Abstract
Background:
Office-based biofeedback therapy (OB) is efficacious for constipation with dyssynergic defecation (DD). However, it requires skilled staff, multiple visits and only available in selected centers. Whether home-based biofeedback therapy (HB) is also effective is not known.
Methods:
In a randomized controlled trial, patients with DD (Rome III) received either OB or HB. OB comprised of therapist-guided six biweekly sessions of pelvic floor training. HB comprised of self-training at home, 20 minutes, twice a day, after self-inserting a probe attached to a display box that provided individual, performance-specific, visual feedback. Anorectal physiology and daily symptom diary were assessed and compared. Subjects with normalization of dyssynergic defecation and ≥1 increase in number of complete spontaneous bowel movements (CSBM)/week at 3 months were considered responders. Cost outcomes were assessed using hospital billing records and questionnaires. ITT non-inferiority analyses and per protocol analyses were performed using the one-sided t-test with a margin (bound) added to the null value. Clinical trials.gov no NCT03202771.
Results:
100 subjects (96 F) participated, 83 completed [Home = 38/50, office= 45/50]. Thirty-four (68%) patients were responders in HB and 35/50 (70%) in OB. The number of CSBM/week, dyssynergia pattern, balloon expulsion time, digital maneuver use and bowel satisfaction improved significantly (p<0.0001) from baseline with both treatments. The effect of HB was non-inferior to OB for the primary subjective and physiologic outcomes of number of CSBM/week, bowel satisfaction, and balloon expulsion time in both ITT and per protocol analyses, and for dyssynergia in the per protocol analysis. Home device was well tolerated. There were no adverse events. HB incurred significantly lower costs than OB (p<0.01), with a saving of $860.00.
Conclusion:
HB significantly improves bowel symptoms and physiology and is as effective as OB. HB is well tolerated, less costly and should be preferred treatment for DD.
Funding:
NIH grant RO1 DK 57100–05 and grant RR00059 from the General Clinical Research Centers program, National Center for Research Resources.
Keywords: Home Biofeedback therapy, dyssynergic defecation, cost-effectiveness, constipation, anorectal function
INTRODUCTION
Constipation is a common digestive complaint that affects approximately 15% of Americans1,2. Dyssynergic defecation is one of three overlapping subtypes of chronic primary constipation that affects one third of all constipated subjects3–6. It is characterized by either impaired propulsion of stool from the rectum or paradoxical anal contraction or inadequate anal relaxation, or a combination of these mechanisms4,7,8.
Several recent controlled studies have shown that biofeedback therapy is effective and is superior to laxatives9, sham feedback treatment and relaxation therapy10. A position paper from the AGA endorsed biofeedback therapy as effective,4 and the American and European societies of Neurogastroenterology and Motility conferred a grade A recommendation for biofeedback therapy in the treatment of dyssynergic defecation11. However biofeedback therapy is not widely available, is labor intensive both from the patient’s and therapist’s perspective, and requires skilled personnel and multiple office visits, all of which affect compliance with this treatment and poses personal hardship. Also, many insurance agencies in USA do not provide coverage for biofeedback therapy. Additionally, with rising health care costs, aside from efficacy, treatments must be cost effective. Consequently, a home-based biofeedback therapy could be an attractive and cost-effective option of delivering this behavioral therapy that could offset some of the potential pitfalls with office biofeedback therapy, and allow greater usage of this treatment modality.
We hypothesized that home-based biofeedback (HB) therapy is as efficacious as office-based biofeedback (OB) therapy, but less costly for patients with dyssynergic defecation. To test this, we performed a parallel arm randomized controlled trial to determine whether a self-administered HB therapy program with a new portable device is as effective as OB therapy in improving bowel symptoms and anorectal physiology in patients with dyssynergic defecation. Additionally, we prospectively assessed whether HB is a more cost-effective alternative to the standard OB therapy.
METHODS
Study design and participants:
Adult outpatients (18–80 years), who were referred to a tertiary care center with a complaint of constipation were eligible to participate in the study. Patients were included if they fulfilled Rome III criteria for functional constipation12, had failed routine management of constipation, and demonstrated a dyssynergic pattern of defecation during attempted defecation with anorectal manometry13, and either had prolonged balloon expulsion time (> 1 minute) or prolonged delay (> 20% radiopaque marker retention) in colonic transit10,13,14,15. They were required to have no evidence of structural or metabolic diseases that could cause constipation, as assessed by colonoscopy/barium enema and routine hematological, biochemical and thyroid function tests. Patients taking drugs known to be constipating, for example opioids, were excluded or they discontinued the drug two weeks before enrollment. Other exclusion criteria included: severe cardiac or renal disease, previous gastrointestinal, spinal or pelvic surgery except cholecystectomy, hysterectomy or appendectomy, neurologic diseases such as multiple sclerosis, stroke, Parkinson’s disease or spinal injury, impaired cognizance (mini-mental score < 15), legal blindness, pregnancy, rectal prolapse, anal fissure, and alternating pattern of constipation and diarrhea.
All subjects were assessed at baseline and after treatment with anorectal manometry, balloon expulsion test10,14,16,17, and colonic transit study in which three different shaped radioopaque markers (Sitzmark ®, Konsyl Pharmaceuticals, Fort Worth, Texas) were administered on three consecutive days and a plain abdomen x-ray taken on day 610,18. We assessed the percentage of subjects with slow colonic transit before and after biofeedback. In a prospective stool diary, starting one week before enrollment subjects recorded the time, consistency (Bristol stool scale19; type 1=hard pellets and 7 = watery stools), straining effort (1=normal, 2=moderately excessive, 3=severe) of each bowel movement and feeling of incomplete evacuation (yes/no) and need for digital assistance (yes/no). Also, they rated the overall satisfaction with bowel function on a 100 mm visual analog scale (VAS)10. Progress and compliance were monitored through bi-weekly phone calls. After completion of treatment, patients in both groups answered a survey that assessed the tolerability, coping skills, instructions on device use and social issues with biofeedback treatments.
