan (CHT), and chitosan/silk fibroin (CHT/SF) microparticles loaded with recombinant human parathyroid hormone (rhPTH) as a formulation. A) Fourier-transform infrared spectroscopy (FTIR) of silk fibroin (SF). B) FTIR of chitosan (CHT). C) FTIR of silk fibroin/chitosan (SF/CHT) microparticles loaded with recombinant human parathyroid hormone (rhPTH) as a formulation. The experiments were carried out in three biological replicates (in triplicate).
Surface morphology studies of the chitosan/silk fibroin microparticles
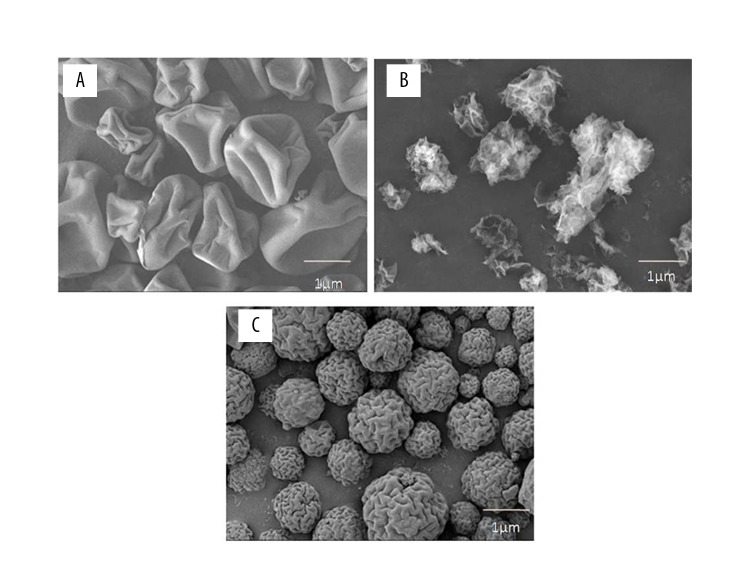
The surface morphology of silk fibroin, chitosan, and the chitosan/silk fibroin microparticles loaded with rhPTH were of variable shapes and are shown in Figure 2A–2C. The surface morphology of silk fibroin (Figure 2A), which was consistent with previously reported findings [24]. A wrinkled surface with loose surface topography was found for chitosan (Figure 2B), which was consistent with previously reported findings [25]. The surface morphology of chitosan/silk fibroin loaded with rhPTH microparticles were almost spherical, but there were observed wrinkles over the surface (Figure 2C). This finding could be due to the electrostatic interactions between the two different polymers. However, the addition of crosslinkers into formulations may also influence the surface morphology of the chitosan/silk fibroin microparticles.
Figure 2.
Scanning electron microscopy (SEM) of the morphology of silk fibroin (SF), chitosan (CHT), and chitosan/silk fibroin (CHT/SF) microparticles loaded with recombinant human parathyroid hormone (rhPTH). (A) Scanning electron micrograph of the morphology of silk fibroin (SF). (B) Scanning electron micrograph of the morphology of chitosan (CHT). (C) Scanning electron micrograph of the morphology of chitosan/silk fibroin (CHT/SF) microparticles loaded with recombinant human parathyroid hormone (rhPTH) formulation. The experiments were carried out in three biological replicates (in triplicate).
Powder X-ray diffraction (PXRD) of the microparticles
Powder X-ray diffraction (PXRD) of the silk fibroin, chitosan, and the chitosan/silk fibroin microparticles loaded with rhPTH microparticle formulation are shown in Figure 3. The pure silk fibroin film (Figure 3(A)) presented a halo PXRD pattern, which is characteristic of amorphous materials, with the absence of characteristics peaks. The PXRD pattern of chitosan (Figure 3(B)) showed broad diffraction peaks at 2θ=31° that represented semi-crystalline chitosan. However, the chitosan/silk fibroin microparticle formulation (Figure 3(C)) showed a PXRD pattern that was typical of an amorphous substance.
Figure 3.
