Introduction
Many people drink heavily, yet only a limited number (~35%) develop more advanced liver diseases (alcoholic hepatitis-AH, or cirrhosis). Thus, there must be modifying factors that either prevent or facilitate disease activity/progression. These modifiers can either be fixed (e.g., genetics) or can undergo intervention (e.g., smoking, diet). Diet is one of the most important potential disease modifiers, and one in which the health care provider and patient can intervene. Here, we discuss advances in our understanding of two important dietary factors-the trace metal, zinc, and the macronutrient, fat.
Overview of zinc metabolism
Zinc is the second most prevalent trace element in the body. Zinc plays a major role in the regulation of gene expression through metal-binding transcription factors and metal response elements in the promoter regions of regulated genes. Zinc also plays a role in zinc-finger motifs that are important to normal hepatic metabolism, such as hepatocyte nuclear factor-4alpha (HNF4α) and peroxisome proliferator activated receptor-alpha (PPARα)[1•].Zinc fingers typically have four cysteines within the finger motif that allow zinc to be bound in a tetrahedral complex. Importantly, with oxidative stress the cysteines can be modified, zinc is then lost from the zinc finger protein, and DNA binding activity is lost.
In the United States, the Recommended Dietary Allowance (RDA) for zinc in adults is 8 mg/d for women and 11 mg/d for men. Dietary intake of zinc and protein directly correlates with each other. Zinc status and the serum zinc levels decrease with low dietary zinc intake. There normally are multiple mechanisms in place to protect against zinc deficiency, including increased absorption and decreased excretion via modification of zinc transporters [2, 3]. Patients with alcoholic liver disease (ALD) often have poor quality diets that are low in protein and low in zinc. Absorption may be impaired in cirrhosis, and typically there is increased urinary loss of zinc in cirrhosis [4]. Zinc absorption, transfer, and excretion are accomplished by two large classes of transporters that tend to have opposing effects (ZnT proteins and Zip transporters)[2, 3, 5, 6]. The Zip family of transporters move zinc from the extracellular space into the cellular cytoplasm. Indeed, Zip4 plays a major role in intestinal zinc absorption, and a lack of this transporter causes the zinc deficiency observed in the disease acrodermatitis enteropathica. The ZnT proteins generally work in opposition to the Zip transporters.
Zinc status is typically assessed by plasma/serum zinc concentration. Tissue concentrations of zinc such as leukocyte zinc can be measured on a research basis, but they are not widely clinically available. Unfortunately, there is no test of zinc enzyme activity that reflects zinc status which is available for clinical use. It is important to note that inflammation/stress hormones may cause a decrease in serum zinc level, with an internal redistribution of the zinc [3, 7]. This stress response is often associated with hypoalbuminemia. Albumin is a major binding protein for zinc, but the serum zinc concentration will decrease with an inflammatory stimulus, even in the absence of hypoalbuminemia [7]. This is mediated, at least in part, by changes in zinc transporters, especially induction of Zipl4 and induction of hepatic metallothionein [5]. Metallothionein is a metal-binding protein that serves many functions, including zinc transport, antioxidant activity, and modulation of zinc absorption [2, 3, 8].
Recent studies demonstrate that zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis and severe inflammation, and zinc supplementation attenuated these findings [9]. Zinc deficiency was also associated with significant increases in the cytokine proinflammatory response and hepatocyte apoptosis, which were attenuated with zinc supplementation. Human patients in the critical care unit, some with sepsis, were also evaluated for altered zinc metabolism and enhanced inflammatory response. Patients in the intensive care unit without sepsis had decreased serum zinc levels compared to controls, while septic patients had the lowest zinc levels and the highest cytokine response [10]. Very recent data from Cousins’ laboratory documented a marked increase in the zinc transporter, ZIP14, associated with experimental polymicrobial sepsis and inflammation [11•] ZIP14−/− mice had improved outcome; zinc supplementation also improved outcome. Because patients with severe alcoholic hepatitis have a systemic inflammatory response syndrome similar to septic patients, it will be interesting to see if these new findings from intensive care unit patients can be translated to alcoholic hepatitis subjects [12].
