Abstract
The hormone estrogen is involved in both female and male reproduction, as well as numerous other biological systems including the neuroendocrine, vascular, skeletal, and immune systems. Therefore, it is also implicated in many different diseases and conditions such as infertility, obesity, osteoporosis, endometriosis, and a variety of cancers. Estrogen works through its two distinct nuclear receptors, Estrogen Receptor alpha (ERα) and Estrogen Receptor beta (ERβ). Various transcriptional regulation mechanisms have been identified as the mode of action for estrogen, mainly the classical mechanism with direct DNA binding but also a non-genomic mode of action and one using tethered or indirect binding. The expression profiles of ERα and ERβ are unique with the primary sites of ERα expression being the uterus and pituitary gland and the main site of ERβ expression being the granulosa cells of the ovary. Mouse models with knockout or mutation of Esr1 and Esr2 have furthered our understanding of the role each individual receptor plays in physiology. From these studies, it is known that the primary roles for ERα are in the uterus and neuroendocrine system, as female mice lacking ERα are infertile due to impaired ovarian and uterine function, whereas female mice lacking ERβ are subfertile due to ovarian defects. The development of effective therapies for estrogen-related diseases has relied on an understanding of the physiological roles and mechanistic functionalities of ERα and ERβ in various human health and disease.
Keywords: Estrogen, estrogen receptor, ER, ERα, ERβ
Introduction
Our understanding of mechanisms by which hormones act has evolved since the first description over 100 years ago; especially, regarding the role of nuclear receptors and receptor-mediated signaling first proposed by Jensen over 50 years ago (Jensen, 1962; Jensen and Jacobson, 1960, 1962). Our knowledge of cellular mechanisms from the initial concepts of ligand receptor binding, activation, direct DNA binding and resulting gene regulation now includes non-DNA binding or tethering, cellular non-genomic signaling and receptor mediated non-ligand hormone activities (Hewitt et al., 2016). Estrogen, one of the first hormone substances identified, was thought to have only female-selective activities important in female reproduction. We now know, however, that estrogen is also involved in male reproduction and in numerous other systems including the neuroendocrine, vascular, skeletal, and immune systems of both males and females. Estrogen influences many physiological processes, as it is also implicated in many different diseases including obesity, metabolic disorder, a variety of cancers, osteoporosis, lupus, endometriosis, and uterine fibroids (Burns and Korach, 2012; Deroo and Korach, 2006).
Cell Mechanisms
It is now accepted that the predominant mechanism of estrogen action is through nuclear estrogen receptor (ER) expression in estrogen target organs (Mangelsdorf et al., 1995). For many years it was thought there was only a single estrogen receptor (ERα), but a second form of estrogen receptor (ERβ) was later discovered (Kuiper et al., 1996). The biological effects of estrogen, described later in this chapter, are mediated through these two distinct ER proteins, ERα and ERβ. Separate genes on non-homologous chromosomes encode these receptors; the expression profiles of which are quite different across tissues and cell types. The predominant expression tissues for ERα include: uterus and pituitary gland with the highest levels, liver, hypothalamus, bone, mammary gland, cervix and vagina. ERβ expression on the other hand is expressed in fewer tissues by most analyses, but tissues with predominant levels include ovary, lung, and prostate (Couse et al., 1997). ERβ expression is especially high in the ovary and is found exclusively in the granulosa cells. Many therapeutic interventions to estrogen related diseases target the functions of ERα and ERβ. Such therapeutic approaches highlight the importance of understanding the physiological role of ERα and ERβ in tissues and their in vivo mechanistic functionality to identify effective treatments and minimize side effects.
The estrogen receptors are members of the nuclear receptor superfamily of hormone receptors, and are composed of several main structural features that are consistent in these proteins (Aagaard et al., 2011). All members of this superfamily are comprised of four structural and functional domains: an amino-terminal domain (A/B-domain), a DNA binding domain (DBD; C-domain), a hinge region (D-domain), and a ligand-binding domain (LBD; E-domain). The ERs have an additional fifth domain: the carboxyl-terminal domain (F-domain) whose function is still unknown (Mangelsdorf et al., 1995). In the case of ERα and ERβ the C and E domains carry a high-degree of homology between the two forms, however the A/B, D and F domains are divergent (Germain et al., 2006; Mangelsdorf et al., 1995). The A/B-domain contains the transcription activation function 1 (AF-1) which is reported to be important for ligand-independent transactivation (Bourguet et al., 2000). The LBD or E-domain of ERs contains the transcription activation function 2 (AF-2) that is important for ligand-dependent transcriptional regulation (Bourguet et al., 2000). Helix 12 is a highly conserved region within the LBD and is the core of the AF-2 functionality. The structural configuration of helix 12 is changed by ligand binding resulting in either an active (agonist bound) or inactive (antagonist bound) form for the transcription regulation (Green and Carroll, 2007; Klinge, 2000).
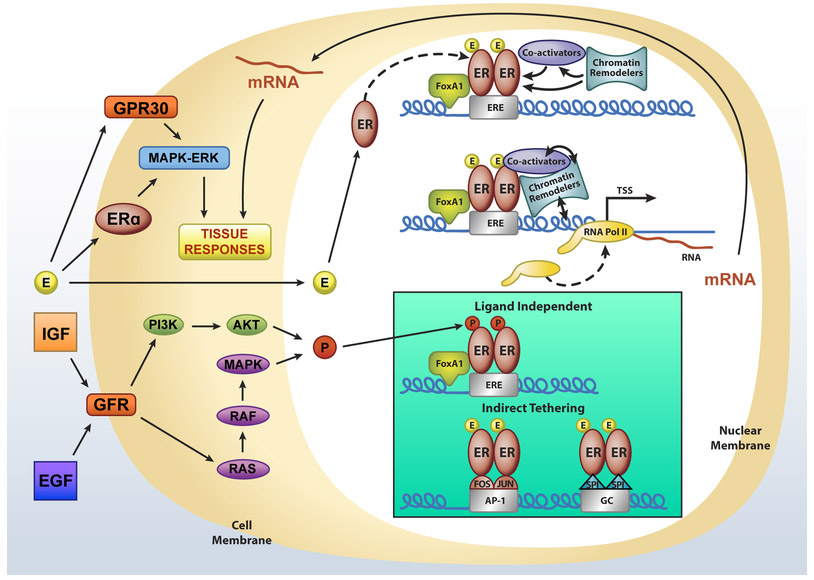
Estrogen works through several possible cellular mechanisms to mediate its biological responses as shown in Figure 1. These include two major cellular actions involving the receptors: rapid non-genomic effects and genomic activities (Hewitt et al., 2016). Several studies, primarily in cell culture, have shown these rapid actions occur within minutes of hormone treatment and can be silenced by inhibition of either the MAPK/ERK or AKT signaling pathways (Clark et al., 2014; Kelly and Levin, 2001). Activation of these intracellular signaling pathways has been shown to involve a plasma membrane-associated process that is mediated by either a G-protein coupled receptor, GPER1 (originally designated GPR30), or a caveolin-associated form of ERα (Levin, 2015; Prossnitz and Hathaway, 2015). Estrogen signaling at the membrane solely involves ERα and is shown to require palmitoylation at cysteine 447 (human) or cysteine 451 (mouse) (Levin, 2015). Mutation of this cysteine in mouse models results in differing phenotypes, effects, and mechanistic interpretations (Adlanmerini et al., 2014; Pedram et al., 2014). How significantly non-genomic signaling contributes to genomic actions of ERα and the biology of hormone responsiveness is still not totally resolved and requires continued study.