Randomization and masking:
Randomization used permuted blocks of 4 with 1:1 assignment into the two parallel study groups. Random numbers generated in advance by the biostatistician were placed into sequentially numbered opaque envelopes, sealed and used for subject assignment. After screening, and once patients met the study inclusion and exclusion criteria, the study coordinator enrolled patients into one of the two treatment arms, by opening the sealed envelope. While the therapist and patient could not be blinded, the manometry reader and the data analyst were unaware of patient assignment or previous data. Standard protocols were employed for each group to ensure that all patients received similar general guidelines for management of their constipation. The study recruitment and biofeedback therapy were performed at University of Iowa Hospitals and Clinics between 2005–2010, and thereafter data analysis and manuscript writing were performed at University of Iowa and Augusta University. All subjects provided written informed consent and the study was approved by the University of Iowa Institutional review Board No 200209080 and registered at Clinical trials.gov no NCT03202771.
Procedures:
A gastroenterologist, nurse therapist and dietitian provided advice regarding bowel habits, exercise, laxatives, dietary fiber and fluid intake, and timed-toilet training during an initial visit that was reinforced during the follow up visits.
All patients were advised to attempt bowel movement for 5 minutes, twice a day, 30 minutes after eating, irrespective of their urge to defecate. The nurse therapist taught subjects how to improve their push effort by using postural and diaphragmatic breathing techniques10,,20,21 and instructed them to practice these maneuvers at home for 15 minutes, three times a day. Magnesium hydroxide (Milk of Magnesia ®, Phillips, Bayer USA, CA) 1–2 tablespoons or magnesium gluconate (Magonate ® 500 mg, Fleming & Company, St. Louis, MO) 2–4 tablets daily was recommended daily as the standard laxative, and subjects were instructed to titrate its use. All subjects were advised to refrain from using manual maneuvers and if employed its use was recorded. Patients having no bowel movement for 48 hours were instructed to use one glycerin suppository, then after 72 hours, a tap water enema and after 96 hours two bisacodyl tablets orally (rescue laxatives). The dietitian advised subjects to consume a balanced, adequate calorie diet, increase fruit and vegetable intake to five servings per day and consume 25 g of dietary fiber from natural food sources daily.
Office biofeedback (OB) treatment:
In addition to the aforementioned general instructions, subjects had an initial training session by a nurse specialist followed by biweekly, one hour OB sessions, up to a maximum of six therapy sessions over 3 months. Biofeedback training was performed by placing a solid state manometry probe (Koningsberg Instruments, Pasadena, CA), and by using a data recorder and software (Nanologger® & Amb B®, Gaeltec Ltd. Dunvegan, Isle of Skye, UK) for displaying and analyzing the manometric data. Biofeedback treatment consisted of three components. First, the patient was instructed on diaphragmatic breathing techniques to improve the push effort21. Second, the patient was trained to improve the rectoanal coordination by increasing the push effort as reflected by a rise in intra-abdominal/intra-rectal pressures on the display monitor, synchronized with anal relaxation as reflected by a decrease in anal sphincter pressure. While seated on a commode, subjects watched the manometric tracings on a computer monitor and received guided instructions using visual and verbal feedback techniques to correct the dyssynergic pattern during attempted defecation7,20. Third, the patient was trained to efficiently expel a 50 ml artificial stool, over three trials of defecation7,20,22. Their posture and breathing techniques were continuously monitored and appropriate feedback was provided to improve the defecatory effort during all 3 components.
Home biofeedback (HB) treatment:
In addition to measures outlined under study protocol, patients randomized to HB were trained on the use of home-training device in a single session. Patients were instructed on how to place a reusable, dual sensor, probe into their rectum. The probe was connected to a hand- held pressure monitor that displayed the patient’s response (Anatoner ®, Protech, Hyderabad, India). Next, the patient was asked to sit on a commode, and attempt 10–15 push maneuvers whilst observing the anal and rectal pressure changes on the hand-held device. When the anal sphincter pressure decreased a greater number of lights would illuminate on the anal panel of the home device. If the patient could not relax then fewer or no lights would be displayed. This provided instant feedback to the patient regarding their anal relaxation effort. Likewise, with an appropriate push effort, more lights would illuminate on the rectal panel providing feedback of their performance. Patients were asked to insert the probe at least twice daily, and practice for 20 minutes and keep a daily log. They returned to the lab for follow up visits at 4 and 8 weeks, and based on their progress, the device’s sensitivity was adjusted and new target goals were set.
Outcomes:
Because constipation is a heterogeneous condition and no single parameter adequately defines constipation or represents an optimal way of assessing clinical outcome10,14,23, a range of subjective and physiologic measures of bowel function were used. Subjective primary outcome measures were the number of complete spontaneous bowel movements (CSBM) per week and the scores on the global bowel satisfaction visual analog scale. A spontaneous bowel movement was defined as a bowel movement that occurred naturally or without use of rescue laxatives, suppositories or enemas within the previous 24 hrs. A CSBM was defined as a bowel movement reported on a stool diary without a feeling of incomplete evacuation. The proportion of subjects who reported ≥ 20 mm positive change on VAS was used as an index of global bowel satisfaction.
Physiologic primary outcome measures were the presence of dyssynergic pattern during attempted defecation7,14 and the balloon expulsion time. Intention to treat and per protocol analyses were performed for all the primary outcome measures.
Secondary subjective outcome measures included stool frequency, stool consistency, straining effort, and proportion of patients needing digital assistance for stooling. The secondary physiologic outcome measures included anal residual pressure, intrarectal pressure and defecation index during attempted defecation, thresholds for first perception and urge to defecate, and the proportion of subjects with slow colonic transit time14,17,18.
Responder Definition:
In this trial, a responder was defined by using a composite measure comprising of a change of ≥ 1 CSBM/week over baseline together with normalization of the dyssynergia pattern of defecation after 3 months. Because a diagnosis of dyssynergic defecation requires both symptoms and altered anorectal physiology13,14, we felt that this composite measure could provide a more robust method of assessing the treatment success.
Cost-outcomes analysis:
Costs were estimated using micro-costing analysis using patient’s electronic medical record, hospital billing records and study questionnaires. We captured costs from a societal perspective by incorporating both direct (healthcare system costs) and indirect costs to the patient (work loss due to appointment). We used the following equation to estimate the cost in each treatment arm:Total costs=hospital costs + physician costs + equipment costs + home treatment costs + work loss costs + travel time costs + transportation costs.