Zinc deficiency and zinc supplementation in experimental models of ALD
Over the last decade, a series ot studies by Zhanxiang Zhou and coworkers in experimental animals and in vitro has markedly expanded our understanding of mechanisms whereby zinc deficiency may exacerbate alcohol-induced liver injury and mechanisms that zinc therapy may prevent or treat alcohol-induced liver injury. They initially confirmed reports showing decreased tissue stores of zinc in alcohol-fed mice. They showed that alcohol feeding caused marked alteration in several zinc transporters, which likely plays a major role in alcohol-induced changes in zinc homeostasis [13]. They performed a series of studies in rats and mice showing that alcohol feedings caused a disruption of intestinal barrier function that was improved with zinc supplementation [14•, 15]. Animals fed the Lieber-DeCarli alcohol diet had increased barrier permeability in the ileum and a decrease in intestinal tight-junction proteins such as claudin-1 and zona occludens-1 [14•]. Alcohol feeding decreased the ileal zinc concentration, and this was associated with an accumulation of reactive oxygen species [14•]. Zinc supplementation corrected all of these abnormalities [14•,15]. Alcohol-fed mice had significantly increased plasma endotoxin levels, while those fed alcohol supplemented with zinc showed no significant increase in endotoxin levels [14•].
Translocation of gut-derived toxins, including endotoxin, typically activate toll-like receptors on Kupffer cells and induce inflammatory cytokine production, such as tumor necrosis factor (TNF), with subsequent hepatic inflammation/injury. Zhou and coworkers documented that zinc interfered with the normal endotoxin-TNF signaling pathways and attenuated NF-kappaB activation in Kupffer cells [16], They showed that zinc attenuated alcohol-induced cell death through multiple mechanisms [17–19]. They initially noted that zinc inhibited alcohol-induced endogenous FAS ligand activation, which is a key component in signaling pathways leading to apoptosis [17, 18]. They also showed that chronic alcohol exposure significantly reduced zinc levels in isolated hepatic endoplasmic reticulum (ER) and mitochondria [19]. Zinc depletion was associated with caspase-3 activation and apoptosis in association with ER stress and mitochondrial dysfunction. These events were attenuated by low concentrations of zinc supplementation but not by supplementation with the antioxidant N-acetylcysteine. Lastly, they showed that critical zinc-finger proteins were inactivated in experimental alcoholic liver disease, and zinc supplementation enhanced liver regeneration by preserving activity of the zinc-finger protein, HNF 4 α [20]. In summary, these studies in rodents and in vitro demonstrate that zinc supplementation acts at multiple levels to prevent/protect against experimental alcohol-induced liver injury (Fig. 1) [21•].
Fig. 1.
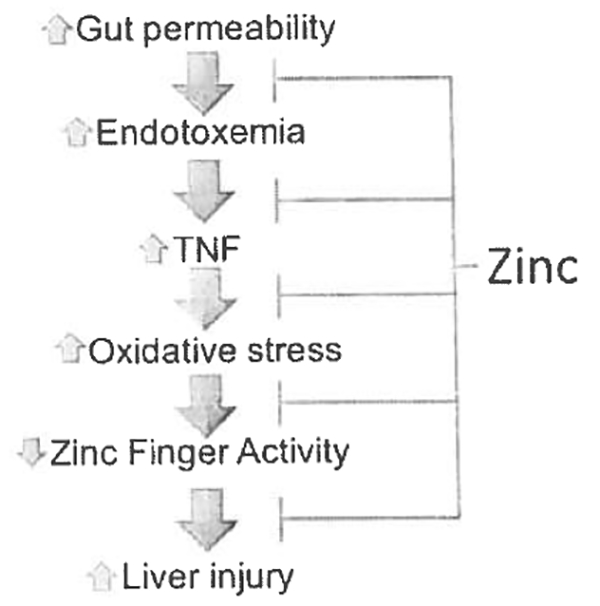
Zinc deficiency and zinc supplementation affect multiple metabolic pathways in the development/progression of ALD
Zinc deficiency and zinc supplementation in human ALD
Vallee, et al. first reported [22] in 1956 on the occurrence of marked hypozincemia in patients with severe alcoholic cirrhosis. This observation was subsequently confirmed by multiple investigators [23, 24]. Hypozincemia was next reported in patients treated for acute and chronic alcoholism without evidence of liver disease [25]. Concentrations of zinc in liver, white blood cells, pancreatic juice, and testes have all been reported to be decreased in alcoholic cirrhotics [26–32].
The mechanism(s) for the zinc deficiency in alcoholics appear to be multifac-torial. We performed careful dietary histories in alcoholics with or without liver disease and in healthy volunteers. While the healthy controls regularly consumed the recommended amount of zinc or more daily, approximately 90% of the alcoholics (with or without liver disease) had an inadequate dietary intake of zinc [33]. Absorption of zinc is also impaired in chronic alcoholics, and ethanol administration to rats decreased zinc absorption [34]. Multiple investigators have reported increased urinary excretion of zinc in alcoholics with or without liver disease, and the greatest excretion was observed in patients with more severe liver disease [23–25]. Lastly, as noted previously, zinc may be lost from zinc finger proteins during oxidative stress, producing functional zinc deficiency.