Figure 1. Cellular Mechanisms of Estrogen Action.
Model of nuclear and non-nuclear estrogen receptor action. Estrogen (E circles) and estrogen receptor (ER) complex binds directly to the regulatory DNA elements (estrogen responsive element (ERE) recruiting additional factors involved in transcriptional regulation. ER can also bind indirectly through a tethering mechanism to AP1 or Sp1 binding sites (GC) to regulate transcription. Growth factors (IGF, EGF) can phosphorylate ER through membrane growth factor receptor (GFR) mediated intracellular signaling pathways (P circles) to regulate gene expression in the absence of ligand (nuclear action). Estrogen also binds and activates membrane ERα or GPR30, inducing the intracellular signaling pathway (non-nuclear action) that is rapid.
Three major genomic ER-mediated transcriptional regulation mechanisms have been characterized. These include the direct binding to regulatory elements of DNA (classical), indirect binding to other existing transcription factors which are bound to DNA (tethering), and ligand-independent receptor activation, which is proposed to involve altered phosphorylation of sites on the receptor protein. Examples of each of these genomic modes of action for estrogen and ER have been published, although most studies have investigated the activity primarily involving ERα. In the first example, hormone-ER binding causes a conformational change in the LBD, allowing helix 12 to accept coactivator interactions. Coactivator binding is required for the resulting genomic response, and is directly proportional to the amplitude of this response. In the absence of hormone, ERα is bound to DNA in an inactive state, as shown in both cell culture and in vivo mouse studies by ChIP-Seq. (Carroll et al., 2005; Hewitt et al., 2012). Hormone binding increases the number of binding peaks in the genome. Mouse models in which the DBD of ERα is mutated indicate that direct DNA binding is required to elicit hormone responses and biological activity. Further research will determine whether it is the only activity that is required or if complementary actions of other signaling mechanisms (Ahlbory-Dieker et al., 2009; Hewitt et al., 2014). As shown in Figure 1, other nuclear factors influence direct binding such as a pioneering factor FoxA1, which is bound at sites to allow the recruitment of chromatin remodeling proteins, opening the chromatin to give ER accessibility to its regulatory DNA sites. Following the assembly of the ER transcription complex, composed of a multitude of components (Carroll and Brown, 2006) gene transcription is initiated by recruitment of polymerase II. A second method, (shown in figure 1 Box) primarily described in cell culture is the indirect or tethered mechanism of hormone receptor action in which the hormone receptor modulates gene expression by protein-protein interactions with existing transcription factors (eg. Fos/jun), which bind directly to their respective response elements (AP1) (Jakacka et al., 2001). Other examples of regulatory elements have included binding to factors in Sp1 sites in GC rich regions of DNA (Kushner et al., 2000). Lastly, ER can have regulatory activities and drive hormone responses in the absence of hormone through ligand-independent activation by growth factors or other intracellular signaling pathways, thought to involve phosphorylation of certain serine residues on the receptor (Smith, 1998). Such a coupling of non-genomic and genomic signaling may be an explanation for the complementation of different cellular signaling pathways, which elicit the broad spectrum of hormone responses of estrogen action.
Uterine Estrogen Response
The ovariectomized (ovexed) mouse uterus is an estrogen responsive organ and therefore a valuable model to study ER-mediated responses. The rodent uterus is a bicornuate tube made up of outer muscle cell layers (myometria), an inner lumen lined with luminal epithelial cells, and a layer of stroma cells between the lumen and myometrium. The uterus also contains glandular structures that are lined with epithelial cells. The endogenous stimulation of the uterus occurs during the transient proestrus surge of the reproductive cycle. Experimentally, a single injection of estrogen can mimic this stimulation by being administered to an ovexed animal, and the uterus will then undergo a series of ordered, well-characterized events that can be divided into an initial (early) phase and subsequent (late) responses culminating specifically in waves of mitosis restricted to only uterine epithelial cells. These responses are mediated by ERα, which is expressed in all uterine cells (luminal and glandular epithelial, stromal and myometrial cells).