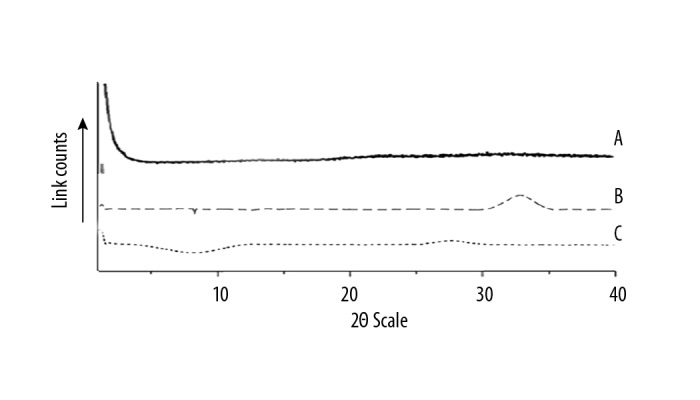
Powder X-ray diffraction (PXRD) pattern of silk fibroin (SF), chitosan (CHT), and chitosan/silk fibroin (CHT/SF) microparticles loaded with recombinant human parathyroid hormone (rhPTH). A) Powder X-ray diffraction (PXRD) pattern of silk fibroin (SF). B) PXRD pattern of chitosan (CHT). C) PXRD pattern of chitosan/silk fibroin (CHT/SF) microparticles loaded with recombinant human parathyroid hormone (rhPTH) formulation. The experiments were carried out in three biological replicates (in triplicate).
Entrapment efficiency (EE) and in vitro release studies
The entrapment efficiency (EE) was found to range from 60.36–74.9% (Figure 4). Previously reported findings have shown that increased concentration in the crosslinker significantly reduced the loading [26]. This finding might be due to the irregular surface of the microparticles, which was further enhanced by spray-drying of the rhPTH-loaded microparticles. The entrapment efficiency of 0.1 w/v chitosan with 10−6 M rhPTH was found to be 68%.
Figure 4.
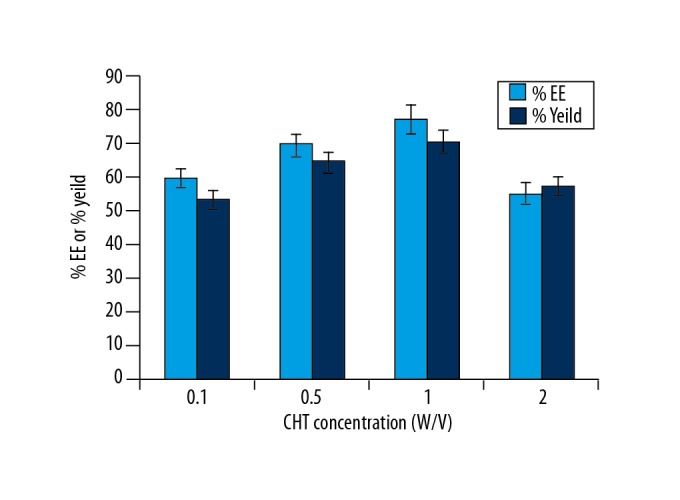
Entrapment efficiency (EE) and in vitro release study findings. The yield (percentage) of microparticles. The experiments were carried out with three biological replicates (in triplicate).
Figure 5 shows the 96-hour release profile of rhPTH-loaded silk fibroin/chitosan microparticle formulation. The mechanism of release might be due to the absorption of the water molecules (imbibition) in the matrix of the polymers, leading to swelling of the microparticle resulting in an inefficient cross-linkage that results in the release of the peptide. The stability of the peptide in the in vitro conditions could be increased following entrapment into the chitosan/silk fibroin polymeric chain, as the enzyme-linked immune sorbent assay (ELISA) detected the amount of the peptide even after 84 hours.
Figure 5.