Knowledge concerning the importance of the metabolic role of zinc was initially derived from manifestations of zinc deficiency in either zinc deficient animals or in patients with acrodermatitis enteropathica, a hereditary disease of impaired zinc absorption [35, 36]. Information from these early studies facilitated the recognition of zinc deficiency in common clinical conditions such as ALD. Some of the potential clinical manifestations of zinc deficiency in ALD) are shown in Table 1. We highlight four examples of zinc deficiency complications that may be observed in ALD.
Table 1. Zinc deficiency may present in multiple ways that are of clinical relevance in ALD.
| 1. Acrodermatitis |
| 2. Anorexia, altered taste and smell |
| 3. Hypogonadism |
| 4. Impaired night vision |
| 5. Impaired immune function |
| 6. Altered protein metabolism, poor wound healing |
| 7. Diarrhea |
| 8. Depressed mental function/encephalopathy |
Severe zinc deficiency may cause crusting skin lesions. This rash tends to occur in a typical distribution, usually starting around the eyes, nose, and mouth, and later over the scrotum, buttocks, and sometimes over the extremities [37]. Chronic alcoholics (especially those with ALD), can develop zinc deficiency skin lesions (acrodermatitis) that correct with oral zinc supplementation [37–39].
Anorexia is an early and major manifestation of zinc deficiency in animals fed a zinc-deficient diet [40, 41]. Similarly, weight loss has been observed in human volunteers fed a zinc-deficient diet [42]. Patients with ALD frequently complain of anorexia with altered taste sensation and have decreased food consumption. Knudsen and Weismann [43] carried out a double-blind clinical trial of zinc supplementation in stable alcoholic cirrhotics, and one of the variables evaluated was taste sensation. Moderate but significant improvement in taste sensation was observed in the zinc-treated group, and major improvements were seen in individual patients [43].
Zinc deficiency is a well-recognized cause of hypogonadism in experimental animals, and it has been postulated to play a pathogenic role in the human hypogonadism observed in certain disease processes such as regional enteritis, sickle cell disease, uremia, and chronic alcoholism [33, 44–49]. Chronic alcoholics, with or without liver disease, may have hypogonadism [50]. Zinc deficient animals have reduced basal testosterone levels and depressed weights of testes and other androgen-sensitive organs as well as decreased muscle mass compared to zincsufficient controls [40, 51, 52]. Uncontrolled studies suggested that zinc supplementation may improve the hypogonadism in ALD [33, 49]. Low testosterone may play a role in the sarcopenia frequently observed in chronic ALD, and testosterone therapy has recently been shown to improve sarcopenia in cirrhosis [53]. Whether or not zinc improves muscle wasting/sarcopenia in ALD has not been evaluated.
Portal systemic encephalopathy (PSE) is a derangement of mental function caused by liver disease or shunting of blood around the liver. Blood toxins such as ammonia, mercaptans, and false-neuro transmitters are postulated to play an etiological role in PSE. Zinc is critically involved in ammonia metabolism. Zinc deficiency by itself can also cause confusion and apathy. Our group and others have shown that serum zinc levels are decreased in patients with compensated ALD and are further decreased in those with PSE (Fig. 2). Multiple trials of zinc supplementation in PSE have been performed, with somewhat inconsistent results. The most recent large study showed a significant improvement in encephalopathy grade, Child-Pugh score, and psychological testing compared to controls [54•]. The most recent systematic review and meta-analysis of the use of zinc in hepatic encephalopathy showed a significant improvement in the number-connection test with zinc, therapy [55]. Because zinc, in theory, can exert its effects biochemically and metabolically via multiple pathways in PSE, and is inexpensive and safe, we frequently use it, at least as adjunct therapy, in patients with PSE.
Fig. 2.