Studies using the ovexed mouse uterine model have examined the regulation of endogenous uterine genes over a 24-hour stimulatory time course, and shown that the gene regulation pattern follows the progression of early (within 2 hours after estrogen was administered) or late (occurring 12–24 hours after estrogen was administered) events. Some gene regulation was seen at intervening time points, but most fall within either the early or late clusters (Hewitt et al., 2003). In experimental analysis of estrogen responsive uterine genes, it is apparent that samplings at 2 or 24-hour time points will represent most of the observed gene responses correlating with tissue physiological actions
Interaction of ERα and RNA polymerase II (PolII) were analyzed using ChIP-seq in uterine tissue (Hewitt et al., 2012) to understand ERα DNA binding within an in vivo system. In vehicle-treated unstimulated samples, more than 5000 peaks were mapped indicating ERα was already bound to the chromatin in the absence of hormone (Hewitt et al., 2012). Estrogen treatment increased the amount of ERα binding at these sites, and also led to ERα binding to additional regions (Hewitt et al., 2012) with a total of more than 17000 sites. The number of active annotated genes (with PolII at the transcription start site (TSS)) within 100 KB of ERα peaks increased from 4672 (1.1 ERα peaks/active gene) to 6519 (2.6 ERα peaks per active gene) thereby showing that in the absence of hormone, ERα is bound to DNA sites, and that hormone treatment increased the number 2.4-fold. Analysis of the ER binding sites for transcription factor binding motifs revealed that ERE motifs were present in 35% of the vehicle sites and were more abundant (59%) in the estrogen-treated sites (Hewitt et al., 2012). Thus, ERE motifs are important for estrogen-dependent ER recruitment. The computed consensus motif for the sequences bound to ERα in uterine chromatin matched the experimentally derived ERE (GGTCAnnnTGACC) (Hewitt et al., 2012), indicating preference for this motif in a biological system. Interestingly, at the sites that were not enriched for ERE motifs, numerous other motifs were seen; notably homeobox (Hox) motifs were highly enriched (Hewitt et al., 2012). Many Hox family members are expressed in the uterus (Hewitt et al., 2012), and Hoxa10 and Hoxa11 have been demonstrated to play key roles in uterine function (Eun Kwon and Taylor, 2004). ERα binding in the uterine tissue was primarily distal from promoters (Hewitt et al., 2012), which has similarly been observed in ERα ChIP-seq in MCF-7 cells. When comparing ChIP-seq data to microarray profiles, up-regulated transcripts at early time points (2h, 6h) were significantly more likely to have ERα binding at their promoters (0 to 10 kb 5’) than down-regulated genes (Hewitt et al., 2012).
Genetic Control of Estrogen Responses
Differences in uterine estrogen sensitivity of two mouse strains, C57Bl6 (more responsive uterus) and C3H (less sensitive uterus) have been mapped to associated quantitative trait loci (QTL) (Roper et al., 1999). Uterine transcriptional profiles of C57Bl6 and C3H mice (basal or 2 or 24 hours after estrogen treatment) include response differences correlated with the QTL on chromosomes 4, 5, 11 and 16 (Wall et al., 2013). For example, Ngfr is in the chromosome 11 QT locus, and its transcript is expressed at a 3-fold higher level in ovexed untreated C57Bl6 than in ovexed untreated C3H uterine samples. Uterine NGF signaling has been shown to impact pregnancy (Hah and Kraus, 2014). Runx1, which was within the chromosome 16 QT locus, and can enhance estrogen responses (Chimge and Frenkel, 2013), was shown to be present at higher levels in C57Bl6 than C3H uterine epithelial cells (Wall et al., 2013). Transcripts that showed strain-selective differences indicated C3H-selective enrichment of apoptosis, consistent with increase in the apoptosis indicator Casp3, and decrease in the apoptosis inhibitor Naip1 (Birc1a) in C3H vs. C57Bl6 following treatment with estrogen (Wall et al., 2013). Mammary gland response differences were also examined (Wall et al., 2014), where an opposite strain sensitivity observation was reported (C3H more sensitive than C57Bl6). Strain-selective transcripts were identified in the mammary samples as well. Most interesting was the opposite pattern of Runx1 expression, with higher levels in C3H than C57Bl6 mammary epithelia, a pattern consistent with higher estrogen sensitivity of C3H mammary glands (Wall et al., 2014). Understanding differences in sensitivity to estrogen is important in understanding genetic contributions to the impact of xenoestrogens on populations of exposed humans and wildlife.
ERα Mutations Demonstrate Uterine Mechanisms
Since ERα is detected in all uterine cells, deletion or mutation of the receptor is expected to have a profound impact on 17β-estradiol (E2) -mediated responses. Mice with ERα deletion or mutation are therefore an optimal biological model in which to dissect details of ERα mechanisms (Table 1). Mice that lack ERα (Esr1−/−; aka αERKO) develop a hypoplastic uterus that includes all uterine cell types and structures, however there is no uterine maturation or growth at puberty, and no response to E2 (Couse and Korach, 1999). Since the female reproductive tract is composed of several tissue types, all expressing ERα, identifying the cell type selectivity of ERα activity related to biological responses is critical. This cell specificity becomes of particular significance when comparing the varying physiological responses of the uterus to its inherent functions involving proliferative to luteal secretory responses and implantation (Wang and Dey, 2006) and the development of diseases, including endometriosis, cystic endometrial hyperplasia, fibroids and endometrial cancer. More specifically, in contrast to adults, neonates and prepubertal experimental animals exhibit both stromal and epithelial tissue proliferation under E2 stimulation, while in adults, E2 selectively stimulates growth only in epithelial cells with the stroma remaining quiescent (Quarmby and Korach, 1984). Two major concepts have been published to explain the mitogenic mechanisms for the E2 activity; one involves direct estrogen action through ER in the epithelial cell, and the second regards a paracrine mechanism of direct stimulation of E2/ER in stromal cells to induce a mitogenic signal (eg. growth factor) on the epithelium. To identify the specific epithelial responses to E2, uterine epithelial-specific ERα knockout mice (Wnt7aCre/+;Esr1f/f or referred to as “epithelial ER cKO”) were generated by crossing Esr1-floxed mice (Hewitt et al., 2010a) with Wnt7aCre/+ mice (Huang et al., 2012). Using the epithelial ER cKO mouse model, it has been shown that ERα in uterine epithelium is dispensable for epithelial growth response to E2. This finding supports mediation by stromal mitogenic paracrine factors, such as IGF-1. However, epithelial ERα is required for a full growth response of endometrial hyperplasia by actively inhibiting epithelial apoptosis in the uterus (Winuthayanon et al., 2010). To dissect the mediators of epithelial ERα response during uterine transcription, microarray analysis was performed to evaluate the differentially expressed genes in the presence (WT control littermates, referred to as WT) or absence (epithelial ER cKO) of epithelial ERα after E2 treatment for 2 h or 24 h in ovexed adult females (Winuthayanon et al., 2014). RNA microarray analysis revealed approximately 20% of the genes differentially expressed at 2 h were epithelial ERα-independent, as they were preserved in the epithelial ER cKO uteri. This indicates that regulation of the early uterine transcripts mediated by stromal ERα is sufficient to promote initial proliferative responses. However, more than 80% of the differentially expressed transcripts at 24 h were not regulated in the epithelial ER cKO uteri, indicating most late transcriptional regulation required epithelial ERα, especially those involved in mitosis. This shows that loss of regulation of these later transcripts results in the blunted subsequent uterine growth after 3 days of E2 treatment. These transcriptional profiles at 2 and 24 h of E2 treatment correlate with previously observed biological responses, in which the initial proliferative response (at 24 h E2 treatment) is independent of epithelial ERα and thus dependent on stromal ERα, yet epithelial ERα is essential for subsequent maintenance of tissue responsiveness during 3 days of E2 treatment.