The hospital costs were estimated taking into consideration patient’s age and insurance reimbursement rate (older=medicare, younger=private insurance) and this value multiplied by 6 to arrive at hospital costs. Because the HB group required just one initial visit to hospital for home training a single visit was calculated. Physician costs were assigned a value of $90.00/visit x 6 for office and x 1 for home. Equipment cost was assigned 0 for office and $244.00 rental fees for home device. Home treatment costs were estimated time spent for home practice (20 mins twice a day) with the patient’s salary per hour multiplied by 0.5. Work loss was calculated by multiplying patient’s salary per hour with no of visits and the time spent including waiting for nurse’s time, car parking etc for each group. Travel time was estimated using MapQuest, multiplied by 2 (round trip), no of sessions and patient’s salary. And likewise transportation costs were estimated with round trip miles multiplied by 0.35. The salary per hour was taken from Bureau of Labor and Statistics website.
Statistical Analysis:
Sample size and power calculations
Based on our previous study10, we estimated that for assessing HB and OB efficacy with a sample size of 50, the paired t-test at the 0.025 significance level can detect a mean change in anorectal function measures of at least 0.45 SD with 0.80 power. This corresponds to at least a 39% decrease in balloon expulsion time, a 103% increase in defecation index, a mean increase of 8 in bowel satisfaction scale (VAS), and 2.2 in CSBM per week. For the non-inferiority study, based on changes in the primary outcome measures, with n=50 per study arm, the test for non-inferiority has 0.70 power to reject, at the 0.05 significance level, the null hypothesis that the mean change due to HB is smaller than office biofeedback (OB) by at least 0.44 SD in favor of the alternative hypothesis that mean change due to HB is no more than 0.44 SD lower than OB (i.e. Ho: ΔHB-ΔOB<−0.44SD vs. Ha: ΔHB-ΔOB≤−0.44SD). Using estimates from our study10, we consider HB no worse than or better than office therapy if the HB group had at least a 58 sec. decrease in balloon expulsion time (111 sec for office, SD=120), 1.0 increase in defecation index (1.4 for office, SD=0.9), 41 point increase in bowel satisfaction scale (49 for office, SD=17), and 1.3 increase in CSBM per week (3.4 for office, SD=4.8).
Based on variations in cost/session of office based therapy ($150-$425), and assuming a range of 3 to 6 office sessions, we expect the total cost of office biofeedback to be between $450 and $2550 respectively. Assuming this range represents 95% of cost values, we estimated that the total cost and standard deviation to be $525 [= (2550–450)/4]. With the proposed sample size of 50 per group, the two-sample t-test at the 0.05 significance level can detect a difference in mean cost of at least $270 with 0.80 power.
An intention to treat (ITT) and a per protocol analyses were performed. The ITT analysis included all subjects that were randomized to each treatment group and who underwent at least one session of treatment. For those with missing end-of-study data, the last observed value was used. The per protocol analysis included only those who completed the required number of biofeedback sessions.
Two major sets of statistical analysis were performed. The first set was study arm specific, and comprised of assessing whether symptoms and anorectal and colonic physiology were significantly different following biofeedback training when compared to those at baseline. The second set compared the effects of biofeedback treatment between the HB and OB study arms, and involved assessing whether HB treatment effect (calculated by subtracting baseline values from post-treatment values) was non-inferior to OB.
In the first set, the null hypothesis was that the mean change from baseline following biofeedback training is zero. By rejecting the null hypothesis we concluded that there is significant change from baseline following biofeedback training. In the second set, the null hypothesis was that the mean change following HB training is worse than OB by at least a threshold value that is clinically significant. This was tested against the alternative hypothesis of non-inferiority that the mean change following HB training is no worse or better than OB. A standard way of testing for non-inferiority of means is the one-sided t-test with a margin (bound) added to the null value. By rejecting the null hypothesis in favor of the alternative hypothesis, we conclude that the mean change in measurement following HB training is non-inferior to OB training. For non-inferiority test of the difference between two independent proportions, the Farrington-Manning score test was used.
The non-inferiority bound for the HB was set as at least 70% of OB for the CSBM/week, and at least 75% of OB for the number of stools per week. The non-inferiority bound for the continuous outcome measures was set at 0.44 of the standard deviation, as what was used for the sample size calculation. The bounds used for the continuous outcomes are listed in Tables 2 and 3. A bound of 0.5 was used for stool consistency which represents 1/12th of the score range of 1–7, and 0.25 for stool strain score which represents 1/8th of the score range of 1–3. A bound of 5 percentage points was used for for the differences in prevalence outcomes for digital assistance, dyssynergia, and abnormal BET, and 10 percentage points for dyssynergia pattern, slow transit and responder rates between the two groups.
Table 2.
Intention-to-treat analysis comparing bowel symptoms and stool diary data between home biofeedback and office biofeedback groups
| Home Biofeedback (n=50) |
Office Biofeedback (n=50) |
Test of non-inferiority | |||||
|---|---|---|---|---|---|---|---|
| Subjective parameters | Time | Mean (SEM or 95% CI) |
Mean (SEM or 95% CI) |
Ho: Bound |
Mean Difference or ratio1 |
90% CI | p-value |
| Baseline | 0.68 (0.17) | 1.20 (0.29) | |||||
| Post | 3.34 (0.37) | 4.74 (0.57) | <0.70 | 0.70 | (0.54, 0.92) | 0.484 | |
| Ratio2 | 4.91 (3.19, 7.56)* | 3.95 (2.55, 6.12)* | <0.70 | 1.24 | (0.75, 2.07) | 0.032 | |
| Baseline | 5.06 (0.49) | 5.71 (0.71) | |||||
| Post | 5.94 (0.53) | 8.36 (0.86) | <0.75 | 0.71 | (0.57, 0.89) | 0.654 | |
| Ratio2 | 1.17 (0.96, 1.43) | 1.46 (1.09, 1.96)* | <0.75 | 0.80 | (0.60, 1.07) | 0.352 | |
| Baseline | 1.89 (0.08) | 1.98 (0.09) | |||||
| Post | 1.68 (0.08) | 1.72 (0.08) | >0.25 | −0.04 | (−0.22, 0.14) | 0.004 | |
| Change2 | −0.21 (−0.36, −0.06)* | −0.26 (−0.45, −0.07)* | >0.25 | 0.05 | (−0.15, 0.25) | 0.050 | |
| Baseline | 3.41 (0.15) | 3.24 (0.20) | |||||
| Post | 3.45 (0.14) | 3.41 (0.19) | <−0.50 | 0.04 | (−0.35, 0.43) | 0.012 | |
| Change2 | 0.04 (−0.27, 0.34) | 0.17 (−0.27, 0.60) | <−0.50 | −0.13 | (−0.57, 0.31) | 0.083 | |
| Baseline | 16.3 (2.4) | 18.4 (2.9) | |||||
| Post | 56.3 (3.9) | 57.5 (3.8) | <−13 | −1.2 | (−10.2, 8.0) | 0.017 | |
| Change2 | 40.0 (31.0, 49.0)* | 39.1 (30.7, 47.5)* | <−13 | 0.9 | (−9.3, 11.1) | 0.013 | |
| Baseline | 24 (48%) | 24 (48%) | |||||
| Post | 6 (12%)* | 5 (10%)* | >5% | 2% | (−9%, 13%) | 0.317 | |
Difference=Home-Office; Ratio=Home/Office
Ratio=Post/Baseline; Change=Post-Baseline
Significant change from baseline
Table 3.