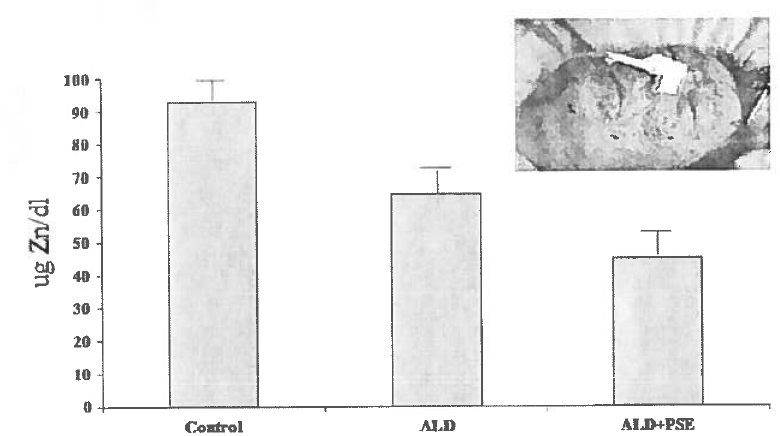
Patients with compensated cirrhosis had decreased serum zinc concentrations compared to normal controls, and alcoholic cirrhotics with clinically-overt PSE had levels that were significantly lower than both controls and compensated cirrhotics. The pictured patient had severe crusting skin lesions of zinc deficiency, very low serum zinc concentrations, and a mental dysfunction that was originally thought to be PSE. However, the skin lesions and mental confusion corrected rapidly with zinc supplementation
While there are abundant studies, but many only case reports, showing that zinc supplementation can correct clinical manifestations of zinc deficiency in ALD, there have been limited well-controlled clinical trials evaluating the efforts of supplementation on clinical outcome in ALD. An early trial showed that zinc supplementation could improve serum zinc concentrations in alcoholics with or without cirrhosis [56]. Studies of oral zinc supplementation to cirrhotics for 2–3 months demonstrated beneficial effects on liver metabolic function and on nutrition parameters [57–59]. Zinc supplementation improved serum albumin, retinol binding protein and insulin-like growth factor 1. Quantitative liver function tests, such as galactose elimination capacity and antipyrine clearance, also improved with zinc supplementation. 1 lowever, there have been few studies evaluating the role of zinc in the development of early ALD, mechanisms of action for zinc therapy in ALD, or effects of zinc on long-term outcome. Three recent studies published only in abstract form address these critical areas [60–63].
The first study evaluated 108 males and females with very heavy drinking histories who were grouped by serum zinc concentrations. Croup One had normal serum zinc levels and consisted of 67 patients; Group Two had depressed serum zinc levels and included 41 patients [60]. All patients were housed at the NIH and were participating in an alcohol treatment program. No patient had clinically-relevant liver injury. Data were collected on demographics, drinking history in the last 90 days, and lifetime drinking history, as well as clinical and laboratory assessments. Liver injury and inflammation as assessed by AST and C-reactive protein were significantly increased in the low-zinc group, and serum zinc correlated with C-reactive protein in the low-zinc group. Serum albumin was also lower in the low-zinc group. Both groups had similar long-term drinking histories and neither group showed evidence of clinical or biochemical malnutrition. Thus, it appears that zinc deficiency may be an early biochemical marker in alcohol-induced liver injury and inflammation, and may play a pathogenic role in early ALD [60].
The second study from Cave and coworkers is an NIH pilot trial in which 22 subjects with Child-Pugh Class A and B alcoholic cirrhosis were randomized to placebo or zinc sulfate 220 mg per day in a single-center, double-blind protocol. Interim 3-month data were presented in two abstracts. Zinc supplemented patients had an increase in serum zinc concentration, a decrease in proinflammatory mediators, improvement in serum endotoxin levels, a modest reduction in Child-Pugh score, as well as a decrease in fibrosis markers such as hyaluronic acid and TGF-beta. These preliminary studies suggest that zinc therapy targets mechanisms in human alcoholic cirrhosis similar to that observed in experimental animal models of ALD [61, 62].
The final study was done in Japan. Two hundred and forty patients with various etiologies of chronic liver disease were studied. One hundred and ninety-two patients received zinc for at least six months and 48 received no zinc supplementation. The zinc-treated group was divided into four subgroups based on their zinc concentration after six months of zinc therapy. The mean follow-up was almost four years. Liver function significantly deteriorated in the no-treatment group but was stable in the zinc group. Similarly, the incidence rate of adverse events was higher in the no-treatment group, and the incidence rate of hepatocellular carcinoma (HCC) was higher in the no-treatment group. Serum zinc concentrations tended to predict outcome. The authors concluded that oral zinc supplementation was effective at maintaining liver function and helped to prevent HCC [63].
In our clinical practice, we typically use 50 mg of elemental zinc per day orally as zinc supplementation in ALD. We most frequently give zinc sulfate 220 mg (50 mg elemental zinc) with a meal. This is the most commonly available zinc supplement, and we give it with a meal because zinc salts can cause gastrointestinal irritation. We usually give zinc long term, for several months to years, or at least until the serum zinc level has normalized. Because the serum zinc level is almost always low in patients with ALD, we do not necessarily obtain serum zinc concentration before starting zinc supplementation. However, if we do supplement patients, it is valuable to have a baseline determination in order to compare with a follow-up zinc concentration to document improvement in the zinc level. If patients cannot tolerate zinc sulfate with a meal, one can switch to another type of zinc supplement such as zinc gluconate or zinc acetate. We usually do not go above 50 mg elemental zinc per day because of potential risks of impaired copper absorption or immune suppression. Response to zinc supplementation can be monitored by resolution of zinc deficiency signs or symptoms, e.g., skin lesions, mental changes, etc., or improvement in serum zinc status.