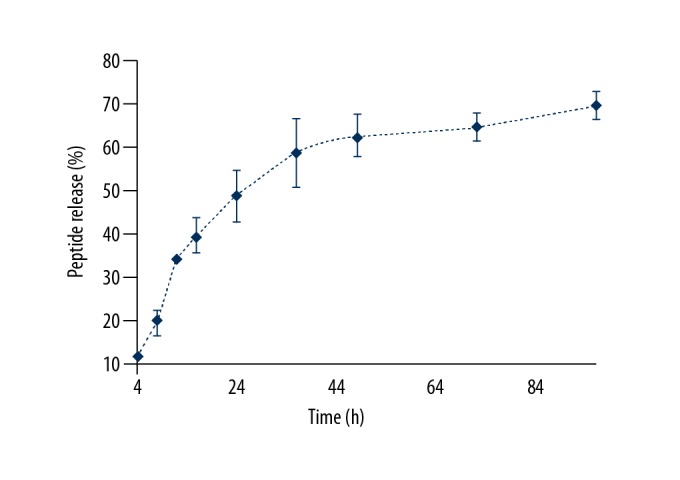
Profile of the percentage release of peptide with time. The experiments were carried out in three biological replicates and expressed as the mean ± standard deviation (SD).
Stability of the chitosan/silk fibroin microparticles in the blood
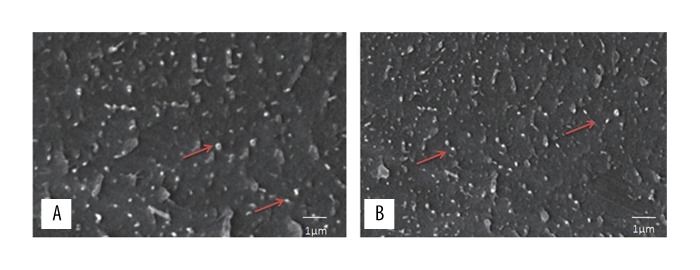
Figure 6A and 6B show the rhPTH loaded chitosan/silk fibroin microparticles at a concentration of 0.1 mg/mL with no aggregation after 2 hours of treatment with human blood plasma. The sample was well-dispersed, spherical, and with a size in the nano range. This finding excluded the possibility of microparticle aggregation in the blood due to the influence of plasma proteins.
Figure 6.
Scanning electron microscopy (SEM) of the morphology of chitosan/silk fibroin (CHT/SF) microparticles loaded with recombinant human parathyroid hormone (rhPTH). (A) Scanning electron microscopy (SEM) of the morphology of chitosan/silk fibroin (CHT/SF) microparticles loaded with recombinant human parathyroid hormone (rhPTH). (B) SEM of the chitosan/silk fibroin (CHT/SF) microparticles loaded formulation after treatment with blood plasma and without particle aggregation.
Blood compatibility studies
To examine the blood compatibility of the chitosan/silk fibroin microparticles, a hemolysis assay was performed after 2 hours of incubation. Figure 7 shows the rhPTH-loaded chitosan/silk fibroin microparticle concentrations together with the negative control (normal saline) and positive control (1% trypsin). The hemolysis assay showed that there was negligible damage to the blood cells associated with chitosan/silk fibroin microparticles. The positive control showed 8% damage to the cells. It was possible to conclude that the use of the chitosan/silk fibroin microparticles hemolysis levels were well within the drug safety limits for biomaterials according to the International Organization for Standardization (ISO) guidelines technical report 7406 (ISO/TR 7406), which indicated that the damage of the sample on the erythrocytes was negligible [27,28].
Figure 7.
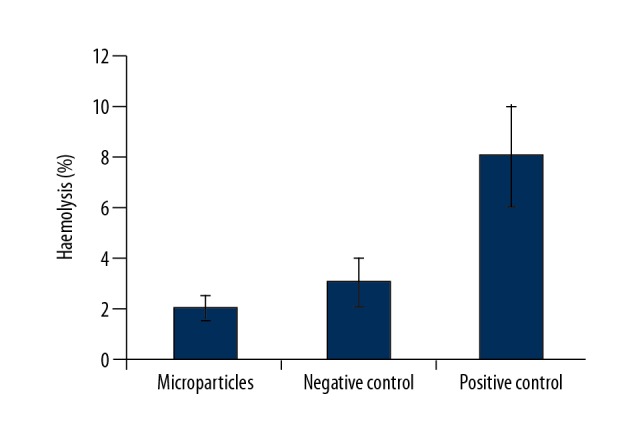
Hemolysis pattern of chitosan/silk fibroin (CHT/SF) microparticles. Negative control. Positive control. N=6. The results are expressed as the mean ± standard deviation (SD).