Table 1.
Uterine Phenotypes of Estrogen Receptor Mutants
| Gene | Mutation | Nick-names | Uterine phenotypes | References |
|---|---|---|---|---|
| Esr1 | Homozygous null for ERα | αERKO, Ex3αERKO | Normal uterine development but exhibits hypoplastic uteri. Insensitive to the proliferative and differentiating effects of endogenous E2, growth factors and exogenous E2. Implantation defect. *lack decidualization. Infertile. |
(Antonson et al., 2012b; Curtis Hewitt et al., 2002; Curtis and Korach, 1999; Dupont et al., 2000; Hewitt et al., 2010a; Lubahn et al., 1993b) |
| Esr1 | One mutated allele of two-point mutation in ERα DBD (E207A, G208A) and one WT allele |
NERKI+/− ERAA/+ |
Normal uterine development but exhibits hyperplastic uteri. Hypersensitive to estrogen. Infertile. |
(Jakacka et al., 2002a) |
| Esr1 | One mutated allele of two-point mutation in DNA binding domain of ERα (E207A, G208A) and one ERα null allele | ERα KIKO, ERAA/− | Normal uterine development. Insensitive to the proliferative effects of exogenous E2 treatment. Infertile. |
(Hewitt et al., 2010b; O’Brien et al., 2006) |
| Esr1 | 4-point mutation of DBD ERα (Y201E, K210S, K214A, and R215A) |
ERαEAAE/EAAE | Normal uterine development but exhibits hypoplastic uteri. Loss of E2-induced uterine transcripts. Infertile. |
(Ahlbory-Dieker et al., 2009) |
| Esr1 | Deletion of amino acids 2-128 including AF2 domain of ERα | ERαAF-10 | Normal uterine development and architecture. Blunted E2 response. Infertile. |
(Abot et al., 2013; Billon-Gales et al., 2009) |
| Esr1 | Deletion of amino acids 543-549 in LBD/AF-2 of ERα | ERαAF-20 | Normal uterine development but exhibits hypoplastic uteri. Insensitive to E2 treatment. Infertile. |
(Billon-Gales et al., 2011) |
| Esr1 | Two-point mutation in LBD/AF-2 of ERα (L543A, L544A) | AF2ERKI/KI | Normal uterine development but exhibits hypoplastic uteri. Insensitive to E2 treatment. ER antagonists and partial agonist (ICI 182,780 and TAM) induced uterine epithelial proliferation. Growth factor did not induce the uterine epithelial cell proliferation. Infertile. |
(Arao et al., 2011a) |
| Esr1 | Point mutation in LBD of ERα (G525L) | ENERKI ERαG525L | Normal uterine development but exhibits hypoplastic uteri. Insensitive to E2 treatment. Synthetic estrogens PPT and DES induce uterine growth. IGF-1 induced patchy uterine epithelial growth. Infertile. |
(Sinkevicius et al., 2008) |
| Esr1 | Female reproductive tract epithelial cell specific deletion of ERα |
Wnt7aCre+;Esr1f/f
Epi ERα cKO WEd/d |
Normal uterine development. Sensitive to E2- and growth factors-induced epithelial cell proliferation. Selective loss of E2-target gene response. Implantation defect. Decidualization defect. Infertile. |
(Pawar et al., 2015; Winuthayanon et al., 2014; Winuthayanon et al., 2010) |
| Esr1 | Uterine specific deletion of ERα | PgrCreCre+;Esr1f/fERα Ut cKO Esrd/d | Normal Uterine development. Insensitive to E2. Decidualization defect. Infertile. |
(Pawar et al., 2015) |
| Esr1 | Point mutation of ERα palmitoylation site (C541A) | C451A-ERα, NOER (nuclear-only ERa) |
C451A-ERα: normal uterine development, E2 growth response NOER: hypoplastic ERα-null like uterus |
(Adlanmerini et al., 2014; Pedram et al., 2014) |
| Esr1 | LBD of ERα fused with multiple palmitoylation sites from the neuromodulin protein | MOER | Normal uterine development but exhibits hypoplastic uteri. | |
| Esr2−/− | Homozygous null for ERβ | Esr2−/− (βERKO, Ex3βERKO, **ERβSTL−/L− | Exhibit grossly normal uterine development and function. Sensitive to E2 treatment. Some Esr2−/− lines reported elevated uterine epithelial proliferation after E treatment. |
(Antal et al., 2008a; Dupont et al., 2000; Krege et al., 1998b; Wada-Hiraike et al., 2006a) (Binder et al., 2013) |
| Esr1 and Esr2 | Homozygous null for both ERα and ERβ | αβERKO | Normal uterine development but exhibit hypoplastic uteri, similar αERKO. Insensitive to E2, infertile | (Couse et al., 1999b; Dupont et al., 2000) |
In addition to uterine response to E2, epithelial ER cKO females were infertile partly due to an implantation defect (Winuthayanon et al., 2010). In addition, they fail to decidualize (Pawar et al., 2015). The role of epithelial ERα during implantation was examined using a uterine receptivity model that has been previously published (Tong and Pollard, 1999) by treating the mice with a series of E2 and P4 injections to mimic the hormonal profile during implantation. E+Pe treatment significantly increases uterine weight in wildtytpe (WT) females, as well as proliferation of stromal cells, but not epithelial cells. In epithelial ER cKO uteri, treatment with E+Pe showed a dampened uterine weight increase when compared to the WT treated group (Winuthayanon et al., 2014), and a slight decrease in stromal cell proliferation. However, epithelial cell proliferation was significantly higher in epithelial ER cKO compared to WT uteri. This suggests that lack of uterine epithelial ERα does not affect stromal cell proliferation but leads to an inability to appropriately arrest epithelial cell proliferation, a key requirement for embryo attachment and implantation. Additionally, leukemia inhibitory factor (Lif) (Stewart et al., 1992) and indian hedgehog (Ihh) (Lee et al., 2006), both required for uterine receptivity and induced in WT uteri, were not induced in the epithelial ER cKO samples, confirming induction of these factors requires epithelial ERα. Comparable expression of PR is seen in WT and epithelial ER cKO uteri using immunohistochemical analysis. Moreover, expression of HAND2, a PR-regulated transcription factor expressed in uterine stromal cells during implantation (Li et al., 2011), showed a similar pattern in the epithelial ER cKO uteri and WT. This indicates that the expression of PR and its downstream effector (HAND2) in the stromal cells were not disrupted by a lack of epithelial ERα. In summary, loss of epithelial ERα disrupted progesterone’s ability to inhibit E2-induced epithelial cell proliferation, but did not affect uterine stromal cell proliferation. Understanding the ERα epithelial cell-specific mechanisms and gene responses for controlling cell growth in the uterus is informative towards understanding a basis for uterine diseases such as endometriosis and endometrial cancer.