In conclusion, there is major zinc dyshomeostasis with ALD. This is mediated, in part, by poor intake and absorption, increased excretion, and altered zinc transporters, especially ZIP 14. Zinc deficiency plays an etiologic role in multiple mechanisms of ALD, ranging from intestinal barrier dysfunction to apoptosis. Zinc supplementation is highly effettive at correcting these ALD mechanisms and preventing/treating experimental ALD. Because human data suggest that zinc deficiency occurs early in the development of ALD, we treat most patients with ALD with zinc sulfate 220 mg/day. Our goal is to improve zinc status and to block multiple mechanisms for liver inflammation/injury/fibrosis in ALD.
Opinion statement.
Many variables, aside from the amount and duration of alcohol consumption, play a role in the development and progression of alcoholic liver disease (ALD). One critical factor that can be modified is diet/nutrition. We have made major recent advances in our under-standing of the interactions of nutrition and ALD. In this article, we review advances made in zinc and fat metabolism/therapy for ALD. There is major zinc dyshomeostasis with ALD which is mediated, in part, by poor intake and absorption, increased excretion, and altered zinc transporters, especially ZIP14. Zinc deficiency plays an etnologie role in multiple mechanisms of ALD, ranging from intestinal barrier dysfunction to hepatocyte apoptosis. Zinc supplementation is highly effective at correcting these ALD mechanisms and preventing/treating experimental ALD. There is no Food and Drug Administration (FDA) approved therapy for any stage of ALD. Because animal and human data suggest that zinc deficiency occurs early in the course of ALD, we treat most ALD patients with daily oral zinc supplementation (220 mg zinc sulfate which contains 50 mg elemental zinc).
Acknowledgements
The work presented in this study was supported by NIH grants K23AA18399 (to Dr. Cave), IR01ES021375 (to Dr. Cave), T35ES014559 (to Drs. Cave and McClain), U01AA022489 (to Dr. McClain), U01AA021901 (to Dr. McClain), U01AA021893 (to Dr. McClain), R01AA023681 (to Dr. McClain), R01AA018869 (to Dr. McClain), the National Institute On Alcohol Abuse And Alcoholism of the National institutes of Health under Award Number P50AA024337, (to Dr. McClain), and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the NIH under grant number P20GM113226. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, and as well by a grant from the Department of Veterans Affairs (to Dr. McClain).
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Vatsalya Vatsalya declares no conflict of interest.
Craig McClain is funded by several federal grants, as noted above, and is a part-time government (VA) employee. Matthew Cave reports the grants noted above.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.•.Mohammad MK. Zhou Z, Cave M, Barve A, McClain CJ. Zinc and liver disease. Nutr Clin Pract 2012:27(1):8–20. doi: 10.1177/0S84533611433534.This is a more comprehensive review of zinc metabolism in multiple types of liver diseases.
- 2.Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J Biol Inorg Chem 2011; 16(7): 1123–34. doi: 10.1007/s00775011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King IC. Zinc: an essential but elusive nutrient. Am J Clin Nutr 2011;94(2):679S–84S. doi: 10.3945/ajcn.110.005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClain CJ, Antonow DR Cohen DA, Shedlofsky SI. Zinc metabolism in alcoholic liver disease. Alcohol Clin Exp Res 1986;10(6):582–9. [DOI] [PubMed] [Google Scholar]
- 5.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 2009;29:153–76. doi: 10.11466/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Zhou B. Dietary zinc absorption: A play of Zips and ZnTs in the gut. IUBMB Life 2010,62(3): 176–82. doi: 10.1002/iub.291. [DOI] [PubMed] [Google Scholar]
- 7.Caetke LM, McClain CJ, Talwalkar RT, Shedlofsky SI.Effects of endotoxin on zinc metabolism in human volunteers. Am I Physiol 1997;272(6 Pt 1):E952–6. [DOI] [PubMed] [Google Scholar]
- 8.Kang YJ, Zhou Z. Zinc prevention and treatment of alcoholic liver disease. Mol Aspects Med 2005:26(4–5): 391–404. doi: 10.1016/j.mam.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Knoell DL, Julian MW, Bao S, Besecker B, Macre JE, Leikauf GD, et al. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med 2009;37(4): 1380–8. doi: 10.1097/CCM.0b013c31819cefe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besecker BY, Exline MC, Hollyfield J, Phillips G, Disilvestro RA, Wewers MD, et al. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am I Clin Nutr 2011;93(6): 1356–64. doi: 10.3945/ajcn.110.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.•.Wessels I, Cousins RJ. Zinc dyshomeostasis during polymicrobial sepsis in mice involves zinc transporter Zipl4 and can be overcome by zinc supplementation. Am J physiol Gastrointest Liver Physiol. 2015;309(9):G768–7S. doi: 10.1152/ajpgi.00179.2015.This study demonstrates the altered zinc metabolism that occurs during sepsis and the critical role for ZIP14, a zinc importer induced by proinflammatoty stimuli, in sepsis-induced zinc dyshomeostasis.