Intention-to-treat analysis comparing the anorectal physiology parameters and colonic transit time results between the home biofeedback and office biofeedback groups
| Home Biofeedback (n=50) |
Office Biofeedback (n=50) |
Test of non-inferiority | |||||
|---|---|---|---|---|---|---|---|
| Physiological parameters | Time | Mean (SEM or 95% CI) |
Mean (SEM or 95% CI) |
Ho: Bound |
Mean Difference or ratio1 |
90% CI | p-value |
| Baseline | 50 (100%) | 50 (100%) | |||||
| Post | 14 (28%) | 10 (20%) | >10% | 8% | (−6%, 22%) | 0.638 | |
| Baseline | 64.1 (12.3) | 57.8 (11.4) | |||||
| Post | 17.5 (3.1) | 17.5 (3.0) | >1.70 | 1.00 | (0.66, 1.51) | 0.018 | |
| Ratio2 | 0.27 (0.18, 0.40)* | 0.30 (0.21, 0.44)* | >1.70 | 0.90 | (0.58, 1.40) | 0.010 | |
| Baseline | 28 (56%) | 26 (52%) | |||||
| Post | 8 (16%) | 8 (16%) | >5% | 0% | (−13%, 13%) | 0.249 | |
| Baseline | 0.48 (0.04) | 0.47 (0.04) | |||||
| Post | 1.64 (0.24) | 1.77 (0.24) | <0.65 | 0.92 | (0.66, 1.29) | 0.042 | |
| Ratio2 | 3.43 (2.50, 4.71)* | 3.74 (2.82, 4.95)* | <0.65 | 0.92 | (0.65, 1.30) | 0.051 | |
| Baseline | 31 (62%) | 23 (46%) | |||||
| Post | 18 (36%)* | 17 (34%) | >10% | 2% | (−14%, 18%) | 0.200 | |
| Responder (%) | Post | 34 (68%) | 35 (70%) | <−10% | −2% | (−17%, 13%) | 0.193 |
Difference=Home-Office; Ratio=Home/Office
Ratio=Post/Baseline; Change=Post-Baseline
Significant change from baseline
Log transformed data
SAS 9.2® TTEST procedure was used to perform both the difference test between baseline and end of active treatment measures, and the non-inferiority test between the changes with treatment in the home-based and office-based study arms. Certain measures, such as balloon volumes for first sensation, balloon expulsion time, and defecation index typically have a log normal distribution, and therefore were log-transformed for the analyses. For the number of CSBM and stool frequency per week, generalized mixed model analysis for Poisson counts (GLIMMIX procedure) was used. The tests for the prevalence outcomes were performed using SAS FREQ procedure.
Effects for the continuous variables were expressed as mean change from baseline (post-pre) with 95% confidence limits for within group changes, and as mean difference between groups (HB-OB) with corresponding one-tailed 95% lower (or upper) limit for non-inferiority test of HB compared to OB. For the log-transformed variables, and CSBM and stool frequency counts, effects were calculated as mean ratio of post-treatment relative to baseline, and as mean ratio of HB to OB.
RESULTS
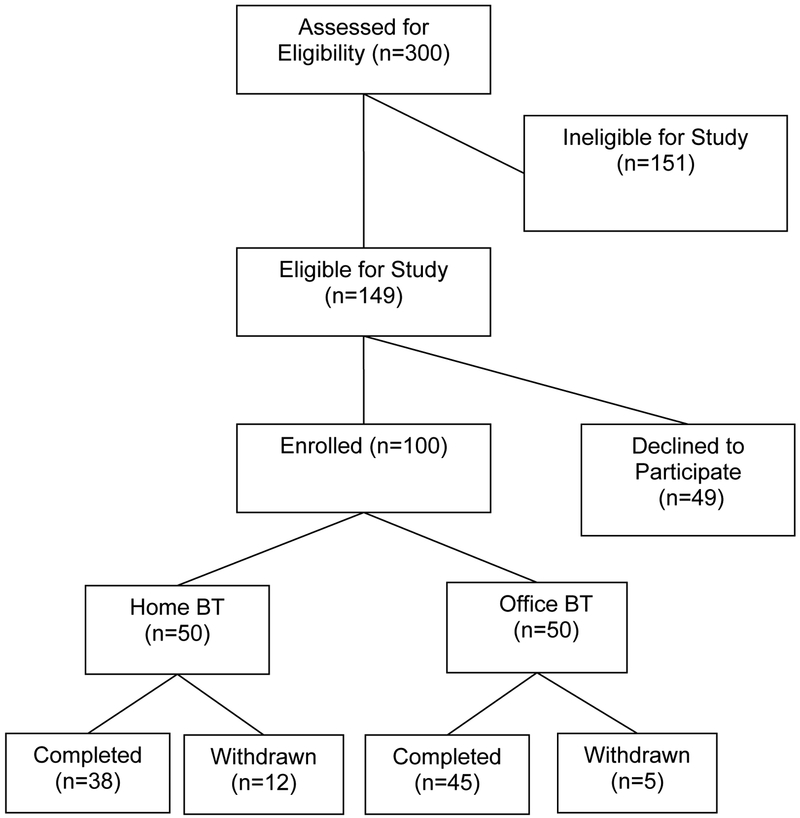
One hundred patients with dyssynergic defecation participated, of whom 50 women were randomized to HB, mean age ± sd =37 ± 12 years, and 46 women and 4 men were randomized to OB, mean age ± sd =42 ±15 years. Among these, 83 subjects completed the study (Home=38 and office =45) and 17 subjects dropped out (Fig 1). The mean duration of constipation for the HB group was 12 years and OB group was 15 years.