Tethered pathway analysis using DNA binding deficient ERα mutants
Studies using in vitro cell culture based models have indicated that estrogen responsive genes that lack the canonical ERE sequence can interact with estrogen receptors via a tethering mechanism whereby ER is recruited by AP1 or SP1 bound to their respective response elements. (Jakacka et al., 2001; Kushner et al., 2000; O’Lone et al., 2004; Safe, 2001) (Figure 1). To study the relative biological roles for tethered and ERE DNA binding mechanisms in vivo, two different ERα “knock in” mouse models have been created that have mutations of the first zinc finger of the ERα DNA binding domain. Both DNA binding mutations were designed to prevent ER-ERE binding, while retaining the ability to regulate genes via the tethered pathway (Ahlbory-Dieker et al., 2009; Jakacka et al., 2001). The first mouse model was referred to as the “nonclassical ER knock in” (Nerki) (Jakacka et al., 2002a). Female mice heterozygous for this mutation are infertile due to ovarian and uterine pathologies (Jakacka et al., 2002a); however, by intercrossing with the Esr1−/− global knockout line, a mouse possessing one copy each of the Nerki ERα allele and one copy of the null ERα allele has been generated (O’Brien et al., 2006) thereby expressing the Nerki mutant as its only ERα protein. The Nerki/αERKO (Esr1AA/-; aka KIKO) uterus is not hypoplastic, however it resembles the Esr1−/− in that estrogen fails to elicit uterine weight increase or cell proliferation (Hewitt et al., 2010b; O’Brien et al., 2006). Microarray comparison of transcripts after estrogen treatment indicated the KIKO uterus retains some of the gene regulation (~24%) also seen in the WT uterus (Hewitt et al., 2009). WT vs. KIKO differentially regulated genes in this microarray profile were enriched for components of the Wnt/Ctnnb signaling pathway as transcripts for Wnt ligands, receptors, transducers and targets were misregulated by E2 in KIKO vs. WT uteri (Hewitt et al., 2009).
Microarray and later ChIP-seq analysis (Hewitt et al., 2014) also showed unexpected results, which were the appearance of numerous estrogen regulated responses in the KIKO that were not observed in normal WT uteri. Evaluation of the KIKO uterine cistrome by ERα ChIP-seq revealed that these transcripts result from an unanticipated “gain of function” of the KIKO DNA binding mutation. Analyses of the sequences bound to KIKO ERα revealed enrichment of hormone response element (HRE) DNA motifs, which typically bind androgen, progesterone and glucocorticoid receptors (AR, PR, GR). Further in vivo and in vitro analyses have shown that the KIKO ERα binds HRE DNA and regulates uterine genes that are normally PR targets (Hewitt et al., 2014), indicating this particular ERα mutation has an aberrant binding activity with loss of ERE binding but a gain of HRE binding and gene regulation (Hewitt et al., 2014). The KIKO ERα was created by introducing two point mutations (E207A G207A) at the base of the first zinc dinger of the DBD. These positions in the PGR, AR and GR are occupied by GS. However, modeling the interaction between ERα and ERE based on structural studies revealed the critical importance of E207 in forming a hydrogen bond with the G/C nucleotides in an ERE at position 2/12 (GGTCAnnnTGACC). Additionally, attempting to interact with the T/A found at the equivalent position of an HRE (GAACAnnnTGTTC) results in significant steric clash, thus excluding ERα/HRE binding. However, by replacing the critical E207 residue with A, the hydrogen binding with ERE and the exclusion of HRE are lost, leading to an ability to interact with HRE (Hewitt et al., 2014)
The second DNA binding mutant ERα mouse model (EAAE), has an ERα that does not bind to HRE or ERE motifs either in vitro or in vivo (Hewitt et al., 2014), but retains AP1 mediated gene induction (Hewitt et al., 2014). It was created by introducing 4 point mutations (Y201A, K210A K214A, R215E) in the first zinc finger and between the first and second zinc finger. Since EAAE ERα maintains the critical E207 residue, it lacks aberrant HRE binding seen with the KIKO ERα. The EAAE mouse uterus is hypoplastic and refractory to estrogen responses, like the αERKO, indicating that the tethering HRE mechanism is not a major physiological regulatory response in the uterus. Microarray profiling of uterine RNA indicated a lack of estrogen responsive transcripts (Hewitt et al., 2014). Altogether, this shows that DNA binding activity of the ERα is critical for uterine function and estrogen response, and DNA binding-independent activity appears to have little role on its own, but may complement the direct DNA binding activity in eliciting the full uterine hormone response.
Induction of IGF1 signaling by E2 is known to be a major mediator of uterine growth in a paracrine manner, whereby uterine stromal cells secrete IGF1, which then stimulates epithelial cell growth (Adesanya et al., 1999; Cunha et al., 2004; Zhu and Pollard, 2007). One major surprising observation in the KIKO and EAAE models was the inability of estrogen to increase the transcript of insulin-like growth factor 1 (Igf1), which had been reported to be regulated via interaction between ERα to AP1 motifs in the Igf1 promoter via tethering. Analysis of Igf1 genomic sequences indicated that the AP1 motif previously identified using the chicken Igf1 gene is absent in mammals. Several potential ERE sequences were identified and tested for ERα binding by ChIP and gel shift (Hewitt et al., 2012; Hewitt et al., 2010b). ChIP-PCR analysis confirmed ER bound to specific ERE sequences of the Igf1 in WT but not KIKO uteri. Interestingly, exogenous treatment with IGF-1 did not restore KIKO uterine growth, indicating additional ERE mediated responses are needed to modulate the stimulatory action of IGF-1 in the full uterine response. Additionally, analysis of our uterine ERα ChIP-seq data revealed an enhancer 50 kb 5’ of the Igf1 promoter that has more estrogen dependent ERα enrichment than the previously tested EREs (Hewitt et al., 2012).