Discussion
This report has described the recent synthesis and characterization of a formulation of recombinant human parathyroid hormone (rhPTH) in a silk fibroin and chitosan matrix (chitosan/silk fibroin microparticle) using spray-drying technology. Human parathyroid hormone (PTH) contains 84 amino acids in its structure and is an endogenous polypeptide [29]. The synthesis of PTH takes place in the cells of the parathyroid gland. Human PTH 1–34 is a bone-forming agent that regulates calcium homeostasis and bone turnover, and can help to stimulate new cortical and trabecular bone formation and improve bone growth and bone density [30]. The 1–34 N-terminal fragment is responsible for the biological activity of PTH and for both catabolic and anabolic effects in bone [31]. Therefore, the manufacture of microparticles using biocompatible polymers can provide the prolonged action of the sustained-release properties of hrPTH combined with chitosan/silk fibroin microparticles. There is a need for improved treatment of osteoporosis and bone trauma that can improve current daily PTH injection treatment regimens and improve patient compliance.
Spray-drying is the most widely used technique to formulate microparticles of the desired particle size and distribution, and is a process that is commonly used in industrial processes involving particle formation and drying [32]. In this study, the spray-drying delivery system has been reported for the first time using silk fibroin and chitosan as biocompatible polymers to deliver rhPTH peptides with sustained release in the treatment of bone trauma. There are most likely to be electrostatic interactions between silk fibroin and chitosan that facilitate microparticle formation, due to the positive charge contributed by chitosan and the negative charge of silk fibroin. Surface properties of microparticles are significantly changed due to the silk fibroin. The blending of silk fibroin in the formulation might also be the factor that prevents the aggregation and decomposition of the microparticles in the blood, due to its robust material properties.
It has previously been reported that both the diffusion and stabilization effect provided by silk fibroin might cause the sustained release of the peptide from the formulation [33]. Previously, poly(lactic-co-glycolic acid) (PLGA) and alginate microspheres were prepared with a surface coating of silk fibroin to obtain the controlled release of the rhPTH, and the use of chitosan and silk fibroin in formulating microparticles for sustained release of a hydrophilic drug using a spray-drying method has been reported to be a method that can be used on a large scale [33]. This delivery system consists of three components, silk fibroin, chitosan, and a crosslinker. These polymers were chosen for their biocompatibility and suitable processing properties. Silk fibroin is a naturally occurring polymer and was chosen as a matrix due to its excellent properties that include its high mechanical strength, biocompatible nature, negligible flammability, and biostability or longer biodegradability. Previous reports described numerous formulation of silk fibroin as a film, scaffold, hydrogel or as non-woven mats [34]. All these formulations have the potential for improved therapy of bone disease or in tissue engineering research.
Chitosan is an abundant natural polysaccharide, second only to cellulose, and has many advantages over other polymers, which include nontoxicity, biocompatibility, and biodegradability. Chitosan is a family of cationic polysaccharides with a basic chemical structure of (1,4)-linked 2-amino-2-deoxy-D-glucans [35]. For commercial purposes, chitosan is produced by the partial deacetylation of chitin. Recently, chitosan and a silica hybrid membrane containing localized bone morphogenic protein (BMP)-2 delivery has been reported to show the healing ability of chitosan.
In the present study, Fourier-transform infrared spectroscopy (FTIR) was used to determine differences in the absorption frequency of samples and demonstrated characteristic peaks of silk fibroin and chitosan. In contrast, rhPTH loaded silk fibroin/chitosan microparticles displayed a slight change in the absorption frequency because of interaction between silk fibroin and chitosan, which is consistent with previously published findings [20]. In this study, absorption peaks of the amide groups in chitosan spectra, including at 1536 cm−1, were observed in silk fibroin/chitosan microparticles. There are also possibilities of interaction between the -COOH group of silk fibroin and -NH2 of chitosan, which caused a shift to 1644 cm−1 for silk fibroin/chitosan microparticles.
In the scanning electron microscopy (SEM) study, crystals of silk fibroin and chitosan were not present following the spray-drying process, which could have been due to the interaction between both polymers, resulting in the different surface morphology of the microparticles. Also, processing parameters of the microparticles could also influence the surface architecture. Powder X-ray diffraction (PXRD) results showed a halo pattern of the formulation that was most likely to be associated with the amorphous substances formed after spray-drying. Due to the presence of many -NH3+ groups present in the chitosan, electrostatic interactions with the negatively-charged amino acids present in the silk fibroin, which was also observed in the sustained-release studies of the peptide from the microparticles.