Figure 1:
Trial profile
There were no differences in the demographic distribution between the two groups (Table 1). The mean (range) number of therapy sessions for OB was 5 (4–6). The baseline bowel symptom profiles and manometric features were comparable and similar between the two groups (Tables 1, 2, 3 and 4). Of the 12 patients who dropped out in the HB group, 2 became pregnant, 6 were lost to follow up, 1 was withdrawn because of hospitalization from diabetic complications, 2 patients had transportation problems and 1 patient, a student who lived in a dormitory found it difficult to use home device. Of 5 patients who withdrew in OB group, one became pregnant, 1 was hospitalized with a leg fracture, 1 had transportation issues, and 2 were lost to follow up. All of these patients were included in the ITT analysis. The non-compliance rate did not differ among the groups (p=0.21), but a type 2 error cannot be excluded.
Table 1.
Demographic characteristics of subjects and baseline symptom patterns in the Home biofeedback and Office biofeedback groups
| Home Biofeedback (n=50) |
Office Biofeedback (n=50) |
|
|---|---|---|
| Sex | ||
| Female | 50 (100%) | 46 (92%) |
| Male | 0 | 4 (8%) |
| Age, mean (SD) years | 37 (12) | 42 (15) |
| Ethnicity | ||
| White | 44 | 42 |
| Black | 2 | 3 |
| Others | 4 | 5 |
| Mean duration of constipation, years (Range) | 12 (2–37) | 15 (3–33) |
| Number of stools/week, mean (SEM) | 5.06 (0.5) | 5.71 (0.7) |
| Stool Consistency (BSFS, Types 1–7), mean (SEM) | 3.41 (0.15) | 3.24(0.2) |
| Stool Straining effort score (1–3), mean (SEM) | 1.89 (0.08) | 1.98(0.09) |
| Number of CSBM/week, mean (SEM) | 0.68 (0.17) | 1.2 (0.29) |
| Use of digital maneuvers to assist stooling, n (%) | 24(48%) | 24 (48%) |
| Bowel satisfaction score- VAS (mm), mean (SEM) | 16.3 (2.4) | 18.4 (2.9) |
BSFS= Bristol stool form scale; VAS= Visual analog scale
Table 4.
Intention-to-treat analysis comparing the rectal sensory thresholds between the home biofeedback and office biofeedback groups
| Home Biofeedback (n=50) |
Office Biofeedback (n=50) |
Home-Office | |||
|---|---|---|---|---|---|
| Sensory Threshold | Time | Median (IQR) |
Median (IQR) |
Median Difference |
90% CI |
| Baseline | 20 (10–30) | 20 (10–30) | |||
| Post | 15 (10–30) | 20 (10–20) | −5 | (−10.5, 0.5) | |
| Post-Baseline | 0 (−10–0)* | 0 (−10–0)* | 0 | (−3.9, 3.9) | |
| Baseline | 75 (60–130) | 80 (70–120) | |||
| Post | 75 (70–120) | 80 (50–100) | −5 | (−21.4, 11.4) | |
| Post-Baseline | 0 (−40–20) | 0 (−30–30) | 0 | (−7.8, 7.8) | |
| Baseline | 170 (100–250) | 160 (110–220) | |||
| Post | 170 (100–200) | 140 (110–180) | 30 | (4.2, 55.9) | |
| Post-Baseline | 0 (−30–20) | −5 (−50–30) | 5 | (−10.9, 20.9) | |
Significant change from baseline
Patients who received HB as well as those who received OB demonstrated a significant increase in the number of CSBMs per week when compared to the baseline period (both p<0.0001; Table 2, Fig 2). Number of CSBMs per week increased nearly 5 times (mean post/base ratio: 4.91; 95% CI: 3.19, 7.56) in the HB group compared to around 4 times of baseline (mean ratio: 3.95; 95% CI: 2.55, 612) in the OB group. Non-inferiority test with home/office ratio bound of 0.70 (i.e. Ho: home/office<0.70 vs. Ha: home/office≤0.70) was significant (p=0.032), indicating that effect of HB was no worse/or better than OB in mean number of CSBMs per week. Response to HB was 1.24 times that of OB, with one-tailed 95% lower limit of 0.75 (i.e. 90% CI: 0.75, 2.07), suggesting that HB response was no lower than 25% of OB (Table 3). The overall satisfaction with bowel function significantly increased in both groups (p<0.0001), with a mean score change difference of 0.9, and with a HB group mean change of no more than 9.3 lower when compared to the OB group (i.e. 90% CI: −9.3, 11.1) (Table 2).
After treatment, the mean stool frequency per week increased by 46% (mean post/pre ratio 1.46; 95% CI: 1.09, 1.96; p=0.011) in the OB group, and by 17% (mean post/pre ratio 1.17; 95% CI: 0.96, 1.43; p=0.109) in the HB group. Non-inferiority of HB to OB was not shown (p=0.352), with a 20% smaller mean response to HB compared OB, and could be as low as 40% smaller than OB (90% CI: 0.60, 1.07) (Table 2). There was significant improvement in straining effort in both HB and OB groups, and a small improvement towards softer stools. Non-inferiority of HB was demonstrated in straining effort improvement and in stool consistency at end of study (Table 2). A need for digital assistance with stooling lessened significantly (p<0.0001) in both HB and OB groups, however, non-inferiority test showed that the proportion requiring digital assistance in HB group could be as high as 13 percentage points more than OB (Table 2). Laxative use in the HB versus OB groups was, none in 17/50 (34%) versus 13/50 (26%), p=0.38, magnesium/senna/stool softener in 26/50 (52%) versus 30/50 (60%),p=0.42, bisacodyl, polyethyleneglycol, lubiprostone in 5/50(10%) versus 6/50 (12%), p=0.75, and enema/suppository use in 2/50 (4%) versus 1/50 (2%), p=0.56 respectively. There was no difference between groups.