Analysis of AF-1 and AF-2 mediated responses
As described in the mechanism section, ERα activity requires interaction with co-regulators through AF-1 and AF-2. To understand how these impact uterine responses, mice with mutations in AF-1 (ERαAF-10) or AF-2 (ERαAF-20 and AF2ER) have been created. ERαAF-10 mice were made by deleting amino acids 2–128, which includes the AF-1. The uterus develops normally, but exhibits a blunted response to E2 (Abot et al., 2013; Billon-Gales et al., 2009). In contrast, mice lacking AF-2 exhibit more severe uterine phonotypes, with development of a hypoplastic uterus and complete insensitivity to E2 treatments (Arao et al., 2011a; Billon-Gales et al., 2011). ERαAF-20 mice were made by deleting amino acids 543–549, whereas AF2ER mice were made using 2 point mutations in the LBD. Both experimental approaches resulted in inactivation of the AF-2 function and lack of response to E2. One advantage of the AF2ER model is that ER antagonists such as tamoxifen and fulvestrant (ICI182780) exhibit agonist activity, resulting in an antagonist-agonist reversal, thus allowing re-activation of some ERα-mediated responses, including uterine epithelial cell proliferation, presumably through the AF-1 function of the mutant ERα. Overall, studies with AF-1 and AF-2 mutant mice demonstrate that AF-2 activity is critical for uterine E2 response, but that AF-1 activity can promote uterine response. Further characterization of these mice has also uncovered the tissue selectivity of ER actions through either AF-1 or AF-2. AF-1 activity is sufficient in promoting uterine and male efferent duct responses; in contrast, AF-2 activity is necessary for pituitary and mammary responses (Arao et al., 2013; Arao et al., 2012; Arao et al., 2011a).
A mouse with G525L mutation in the LBD called the estrogen-nonresponsive ER knock-in (ENERKI), shows lack of response to E2 (Sinkevicius et al., 2008). Like the AF2ER mice, high doses of the synthetic ERα selective agonist propyl pyrazole triol (PPT) and the ER agonist diethylstilbestrol (DES) are able to induce uterine growth (Sinkevicius et al., 2008). These observations support the findings from mice with mutations in AF-1 or AF-2 activities, that full ER function is needed for optimal uterine response.
Analysis of biological impact of membrane initiated signaling
To address the impact of cell membrane-associated signals, two mouse models with an identical mutation of the palmitoylation site (C451A) have been made which prevent membrane localization of ERα; Nuclear-only ER (NOER) (Pedram et al., 2014) and C451A-ERα (Adlanmerini et al., 2014). The two models differed in their uterine phenotypes, the NOER having a hypoplastic uterus that lacks E2 responses, and the C451A-ERα having normal uterine development and E2 responses. The C451A-ERα model only had ~55% reduction in membrane ERα (measured only in hepatocytes) which perhaps explains the different phenotypes {Pedram, 2014 #9}Conversely, another mouse model was created that prevented nuclear localization (Membrane-only ER, MOER) (Pedram et al., 2009) by expressing the LBD fused with multiple palmitoylation sites from the neuromodulin protein, resulting in a hypoplastic, E2-insensitive uterus (Pedram et al., 2009). Clearly, nuclear ERα is critical to uterine function, however the role of membrane-localized ERα is uncertain and the focus of ongoing investigations is to identify the intracellular signaling pathways involved.
ERβ does not impact uterine responses
Observation from ERβ-null females indicates their subfertility is due to diminished ovarian responses, with normal uterine development, function, and responses to E2 (Hewitt et al., 2003; Krege et al., 1998b). Although females with deletion of both ERα and β have more severe ovarian defects, the uterine phenotypes are similar to that observed in ERα-null mice (Couse, 1999).
Importance of ERα to uterine function informs mechanisms of disease
Owing to the biological and molecular events that require ERα, the mouse uterus has enabled advancement of our understanding of the details underlying estrogen-initiated responses. Studies in the many mouse models developed with deletion or mutation of ERα have highlighted the essential role of DNA binding and AF-1 and 2 functions in achieving optimal development and response. Additionally, ERα has cell type dependent roles. Our increased understanding of these molecular details and their roles in normal uterine function are critical to understanding perturbation that leads to impaired embryo implantation or endometrial diseases including endometriosis, endometrial cancer and leiomyoma.
Estrogen Receptor in the Ovary
Both known forms of nuclear estrogen receptor, ERα and ERβ, are expressed in mammalian ovaries but localized to distinct functional compartments. ERβ is highly expressed but limited to the granulosa cells of growing follicles, while ERα is generally localized to the interstitium and theca cells. This expression pattern is highly conserved among several mammalian species. Adult estrogen receptor alpha null (αERKO) females are anovulatory, possessing pre- and small antral follicles but lacking corpora lutea, resulting in infertility. By 50 days of age, αERKO mice are infertile and have ovaries that exhibit multiple enlarged, hemorrhagic, and cystic follicles, with increased gonadotropin and gonadotropin receptor levels, elevated steroid synthesis, and hypertrophied theca cells (Couse and Korach, 1999, 2001; Schomberg et al., 1999). Adult estrogen receptor beta null (βERKO) females are sub-fertile, as evidenced by reduced litter number and size (Couse et al., 2005). Despite speculated roles of ER in granulosa cells, βERKO ovaries appear relatively normal and possess follicles at all stages of growth and are not overtly impaired by losing ERβ (Couse et al., 2005; Krege et al., 1998b). Consistent with the subfertility, superovulatory treatments in βERKO females result in significantly fewer ovulations and observation of trapped oocyte follicles (Couse et al., 2005; Krege et al., 1998b). In addition, reduced expression of PR and Cox2, increased rates of follicle atresia, and a paucity of corpora lutea in βERKO ovaries indicate that the subfertility is likely due to a reduced ovulatory frequency (Emmen et al., 2005; Krege et al., 1998b).