The findings of this study are supported by the findings from previous studies. Lee et al. reported that the influence of the silk fibroin to chitosan concentration ratio affected the release profile of tetracycline, as the percentage cumulative release of tetracycline was observed to range from between 67–80% at various silk fibroin/chitosan ratios [36]. At a ratio of 10: 4 the drug release was sustained with released for four days, while that of the silk fibroin microparticle alone had a maximum sustained release duration of two days [36]. This pattern of sustained release might be explained because of the high concentration of polymers, including the stability of chitosan nanoparticles [5]. This previous study also reported that there was no agglutination of blood after two hours of treatment and examination of human blood plasma [5].
Because exposure of biomaterials to the blood could result in damage to erythrocytes and could also lead to thrombus formation, the use of a hemocompatibility index is essential in the evaluation of sustained-release bioengineered microparticles for drug delivery. This fact is highlighted by reports from several groups that have investigated the blood compatibility of unmodified chitosan in microspheres and emulsions in terms of hemolysis [37–39]. However, the silk fibroin/chitosan microparticles showed excellent results with negligible hemolysis, which means that this formulation has the potential to improve the current bone trauma therapy with improved patient compliance and without hematological safety concerns.
Conclusions
Sustained-release microparticles of silk fibroin and chitosan have been shown to act as a delivery system for sustained-release recombinant human parathyroid hormone (rhPTH), with blood hemolysis levels that were within drug safety limits for biomaterials. This sustained-release formulation of rhPTH may provide an alternative administration route for patients who require treatment with PTH, which currently requires frequent intravenous and subcutaneous dosing, reducing dosing frequency and improving patient compliance during PTH treatment. However, further studies are required to evaluate this formulation in controlled clinical studies.
Footnotes
Source of support: This project was supported by funding from the Health and Family Planning Commission of Hubei Province (WJ2017M174; WJ2017M240)
References
- 1.van de Graaf GMM, Zoppa D, do Valle AL, et al. Morphological and mechanical characterization of chitosan-calcium phosphate composites for potential application as bone-graft substitutes. Res Biomed Eng. 2015;31:334–42. [Google Scholar]
- 2.Venkatesan J, Kim S-K. Chitosan composites for bone tissue engineering. An overview. Mar Drugs. 2017;8:2252–66. doi: 10.3390/md8082252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narayanan D, Anitha A, Jayakumar R, et al. Synthesis, characterization and preliminary in vitro evaluation of PTH 1–34 loaded chitosan nanoparticles for osteoporosis. J Bio Nanotech. 2012;8:98–106. doi: 10.1166/jbn.2012.1367. [DOI] [PubMed] [Google Scholar]
- 4.Codrons V, Vanderbist F, Verbeeck RK, et al. Systemic delivery of parathyroid hormone (1–34) using inhalation dry powders in rats. J Pharm Sci. 2013;92:938–50. doi: 10.1002/jps.10346. [DOI] [PubMed] [Google Scholar]
- 5.Narayanan D, Anitha A, Jayakumar R, Chennazhi K. PTH 1–34 loaded thiolated chitosan nanoparticles for osteoporosis: Oral bioavailability and anabolic effect on primary osteoblast cells. J Bio Nanotech. 2014;10:166–78. doi: 10.1166/jbn.2014.1700. [DOI] [PubMed] [Google Scholar]
- 6.Palumbo FS, Pitarresi G, Mandracchia D, et al. New graft copolymers of hyaluronic acid and polylactic acid: Synthesis and characterization. Carbohydr Polym. 2006;66:379–85. [Google Scholar]