- 12.Mitchell MC, Friedman LS, McClain CJ. Medical Management of Severe Alcoholic Hepatitis: Expert Review from the Clinical Practice Updates Committee of the AGA Institute. Clin Castroenterol Hepatol. 2017; 15(1):5–12. doi: 10.1016/j.cgh.2016.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Q, Li Q, Zhong W, Zhang J, Sun X, Tan X, et al. Dysregulation of hepatic zinc transporters in a mouse model of alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 2014;307(3):C313–22. doi: 10.1152/ajpgi.00081.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.•.Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol 2010;298(5):G625–33. doi: 10.1152/aipgi.00350.2009.This article documents multiple mechanisms whereby zinc deficiency may alter gut-barrier function, and it indicates the multiple implications for gut-barrier dysfunction in ALD.
- 15.Zhong W, Zhao Y, McClain CJ, Kang YJ, Zhou Z. Inactivation of hepatocyte nuclear factor-4 {alpha} mediates alcohol-induced dowmegulation of intestinal tight junction proteins. Am J Physiol Gastrointest Liver Physiol 2010;299(3):G643–51. doi: 10.1152/ajpgi.00515.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Abrogation of nuclear factor-kappaB activation is involved in zinc inhibition of lipopolysaccharide-induced tumor necrosis factor-alpha production and liver injury. Am J Pathol 2004;164(5):1547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Z, Liu J, Song Z, McClain CJ, Kang YJ. Zinc supplementation inhibits hepatic apoptosis in mice subjected to a long term ethanol exposure. Exp Biol Med (Maywood). 2008;233(5):540–8. doi: 10.3181/0710-RM-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert JC, Zhou Z, Kang YJ. Suppression of Fasmediated signaling pathway is involved in zinc inhibition of ethanol-induced liver apoptosis. Exp Biol Med (Maywood). 2003;228(4):406–12. [DOI] [PubMed] [Google Scholar]
- 19.Sun Q, Zhong W, Zhang W, Li Q, Sun X, Tan X, et al. Zinc deficiency mediates alcohol induced apoptotic cell death in the liver of rats through activating ER and mitochondrial cell death pathways. Am J Physiol Gastrointest Liver Physiol 2015;308(9):G757–66. doi: 10.1152/ajpgi.00442.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang X, Song Z, McClain CJ, Kang YJ, Zhou Z. Zinc supplementation enhances hepatic regeneration by preserving hepatocyte nuclear factor-4alpha in mice subjected to long-term ethanol administration. Am J Pathol 2008; 172(4):916–25. doi: 10.2353/ajpath.2008.070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.•.Zhong W, Zhao Y, Sun X, Song Z, McClain CJ, Zhou Z. Dietary zinc deficiency exaggerates ethanol-induced liver injury in mice: involvement of intrahepatic and extrahepatic factors. PLoS One. 2013;8(10):e76522. doi: 10.1371/journal.pone.0076522.This is an excellent overview of how zinc deficiency may worsen alcohol-induced liver injury and how zinc supplementation may attenuate alcohol-induced liver injury in experimental animals.