Ovarian Phenotypes of ERα Mutant Mice
Although αERKO females are anovulatory, it is generally thought to be due to the cystic ovarian phenotype seen in these mice (Couse and Korach, 1999, 2001; Schomberg et al., 1999). Accompanying the pathology is a severe disruption in steroid hormone levels; mainly, αERKO mice have chronically elevated luteinizing hormone (LH), E2 and testosterone (T) due to a disruption of negative feedback (Couse et al., 1999a; Couse et al., 2003). Treatment of αERKO females with a gonadotropin-releasing hormone (GnRH)- antagonist (antide) corrected the cystic follicle and elevated steroidogenesis levels and is therefore thought to be the primary cause of the ovarian phenotypes seen in αERKO females (Couse et al., 1999a; Couse et al., 2005). One distinct phenotype of the ERα null females is the aberrant expression and extremely high levels of the enzymes involved in androgen biosynthesis (Couse et al., 2003). Aberrant expression of Hsd17b3, a testis specific gene, is observed in the αERKO female ovary. HSD17B3 catalyzes the conversion of androstendione to testosterone (T), is expressed in theca cells, and contributes to the high serum T in the αERKO female. T produced by HSD17B3 in theca cells is not converted to E2 by granulosa Cyp19 (aromatase), even though higher expression of Cyp19 was observed in αERKO female ovary. The expression of Hsd17b3 in the αERKO ovary is regulated by LH and treatment with a GnRH-antagonist can normalize T levels in the αERKO female (Couse et al., 2003). Cyp17, the enzyme necessary for androstendione synthesis, is increased 3-fold in αERKO females when compared to WT (Couse et al., 2003), resulting in increased serum levels of E2 and T. Cyp17 is found in the theca cells, where ERα is also localized, and therefore it is speculated that ERα mediates thecal cell steroidogenesis. Additionally, our data shows a very modest increase in Cyp17 in WT females with chronic LH expression (Couse et al., 2003). ERα null follicles grown in culture produce more androgens relative to wild type follicles under a controlled gonadotropin environment (Emmen et al., 2005), further supporting a role for ovarian ERα in T production. Taken together, these data suggest that the role of ERα in ovulation is through regulation of androgen biosynthesis by way of a short loop feedback mechanism in theca cells within the ovary.
It is also important to note the ovarian phenotypes of various ERα mutants that have been described (Table 2). As discussed earlier, there are a group of mutants that were developed to limit the genomic activity of ERα and their mutations only allow estrogen signaling through the non-classical mechanism. One such model is the Nerki. Female mice that are heterozygous for this mutation are infertile and the ovaries contain follicles of all stages but no corpora lutea and lipid-filled cells in the ovarian stroma (Jakacka et al., 2002a). When superovulated, Nerki heterozygous females develop large hemorrhagic cysts like those seen in the Esr1−/− and ovulation does not reach the level seen in WT mice; however, they do not display the altered steroidogenic enzyme or hormone profile (Jakacka et al., 2002a). Jackacka et al. speculated that the ovarian phenotypes result from the Nerki ERα acting in a dominant-negative matter (Jakacka et al., 2002a). As described earlier, more recent research revealed that Nerki ERα has aberrant DNA binding activity and binds to steroid hormone responsive elements (HRE) sequences including progesterone receptor responsive element (PRE) (Hewitt et al., 2014). Unexpected PRE-mediated gene regulation together with the ERE-mediated gene regulation by WT and heterozygote mice may be a cause of the disrupted phenotype described in the Nerki ovary.
Table 2.
Ovarian Phenotypes of Estrogen Receptor Mutants
| Gene | Mutation | Nickname | ovarian phenotypes | Hormone Levels | References |
|---|---|---|---|---|---|
| Esr1 | Homozygous null for ERα | αERKO or Ex3αERKO | -Anovulatory and Infertile Hemorrhagic and cystic ovaries with no CLs present in histological sections. -Increased expression of steroidogenic enzymes Lack of response to superovulation. |
Elevated T, E2 and LH Normal FSH & P |
(Antonson et al., 2012a; Curtis Hewitt et al., 2002; Curtis et al., 1999; Dupont et al., 2000; Hewitt et al., 2010a; Lubahn et al., 1993a) |
| Esr1 | One mutated allele of two-point mutation in ERα DBD and one WT allele | NERKI+/− | -Anovulatory and Infertile -Lack of plugs in NERKI females after superovulation and natural mating -Superovulation partially restored ovulation while increasing cyst presence |
Normal LH, FSH and E2 Reduced P |
(Jakacka et al., 2002b) |
| Esr1 | One mutated allele of two-point mutation in DNA binding domain of ERα and one ERαKO allele | KIKO (ERAA/−) | -Anovulatory and Infertile -No CLs present in histological sections |
Normal E2 and P | (Hewitt et al., 2010b; O'Brien et al., 2006) |
| Esr1 | Homozygous animal of 4-point mutation of DBD ERα | ERαEAAE/EAAE | -Infertile -Hemorrhagic ovaries |
Not reported | (Ahlbory-Dieker et al., 2009) |
| Esr1 | Homozygous animal of one point mutation in LBD of ERα | ENERKI (ERαG525L | -Anovulatory -Hemorrhagic and cystic ovaries with increased atretic antral follicles and No CLs in histological sections. -Hyperplastic theca cells in response to LH (data not shown) |
Elevated serum E2, T and LH Normal FSH |
(Sinkevicius et al., 2008) |
| Esr1 | Homozygous knock-in of two-point mutation in LBD of ERα | AF2ERKI/KI | -Anovulatory and Infertile -Hemorrhagic and cystic ovaries with no CLs present in histological sections. -Lack of reseponse to superovulation. |
Elevated serum LH and E2 | (Arao et al., 2011b) |
| Esr2 | Homozygous null alleles for ERβ | Esr2−/− βERKO, Ex3βERKO, and **ERβSTL−/L | -Subfertile – Infertile (lines vary) -Reduction or failure to respond to superovulation -Lack of COC expansion |
Normal LH and FSH | (Antal et al., 2008b; Dupont et al., 2000; Krege et al., 1998a; Wada-Hiraike et al., 2006b) (Binder et al., In Reivew) |
| Esr1 and Esr2 | Homozygous null for both ERα and ERβ | αβERKO | -Anovulatory and infertile -No CLs and few large follicles -Ovarian transdifferentiation to Sertoli-like cells -Altered expression of steroidogenic enzymes |
Elevated LH and T, Normal FSH and P |
(Couse et al., 1999b; Dupont et al., 2000) |
| Cyp19a1 | Homozygous null aromatase: ArKO Unable to synthesize endogenous E2. |
Cyp19a1−/− | -Anovulatory and infertile -Hemorrhagic and cystic ovaries with no CLs present in histological sections. -Failure to respond to superovulation with partial rescue with E2 treatment. Ovarian transdifferentiation to Sertoli-like cells that express Sox9 |
No E2 Elevated LH, FSH and T |
(Fisher et al., 1998; Honda et al., 1998; Toda et al., 2001a, b) (Britt et al., 2000; Britt et al., 2002; Fisher et al., 1998; Honda et al., 1998; Toda et al., 2012; Toda et al., 2001a) |
| Esr1 | Theca cell specific ERα knockout | Cyp17cre;ERaflox/flox | Fertility normal in young mice, but 6 month old animals have reduced fertility and longer estrous cycle | Elevated T at both 2 & 6 mo. Normal FSH Decreased LH at 2 mo. with further decrease at 6 mo. |
(Bridges et al., 2008; Lee et al., 2009) |
| Esr1 | palmitoylation deficient mutants | Esr1 C541A | Cystic ovaries Cystic ovaries |
LH elevated, E2 normal LH and E2 elevated |
(Adlanmerini et al., 2014) (Pedram et al., 2014) |
As described in the uterine section of this chapter, the ERαAA/- (KIKO) and ERαEAAE/EAAE (EAAE) mouse models were developed to further understand the role of ERα in non-classical estrogen signaling (Ahlbory-Dieker et al., 2009; O’Brien et al., 2006; Sinkevicius et al., 2008). These models both express ERα that is unable to bind to ERE sequences. The ovaries of KIKO mice have follicles with most stages of development, but lack corpora lutea (O’Brien et al., 2006). EAAE mice have hemorrhagic and cystic ovaries and are infertile, similar to the Esr1−/− mice (Ahlbory-Dieker et al., 2009). Taken together, these data suggest that direct ERE binding by ERα is critical for ovarian ERα functionality and regulation required for proper ovulation and therefore fertility.