- 7.Pitarresi G, Pierro P, Palumbo FS, et al. Photo-cross-linked hydrogels with polysaccharide-poly (amino acid) structure: new biomaterials for pharmaceutical applications. Biomacromolecules. 2006;7:1302–10. doi: 10.1021/bm050697m. [DOI] [PubMed] [Google Scholar]
- 8.Gaetani P, Torre ML, Klinger M, et al. Adipose-derived stem cell therapy for intervertebral disc regeneration: An in vitro reconstructed tissue in alginate capsules. Tis Eng Part A. 2014;14:1415–23. doi: 10.1089/ten.tea.2007.0330. [DOI] [PubMed] [Google Scholar]
- 9.Roche ET, Hastings CL, Lewin SA, et al. Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart. Biomaterials. 2014;35:6850–58. doi: 10.1016/j.biomaterials.2014.04.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wischke C, Tripodo G, Choi NY, et al. Hydrolytic degradation behavior of poly (rac-lactide)-block-poly (propylene glycol)-block-poly (rac-lactide) dimethacrylate derived networks designed for biomedical applications. Macromol Biosci. 2011;11:1637–46. doi: 10.1002/mabi.201100226. [DOI] [PubMed] [Google Scholar]
- 11.Tripodo G, Wischke C, Lendlein A. Highly flexible poly (ethyl-2-cyanoacrylate) based materials obtained by incorporation of oligo (ethylene glycol) diglycidylether. Macromolec Symposia: Wiley Online Library. 2011;1:49–58. [Google Scholar]
- 12.Tripodo G, Wischke C, Lendlein A. MRS Proceedings. Cambridge Univ Press; 2012. Design of semi-interpenetrating networks based on poly (ethyl-2-cyanoacrylate) and oligo (ethylene glycol) diglycidyl ether; pp. 1403–11. [Google Scholar]
- 13.Fortunati D, San Chau DY, Wang Z, et al. Cross-linking of collagen I by tissue transglutaminase provides a promising biomaterial for promoting bone healing. Amino Acids. 2014;46:1751–61. doi: 10.1007/s00726-014-1732-0. [DOI] [PubMed] [Google Scholar]
- 14.Chlapanidas T, Tosca M, Faragò S, et al. Formulation and characterization of silk fibroin films as a scaffold for adipose-derived stem cells in skin tissue engineering. Int J Immun Pharmacol. 2013;26:43–49. doi: 10.1177/03946320130260S106. [DOI] [PubMed] [Google Scholar]
- 15.Innocente F, Mandracchia D, Pektok E, et al. Paclitaxel-Eluting biodegradable synthetic vascular prostheses a step towards reduction of neointima formation? Circulation. 2009;120:37–45. doi: 10.1161/CIRCULATIONAHA.109.848242. [DOI] [PubMed] [Google Scholar]
- 16.Douglas KL, Piccirillo CA, Tabrizian M. Effects of alginate inclusion on the vector properties of chitosan-based nanoparticles. J Control Rel. 2006;115:354–61. doi: 10.1016/j.jconrel.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Wu P, He X, Wang K, et al. A novel methotrexate delivery system based on chitosan-methotrexate covalently conjugated nanoparticles. J Bio Nanotech. 2009;5:557–64. doi: 10.1166/jbn.2009.1073. [DOI] [PubMed] [Google Scholar]
- 18.MacLaughlin FC, Mumper RJ, Wang J, et al. Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery. J Controlled Rel. 1998;56:259–72. doi: 10.1016/s0168-3659(98)00097-2. [DOI] [PubMed] [Google Scholar]
- 19.Mao S, Shuai X, Unger F, et al. The depolymerization of chitosan: Effects on physicochemical and biological properties. Int J Pharm. 2004;281:45–54. doi: 10.1016/j.ijpharm.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Chung T-W, Chang C-H, Ho C-W. Incorporating chitosan (CS) and TPP into silk fibroin (SF) in fabricating spray-dried microparticles prolongs the release of a hydrophilic drug. J Taiwan Inst Chem Eng. 2011;42:592–97. [Google Scholar]
- 21.Yang M-C, Wang S-S, Chou N-K, et al. The cardiomyogenic differentiation of rat mesenchymal stem cells on silk fibroin – polysaccharide cardiac patches in vitro. Biomaterials. 2009;30:3757–65. doi: 10.1016/j.biomaterials.2009.03.057. [DOI] [PubMed] [Google Scholar]