- 22.Bartholomay AF, Robin ED, Vallee RL Wacker WE. Zinc metabolism in hepatic dysfunction. 1. Serum zinc concentrations in Laennec’s cirrhosis and their validation by sequential analysis. N Engl J Med 1956;255(9):403–8. doi: 10.1056/NEJM195608302550901. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan JF, Heaney RP. Zinc metabolism in alcoholic liver disease. Am J Clin Nutr 1970;23(2):170–7. [DOI] [PubMed] [Google Scholar]
- 24.Kahn AM, Helwig HL, Redeker AG, Reynolds TB. Urine and serum zinc abnormalities in disease of the liver. Am J Clin Pathol 1965;44(4):426–35. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan JF, Lankford HG. Zinc Metabolism and Chronic Alcoholism. Am J Clin Nutr 1965;17:57–63. [DOI] [PubMed] [Google Scholar]
- 26.Vallee BL, Wacker WF, Bartholomay AF, Hoch FI. Zinc metabolism in hepatic dysfunction. II. Correlation of metabolic patterns with biochemical findings. N Engl I Med 1957;257(22): 1055–65. doi: 10.1056/NEjM195711282572201. [DOI] [PubMed] [Google Scholar]
- 27.Boyett JD, Sullivan IF. Zinc and collagen content of cirrhotic liver. Am J Dig Dis 1970;15(9):797–802. [DOI] [PubMed] [Google Scholar]
- 28.Kiilerich S, Dielrichson O, Loud FB, Naestoft I, Christoffersen P, luhl I., et al. Zinc depletion in alcoholic liver diseases. Scand I Gastroenterol 1980;15(3):363–7. [DOI] [PubMed] [Google Scholar]
- 29.Fredricks RE, Tanaka KR, Valentine WN. Zinc in human blood cells: normal values and abnormalities associated with liver disease. J Clin Invest 1960;39:1651–6. doi: 10.1172/101104188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keeling PW, Jones RB, Hilton PJ, Thompson RP. Re-duced leucocyte zinc, in liver disease. Gut 1980;21(7):561–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan JF, O’Grady J, Lankford HG. The Zinc Content of Pancreatic Secretion. Gastroenterology. 1965;48:438–43. [PubMed] [Google Scholar]
- 32.Trentini CP, Dalla Pria AF. Ferrari De Gaetani C, Vianello A. [Hepatic cirrhosis and changes of the zinc content of the testis. Possible role of serum zinc deficiency in the pathogenesis of hypogonadism in cirrhotic patients]. Arch De Vecchi Anat Patol 1968;52(2):657–70. [PubMed] [Google Scholar]
- 33.McClain CJ, Van Thiel DH, Parker S, Badzin LK, Gilbert H. Alterations in zinc, vitamin A, and retinol-binding protein in chronic alcoholics: a possible mechanism for night blindness and hypogonadism. Alcohol Clin Exp Res 1979;3(2): 135–41. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan JF, Jetton MM, Burch RE. A zinc tolerance test. J lab Clin Med 1979;93(3):485–92. [PubMed] [Google Scholar]
- 35.Luecke RW. Domestic animals in the elucidation of zinc’s role in nutrition. Fed Proc 1984;43(13):2823–8. [PubMed] [Google Scholar]
- 36.Moynahan EJ, Letter: Acrodermatitis enteropathica: a lethal inherited human zinc-deficiency disorder. Lancet. 1974;2(7877):399–400. [DOI] [PubMed] [Google Scholar]
- 37.McClain Cl, Soutor C, Steele N, Levine AS, Silvis SE. Severe zinc deficient presenting with acrodermatitis during hyperalimentation: diagnosis, pathogenesis, and treatment. I Clin Gastroenterol 1980;2(2): 125–31. [DOI] [PubMed] [Google Scholar]
- 38.Weismann K, Roed-Petersen J, Hjorth N, Kopp H Chronic zinc deficiency syndrome in a beer drinker with a Billroth II resection. Int J Dermatol 1976;15:757–61. [DOI] [PubMed] [Google Scholar]
- 39.Weismann K, Hoyer H, Christensen E. Acquired zinc deficiency in alcoholic liver cirrhosis: report of two cases. Acta Derm Venereol 1980;60(5):447–9. [PubMed] [Google Scholar]
- 40.O’Leary MJ, McClain Cl, Hegarty PV. Effect of zinc deficiency on the weight, cellularity and zinc concentration of different skeletal muscles in the post-weanling rat. Bri Nutr 1979;42(3):487–95. [DOI] [PubMed] [Google Scholar]
- 41.Essatara MB, Levine AS, Morley JE, McClain CJ. Zinc deficiency and anorexia in rats, normal feeding patterns and stress induced feeding. Physiol Behav 1984;32(3):469–74. [DOI] [PubMed] [Google Scholar]
- 42.Prasad AS, Rabbani P, Abbasii A, Bowersox E, Fox MR. Experimental zinc deficiency in humans. Ann Intern Med 1978;89(4):483–90. [DOI] [PubMed] [Google Scholar]
- 43.Knudsen L, Weismann K. Taste dysfunction and changes in zinc and copper metabolism during penicillamine therapy for generalized scleroderma. Acta Med Scand 1978;204(1–2):75–9. [DOI] [PubMed] [Google Scholar]