Dyssynergia pattern of defecation significantly improved (p<0.0001) and was corrected in 72% receiving HB, and in 80% receiving OB therapy (Fig 2). Non-inferiority of HB to OB was not shown (Table 3). Balloon expulsion time decreased significantly in both groups when compared to their baseline (p<0.0001). Expulsion time at the end of study decreased to 27% of baseline in HB group, and 30% of baseline in OB group.
The percentage of subjects with abnormal balloon expulsion time improved in both groups (p<0.0001). However, non-inferiority of HB to at most 5 percentage points worse than OB could not be ruled out (p=0.249; 95% upper limit of 13%) (Table 3).
The defecation index, an overall measure of recto-anal coordination during defecation, improved significantly (p<0.0001) in both the HB and OB groups (Fig 2). The mean increase in defecation index in HB was not inferior when compared to OB (Table 3). At baseline, 62% in HB and 48% in OB had slow colonic transit time. After treatment, the proportion of subjects with slow colonic transit decreased significantly in HB to 36% (p=0.0002), but not in OB (34%; p=0.109). Non-inferiority of HB in slow colonic transit after treatment to at most 10 percentage points worse than OB could not be ruled out (p=0.200; 90% CI: −14%, 18%) (Table 3).
Sensory thresholds for first sensation significantly shortened, with no significant change in desire to defecate and urgency to defecate in both HB and OB groups. Difference between HB and OB in change in median sensory threshold was about 4 cc for first sensation, 8 cc for desire to defecate, and 21 cc for urge to defecate (Table 4).
Responder analysis showed that 68% (34/50) of patients who received HB responded to treatment when compared to 70% (35/50) who received OB. Non-inferiority of HB to at most 10 percentage points worse than OB could not be ruled out (p=0.193; percentage difference 90% CI: −17%, 13%) (Table 3).
Results of the per protocol analysis of the outcome measures are presented in Supplemental Tables S1, S2, and S3. Per protocol analyses showed similar findings as in the ITT analysis for the primary outcome measures of CSBM frequency, bowel satisfaction, and balloon expulsion time, and for all secondary subjective measures.
In the per protocol analysis, wherein subjects were excluded due to non-completion of assigned treatment, the per protocol physiologic outcomes in the HB group were relatively better than in the ITT analysis. Dyssynergia pattern was corrected in 92% receiving HB compared to 84% receiving OB therapy. Non-inferiority of HB to OB was shown with 8% less of HB having dyssynergia at the end of study (Table S2). Non-inferiority of HB to OB was also observed for all physiologic measures, except for slow transit with a 95% upper bound of +12% in the percentage difference with slow transit (90% CI: −21%, +12%). The difference between HB and OB for a change in median sensory threshold was larger in per protocol analysis with 95% upper limit for median difference of 4 cc for first sensation, 25 cc for desire to defecate, and 32 cc for urge to defecate. Responder analysis showed that 32/38 (84%) subjects in the HB group responded to treatment compared to 35/45 (78%) subjects in the OB group, with a responder percentage difference that was no smaller than 8% for the HB group (90% CI: −8%, 21%).
Overall both treatments were well tolerated, and patients found the training and instructions to be helpful and rewarding (Table 5). About 90% reported that they would recommend biofeedback therapy (Table 5). Significantly more patients in the HB group (p=0.008) reported that home training created social issues and device use could be messy. Likewise, significantly more patients in the OB group (p=0.03) had to make special arrangements to receive office treatment. Otherwise there were no differences suggesting that both treatments were comparable and satisfactory.
There was no device-related or procedure-related adverse event. As expected, few patients reported anal discomfort from probe placement. There were 2 serious adverse events unrelated to the study; one patient in the HB group was hospitalized for 3 days because of diabetic complications and one patient in the OB group had a leg fracture and was hospitalized for 2 days.
A cost-outcome analysis requires complete diaries and questionnaires and therefore could only be performed in patients who completed the trial (HB=38, OB=45). Due to the lack of normal distribution of data, Wilcoxon-rank sum test were used to evaluate the statistical significance. From a societal perspective, home biofeedback incurred a significantly lower (p<0.001) cost of $1081.70 (Q1 794.90, Q3 $1399.30) in contrast to the office biofeedback cost of $1942.50 (Q1 $1621.70, Q3 $2369.00), with a saving of $860.00 for HB, (Table 6).
Table 6.
Summary of treatment costs in each treatment arm:
| Computation | Cost (median, Q1,Q3) |
Computation | Cost (median, Q1,Q3) |
|
|---|---|---|---|---|
| Hospital costs | 6 sessions*HC per session | $732.78 | 4 sessions* HC per session | $0 |
| Physician costs | 6 sessions*PC per session | $625.50 | 4 sessions* PC per session | $90.00 |
| Equipment costs | 0 | $0 | $280 = $100 (device) + 1.5 (120/probe) | $244.00 |
| Home treatment | 0 | $0 | SPH* (estimated data)*0.5 | $185.80 ($112.30, $325.20) |
| Work loss due to appointments | 2.25 hrs.*6 sessions*SPH | $134.73 ($101.79, $357.21) | 1.75 hrs.*4 sessions*SPH | $142.20 ($79.17, $402.50) |
| Travel time costs | Round trip distance*6 sessions*SPH | $132.50 ($56.60, $345.60) | Round trip distance* 4 sessions*SPH | $149.40 ($71.50, $276) |
| Transportation costs | Round trip distance*6 sessions*0.35 | $203.70 ($48.50, $424) | Round trip distance*4 sessions*0.35 | $179.20 ($78.10, $259.80) |
| Total costs* | $1942.50 ($1621.70, $2369) | $1081.70 ($794.90, $1399.30) |
Pvalue (total costs): <0.001 SPH = salary per hour, taken from the Bureau of labor and statistics (2007) using patient’s job description and state of residence; HC = hospital costs; and PC = physician costs
DISCUSSION
In this randomized controlled parallel arm trial, we found that home biofeedback therapy was as efficacious as office biofeedback therapy and equivalently improved bowel function in subjects with chronic constipation and dyssynergic defecation. Patients who received HB had a significantly greater number of complete spontaneous bowel movements and greater satisfaction with bowel function when compared to their baseline. Likewise, patients who were randomized to OB also showed similar significant improvements in their bowel function including the number of CSBM/week and bowel satisfaction score.