- 22.Rockwood DN, Preda RC, Yücel T, et al. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011;6:1612–31. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rejinold NS, Sreerekha P, Chennazhi K, et al. Biocompatible, biodegradable and thermo-sensitive chitosan-g-poly (N-isopropylacrylamide) nanocarrier for curcumin drug delivery. Int J Bio Macromol. 2011;49:161–72. doi: 10.1016/j.ijbiomac.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Yeo J-H, Lee K-G, Lee Y-W, et al. Simple preparation and characteristics of silk fibroin microsphere. Eur Polym J. 2003;39:1195–99. [Google Scholar]
- 25.Hou J, Wang J, Cao L, et al. Segmental bone regeneration using rhBMP-2-loaded collagen/chitosan microspheres composite scaffold in a rabbit model. Bio Mat. 2012;7:035002. doi: 10.1088/1748-6041/7/3/035002. [DOI] [PubMed] [Google Scholar]
- 26.Ko J, Park HJ, Hwang S, et al. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int J Pharm. 2002;249:165–74. doi: 10.1016/s0378-5173(02)00487-8. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Wang L, Fan Y, et al. Biocompatibility and toxicity of nanoparticles and nanotubes. J Nanomat. 2012;2012:6. [Google Scholar]
- 28.Shalumon K, Chennazhi K, Nair SV, et al. Development of small diameter fibrous vascular grafts with outer wall multiscale architecture to improve cell penetration. J Bio Nanotech. 2013;9(7):1299–305. doi: 10.1166/jbn.2013.1630. [DOI] [PubMed] [Google Scholar]
- 29.Tregear GW, Rietschoten JV, Greene E, et al. Bovine parathyroid hormone: Minimum chain length of synthetic peptide required for biological activity. Endocrinology. 1973;93:1349–53. doi: 10.1210/endo-93-6-1349. [DOI] [PubMed] [Google Scholar]
- 30.Liu CC, Kalu DN. Human parathyroid hormone-(1–34) prevents bone loss and augments bone formation in sexually mature ovariectomized rats. J Bone Mineral Res. 2009;5:973–82. doi: 10.1002/jbmr.5650050911. [DOI] [PubMed] [Google Scholar]
- 31.Jüppner H, Schipani E, Bringhurst F, et al. The extracellular amino-terminal region of the parathyroid hormone (PTH)/PTH-related peptide receptor determines the binding affinity for carboxyl-terminal fragments of PTH-(1–34) Endocrinology. 1994;134:879–84. doi: 10.1210/endo.134.2.8299582. [DOI] [PubMed] [Google Scholar]
- 32.Gouin S. Microencapsulation: Industrial appraisal of existing technologies and trends. Trends Food Sci Tech. 2004;15:330–47. [Google Scholar]
- 33.Wang X, Wenk E, Hu X, et al. Silk coatings on PLGA and alginate microspheres for protein delivery. Biomaterials. 2007;28:4161–69. doi: 10.1016/j.biomaterials.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdulkhani A, Sousefi MD, Ashori A, et al. Preparation and characterization of sodium carboxymethyl cellulose/silk fibroin/graphene oxide nanocomposite films. Polym Testing. 2016;52:218–24. [Google Scholar]
- 35.Gutowska A, Jeong B, Jasionowski M, et al. Injectable gels for tissue engineering. Anat Rec. 2001;263:342–49. doi: 10.1002/ar.1115. [DOI] [PubMed] [Google Scholar]
- 36.Lee S-W, Park Y-T, Kim S-G, et al. The effects of tetracycline-loaded silk fibroin membrane on guided bone regeneration in a rabbit calvarial defect model. Maxi Plastic Reconst Surg. 2012;34:293–98. [Google Scholar]
- 37.Lee D-W, Powers K, Baney R. Physicochemical properties and blood compatibility of acylated chitosan nanoparticles. Carbohydr Polym. 2004;58:371–77. [Google Scholar]
- 38.Amiji MM. Surface modification of chitosan membranes by complexation-interpenetration of anionic polysaccharides for improved blood compatibility in hemodialysis. J Biomat Sci, Polymer Edition. 1997;8:281–98. doi: 10.1163/156856296x00309. [DOI] [PubMed] [Google Scholar]
- 39.Yang M-C, Lin W-C. Surface modification and blood compatibility of polyacrylonitrile membrane with immobilized chitosan – heparin conjugate. J Polym Res. 2002;9:201–6. [Google Scholar]