- 44.Sandstead HH, Prasad AS, Schulert AR, Farid Z, Miale A Jr, Bassilly S, et al. , Human zinc deficiency, endocrine manifestations and response to treatment. Am J Clin Nutr 1967;20(5):422–42. [DOI] [PubMed] [Google Scholar]
- 45.Millar MJ, Fischer Ml, Elcoate PV, Mawson CA. The effects of dietary zinc deficiency on the reproductive system of male rats. Can J Biochem Physiol 1958;36(6):557–69. [PubMed] [Google Scholar]
- 46.McClain C, Soutor G, Zieve L. Zinc deficiency: a complication of Crohn’s disease. Gastroenterology. 1980;78(2):272–9. [PubMed] [Google Scholar]
- 47.Antoniou LD, Shalhoub RJ, Sudhakar T, Smith JC Jr. Reversal of uraemic impotence by zinc. Lancet. 1977;2(S044):895–8. [DOI] [PubMed] [Google Scholar]
- 48.Abbasi AA, Prasad AS, Ortega J, Congco E, Oberleas D Gonadal function abnomalities in sickle cell anemia. Studies in adult male patients. Ann Intern Med 1976;85(5):601–5. [DOI] [PubMed] [Google Scholar]
- 49.Leevy CM, Kanagasundaram N, Smith F. Treatment of liver disease of the alcoholic: a composite approach. Semin Liver Dis 1981; 1(3):254–6. doi: 10.1055/s-2008-1041752. [DOI] [PubMed] [Google Scholar]
- 50.Van Thiel DH, Cavaler IS, Schade RR. Liver disease and the hypothalamic pituitary gonadal axis. Semin Liver Dis 1985;5(1):35–45. doi: 10.1055/s-2008-1041756. [DOI] [PubMed] [Google Scholar]
- 51.McClain CJ, Cavaler JS, Van Thiel DH. Hypogonadism in the zinc-deficient rat: localization of the functional abnormalities. J lab Clin Med 1984; 104(6): 1007–15. [PubMed] [Google Scholar]
- 52.Lei KY, Abbasi A, Prasad AS. Function of pituitary-gonadal axis in zinc-deficient rats. Am J Physiol 1976;230(6): 1730–2. [DOI] [PubMed] [Google Scholar]
- 53.Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. Hlepatol. 2016;65(5):906–13. doi: 10.1016/j.ihep.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 54.•.Takuma Y, Nouso K, Makino Y, Hayashi M, Takahashi H. Clinical trial: oral zinc in hepatic encephalopathy. Aliment Pharmacol Ther 2010;32(9):1080–90. doi: 10.1111/j.1365-2036.2010.04448.x.A large randomized clinical trial of zinc supplementation in PSE which shows beneficial effects of zinc not only on mental funaioli but also on Child-Pugh scores.
- 55.Chavez-Tapia NC, Cesar-Arce A, Barrientos-Gutierrez T, Villegas-Lopez FA, Mendez-Sanchez N, Uribe M. A systematic review and meta analysis of the use of oral zinc in the treatment of hepatic encephalopathy. Nutr J 2013:12:74. doi: 10.1186/1475-2891-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zarski JP, Amaud J, Labadic H, Beaugrand M, Favier A, Rachail M. Serum and tissue concentrations of zinc after oral supplementation in chronic alcoholics with or without cirrhosis. Gastroenterol Clin Biol 1987; 11 (12);856–60. [PubMed] [Google Scholar]
- 57.Marchesini G, Fabbri A, Bianchi G, Brizi M, Zoli M. Zinc supplementation and amino acid-nitrogen metabolism in patients with advanced cirrhosis. Hepatology. 1996;23(5): 1084–92. doi: 10.1053/jhep.1996S.v23.pm0008621138. [DOI] [PubMed] [Google Scholar]
- 58.Bianchi GP, Marchesini G, Brizi M, Rossi B, Forlani G, Boni P et al. Nutritional effects of oral zinc supplementation in cirrhosis. Nutrition Research.20(8):1079–89. doi: 10.1016/S0271-5317(00)00194-9. [DOI] [Google Scholar]
- 59.Marchesini G, Bugianesi E., Ronchi M, Flamia R, Thomaseth K, Pacini G. Zinc supplementation improves glucose disposal in patients with cirrhosis. Metabolism. 1998;47(7):792–8. [DOI] [PubMed] [Google Scholar]
- 60.Vatsalya V, Liu N, Liaquat H, Ramchandani V, McClain C. Role Of Scrum Zinc In The Progression Of Liver Injury In Very Heavy Drinking Alcohol Dependent Patients ACER. 2016;40(S1):89A [Google Scholar]
- 61.Mohammad MK, Song M, Falkner K, McClain C, Cave M. Zinc Sulfate for Alcoholic Cirrhosis (ZAC) Clinical Trial - Interim Analysis of Fiver Injury/Inflammation Biomarkers, Hepatology. 2014;60(S1):794A. [Google Scholar]
- 62.Song M, Mohammad MK, Falkner K, McClain C, Cave M. Zinc Sulfate for Alcoholic Cirrhosis (ZAC) Clinical Trial - Interim Analysis of Clinical Parameters, Intestinal Per-meability and Liver Fibrosis Biomarker. Hepatology. 2014;60(S1):787A. [Google Scholar]
- 63.Hosui A, Hiramatsu N. Oral zinc supplementation improves liver function and decreases the risk of he-patocellular carcinoma development. Flepatologv. 2016;64(S1):92A. [Google Scholar]