We also found that there were 68% responders in the HB and 70% in the OB groups, and there was no difference between the two treatments. Unlike previous RCTs of biofeedback therapy9,10,11, here we used a more stringent and a new composite measure to define the treatment success. Because a diagnosis of dyssynergic defecation comprises of two key components, symptoms of constipation with difficult evacuation together with the presence of dyssynergic pattern on manometric testing4,13–15, any improvement in this condition is best assessed by the use of both symptoms and manometric changes. Consequently, here by using a composite responder criteria, we have demonstrated that biofeedback therapy whether administered at home or in an office setting were equally effective for the treatment of DD.
Biofeedback therapy also improved a number of subjective outcome measures; the overall (global) improvement in bowel satisfaction was significantly greater, and the stool straining effort significantly decreased in both groups when compared to baseline, although stool consistency did not change. Likewise, approximately one half of patients were using digital maneuvers to assist defecation at baseline, and after biofeedback its use decreased significantly to 12% and 10% respectively in HB and OB groups.
The improvement in bowel symptoms with HB or OB was equally matched by the improvement in anorectal and colonic function. The abnormal pattern of dyssynergic defecation was corrected (normalized) in 68% of patients in HB and 78% in OB groups, and in 92% and 86% of patients who completed the treatments. Likewise, the manometric indices of dyssynergic defecation such as the defecation index and the time taken to expel a balloon significantly improved when compared to baseline in both treatment arms. Over 60% of patients with DD have coexisting slow transit constipation5,7,24, and this was further confirmed here. Additionally, after treatment, the proportion of subjects with slow colonic transit time improved in both groups, but it was only significant in HB group, as there were fewer subjects with slow colon transit in OB group at baseline.
Overall these findings not only confirm reports of previous RCTs11 but provide new information that biofeedback therapy is equally efficacious when administered at home as solo treatment and not as adjunctive treatment to OB,25 and remedies both the subjective and physiologic dysfunctions in patients with dyssynergic defecation.
A post-treatment survey of patients who completed the treatments showed that 92% versus 80% would recommend biofeedback training and 87% versus 88% felt the training was rewarding in the HB and OB groups respectively. Although home device was felt to be messy by some, overall it was very well tolerated, with no adverse events.
Although HB treatment is equally efficacious as OB therapy, it was significantly less costly as measured from a societal and individual perspective. There was a clear saving of $860.80 per patient towards their overall health care costs, if biofeedback treatment was administered at home as opposed to an office setting. Furthermore, this assessment did not take into account all the other inconveniences involved with a hospital appointment including effects on quality of life and transportation/parking issues. Thus, HB appears to be a more cost-effective treatment option for patients with dyssynergic defecation. Also there are very few centers that offer office biofeedback treatment across USA and reimbursement remains problematic. Consequently, given the less costly option of HB, and the advances in tele-monitoring of care, this treatment modality can be immediately available to millions of patients with DD, who currently either do not have access to biofeedback or are unable to undergo this treatment because of costs, insurance, social and other constraints.
Although this is the first study to evaluate home biofeedback therapy in DD, our study has limitations including smaller sample size, and referral bias to a tertiary care center. Also, we had a higher screen failure and drop out rate, and the findings may not be generalizable to men, because only 4 men were enrolled in the study, but these observations are comparable to previous trials of biofeedback therapy10,11. This was a single-blinded RCT where there may be a risk of bias in subject selection, but consecutive patients were recruited and randomized to either study arm by a study coordinator using concealed allocation method, and the physician investigators were not involved with randomization. Also, the baseline subjective parameters were comparable between the two groups, and if anything patients randomized to HB group had fewer CSBMs/week (slightly worse disease), suggesting a bias is unlikely. Furthermore, biofeedback is a labor intensive program, and requires motivation and multiple hospital visits (many patients lived at least two hours away); these factors contributed to the variation in non-compliance and drop out rate. A more detailed cost-effectiveness analysis that includes quality of life domains and quality-adjusted life years, is currently being analyzed and will be reported separately.
In conclusion, home biofeedback therapy was equally efficacious as office biofeedback therapy, and both treatments were effective in relieving chronic constipation in 70% of patients. Significantly, HB is also less costly than OB, and hence should be the preferred treatment option for patients with dyssynergic defecation.
Supplementary Material
RESEARCH IN CONTEXT:
-
(1)
“Evidence before this study”: Dyssynergic defecation affects one third of patients with chronic constipation, and these patients generally do not respond to laxative therapy. Recently, randomized controlled trials have shown that office-based biofeedback therapy is useful for this condition, and both American and European Gastroenterology Societies have recommended this treatment for dyssynergic defecation. However, office-biofeedback therapy is only performed in limited centers, and not widely available, in part because it is labor intensive, requires skilled personnel and multiple office visits. Whether a home-based biofeedback therapy is useful in the management of patients with chronic dyssynergic defecation has not been assessed.
-
(2)
“Added value of this study”: This study is one of the largest randomized controlled trials in a well-characterized cohort of patients with dyssynergic defecation. A new portable biofeedback device, and probe that can be used for this training at home were developed and tested in this trial. Also, because a diagnosis of dyssynergic defecation requires both symptoms and manometric criteria for diagnosis, both subjective and objective outcome metrics were assessed alongside a robust composite measure of defining responders in this trial. Also, this is the first RCT to assess the safety of a home device and perform a comparative assessment of the tolerability of home and office-based biofeedback therapies. Also, with rising health care costs, aside from efficacy, treatments must be cost effective. Whether, a home-based biofeedback therapy is efficacious and cost-effective when compared to an office-based biofeedback therapy program is not known.
-
(3)
“Implications of all the available evidence”: We found that home biofeedback therapy was as effective as office biofeedback therapy (non-inferior) in remedying both the bowel symptoms associated with constipation, and in correcting the problem of dyssynergic defecation in 70% of patients. The home device was well tolerated without adverse events. Significantly, home biofeedback therapy was less costly than office biofeedback therapy with a saving of $860.00. These findings should help guide future studies in the field regarding objective metrics for the assessment of clinical outcome. Biofeedback therapy is efficacious and safe for the treatment of patients with dyssynergic defecation, and given the ease of administering the home biofeedback treatment and cost implications, this should be the preferred treatment approach for these patients. A home-based program could significantly broaden the availability and use of this treatment modality for millions of patients suffering with this condition.
Acknowledgement:
This work was supported by NIH grant RO1 DK 57100–05 and grant RR00059 from the General Clinical Research Centers program, National Center for Research Resources. The study was conducted at the University of Iowa and data analysis and manuscript writing was completed at Augusta University and University of Iowa. We sincerely acknowledge the expert assistance of Mrs. Mary Stessman, RN, and M/s Kara Seaton with anorectal physiology tests and biofeedback therapy, the data extraction, and statistical analysis support of Mr Kice Brown, and the cost effectiveness design and analysis performed by Dr John Schneider, Health Economics Consulting group LLC, Morristown, NJ and Dr Jorge Go, and the superb secretarial support of Mrs. Helen Smith.
Footnotes
ClinicalTrials.Gov: Registered at Clinical trials.gov no NCT03202771.
Conflicts of Interest: All authors declare no conflicts of interests with this study.
REFERENCES
- 1.Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004;99:750–9. [DOI] [PubMed] [Google Scholar]
- 2.Rao SSC, Camilleri M. Clinical approach to constipation In Yamada’s Text Book of Gastroenterology, 6th Edition, Edited by Podolsky D et al. , Chapter 42, John Wiley & Sons, Ltd; 2016. Pp 757–780. [Google Scholar]
- 3.Mertz H, Naliboff B, Mayer E. Physiology of refractory chronic constipation. Am J Gastroenterol 1999;94:609–15. [DOI] [PubMed] [Google Scholar]
- 4.Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology 2013;144:211–17. [DOI] [PubMed] [Google Scholar]
- 5.Grotz RL, Pemberton JH, Talley NJ, Rath DM, Zinsmeister AR. Discriminant Value of Psychological Distress, Symptom Profiles, and Segmental Colonic Dysfunction in Outpatients with Severe Idiopathic Constipation. Gut 1994;35:798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surrenti E, Rath DM, Pemberton JH, Camilleri M. Audit of Constipation in a Tertiary Referral Gastroenterology Practice. Am J Gastroenterol 1995;90:1471–5. [PubMed] [Google Scholar]
- 7.Rao SSC, Welcher KD, Leistikow JS. Obstructive defecation: A failure of rectoanal coordination. Am J Gastroenterol 1998;93:1042–50. [DOI] [PubMed] [Google Scholar]
- 8.Halligan S, Thomas J, Bartram C. Intrarectal Pressures and Balloon Expulsion Related to Evacuation Proctography. Gut 1995;37:100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiarioni G, Whitehead WE, Pezza V, Morelli A, Bassotti G. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006;130:657–64. [DOI] [PubMed] [Google Scholar]
- 10.Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol 2007;5:331–8. [DOI] [PubMed] [Google Scholar]
- 11.Rao SS, Benninga MA, Bharucha AE, Chiarioni G, Di Lorenzo C, Whitehead WE. ANMS-ESNM position paper and consensus guidelines on biofeedback therapy for anorectal disorders. Neurogastroenterol Motil 2015;27:594–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longstretch GF, Thompson WG, Chey WD et al. Functional bowel disorders. Gastroenterology 2006;130:1480–91. [DOI] [PubMed] [Google Scholar]
- 13.Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology 2006;130:1510–8. [DOI] [PubMed] [Google Scholar]
- 14.Rao SSC, Mudipalli RS, Stessman M, Zimmerman B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus). Neurogastroenterol Motil 2004;16:589–96. [DOI] [PubMed] [Google Scholar]
- 15.Rao SSC, Dyssynergic Defecation and Biofeedback Therapy In Disorders of the Pelvic Floor and Anorectum. (Edited by Rao SSC) W.B. Saunders Company/Elsevier Inc., Philadelphia: 2008, pp 569–586. [Google Scholar]
- 16.Rao SS, Hatfield R, Soffer E, Rao S, Beaty J, Conklin JL. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol 1999;94:773–83. [DOI] [PubMed] [Google Scholar]
- 17.Rao SSC, Azpiroz F, Diamant N, Enck P, Tougas G, Wald A. Minimum standards of anorectal manometry. Neurogastroenterol Motil 2002;14:553–9. [DOI] [PubMed] [Google Scholar]
- 18.Evans RC, Kamm MA, Hinton JM, Lennardjones JE. The Normal Range and a Simple Diagram for Recording Whole Gut Transit-Time. Int J Colorectal Dis 1992;7:15–7. [DOI] [PubMed] [Google Scholar]
- 19.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920–4. [DOI] [PubMed] [Google Scholar]
- 20.Rao SSC, Welcher KD, Pelsang RE. Effects of biofeedback therapy on anorectal function in obstructive defecation. Digest Dis Sci 1997;42:2197–205. [DOI] [PubMed] [Google Scholar]
- 21.Rao SSC. Biofeedback therapy for constipation in adults. Best Pract Res Cl Ga 2011;25:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelsang RE, Rao SSC, Welcher K. FECOM: A new artificial stool for evaluating defecation. Am J Gastroenterol 1999;94:183–6. [DOI] [PubMed] [Google Scholar]
- 23.Rao SSC, Ozturk R, Laine L. Clinical utility of diagnostic tests for constipation in adults: A systematic review. Am J Gastroenterol 2005;100:1605–15. [DOI] [PubMed] [Google Scholar]
- 24.Karlbom U, Pahlman L, Nilsson S, Graf W. Relationships between Defecographic Findings, Rectal Emptying, and Colonic Transit-Time in Constipated Patients. Gut 1995;36:907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heymen S, Wexner SD, Vickers D et al. Prospective randomized trial comparing four biofeedback techniques for patients with constipation. Dis Colon Rectum 1999; 42:1388–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.