There are also two notable ERα mouse lines that have mutations in the LBD of ERα. In one model, the ENERKI, a single point mutation was created by switching a glycine to a leucine at residue 525 creating an altered ligand binding pocket which prevents ligand binding (Sinkevicius et al., 2008). ENERKI has hemorrhagic and cystic ovaries and does not ovulate based on the lack of corpora lutea found in ovarian sections (Sinkevicius et al., 2008). The ovarian defects and lack of ovulation in the ENERKI reveal the importance of estrogen hormone binding for normal ovarian function and neuroendocrine negative feedback (Sinkevicius et al., 2008). A second line, the AF2ER, has two point mutations in the AF-2 region of ERα and, while E2 can be bound by this mutant, it is not able to engage transcriptional machinery due to the inability to interact with coactivators (Arao et al., 2011a). The ovarian phenotype of AF2ER females looks very similar to the ERα null mice, as they have hemorrhagic and cystic ovaries and ovarian sections have no corpora lutea (Arao et al., 2011a). AF2ER female mice also have a disruption in negative feedback and have elevated E, T, and LH (Arao et al., 2011a). This suggests that the AF-2 region of ERα is critical for regulating ovarian function, neuroendocrine control and ovulation.
Ovary-Specific ERα knockouts
The global ERα knockout mouse has shown that loss of estrogen is detrimental to ovarian function, as evidenced by large hemorrhagic cysts and infertility. However, this approach does not allow us to dissect the role of ERα in the ovary due to confounding factors, such as the high persistent steroid and gonadotropin serum levels following disruption of negative feedback. However, theca cell-specific ERα knock-out mice (thEsr1KO) were generated by crossing the floxed ERα strain with Cyp17 cre mice (Bridges et al., 2008). At 2 months of age, thEsr1KO mice had comparable fertility to WT mice and displayed a normal estrus cycle despite a reduction of serum LH. After superovulation, the same numbers of oocytes were found in the oviduct of the cKO and WT suggesting there was no defect in ovulation (Lee et al., 2009). However, by 6 months of age the thEsr1KO ovaries displayed hemorrhagic cysts and superovulation resulted in significantly fewer oocytes (Lee et al., 2009). Females had an even further reduction of serum LH, suggesting that ERα expression in the theca cells of the ovary is important for feedback regulation and control of LH expression. At both time points, testosterone was elevated, confirming the original role of ovarian ERα regulation of androgen production in the ovary as proposed in the Esr1−/− mice (Couse et al., 2006) (Lee et al., 2009). These data suggest that thecal cell ERα is associated with an age related reduction in ovarian function, but is not required for ovulation and normal ovarian function in young mice.
Ovarian Phenotypes of ERβ Mutant Mice
The role of ERβ in the maintenance of normal reproduction and fertility has not been fully elucidated. It has been reported that female βERKO mice are subfertile compared to WT, as characterized by fewer pregnancies, fewer litters, and smaller litter sizes (Dupont et al., 2000; Krege et al., 1998b). Ovaries of adult βERKO mice contain a reduced number of corpora lutea, indicating fewer ovulations, and ovulation rates cannot be rescued by exogenous gonadotropins (Couse et al., 2005; Emmen et al., 2005; Krege et al., 1998b). ERβ deletion results in impaired follicular maturation and a reduced number of follicles responsive to LH, which could explain why βERKO mice are poor responders to ovulatory stimulation and have smaller litters. The fact that βERKO mice have fewer pregnancies and produce fewer litters may also be due to fewer adequate ovulatory signals (i.e., LH surges). βERKO ovaries and granulosa cells isolated from βERKO mice have an attenuated cAMP accumulation in response to FSH and altered expression of several genes including Lhcgr (LH receptor) and Cyp19a1 (aromatase) (Couse et al., 2005; Emmen et al., 2005). Cultured follicles from βERKO follicles produce less estrogen than WT follicles (Emmen et al., 2005; Rodriguez et al., 2010) and may fail to provide a sufficient stimulus required to trigger physiologically relevant LH surges. The amplitude and timing of the naturally occurring LH surge was measured in individual intact βERKO and WT mice (Jayes et al., 2014) and it was determined that while the pituitary levels of LH revealed no differences, the amplitude of the LH surge was severely blunted in βERKO mice compared to WT. The βERKO mice did not produce an adequate preovulatory E2 surge. To determine if the smaller LH surges and the reduced number of litters in βERKO were due to the lack of ERβ in the hypothalamic-pituitary axis or ovary, ovaries were transplanted from WT into βERKO mice and vice versa. The size of the LH surge was reduced only in mice lacking ERβ within the ovary, and these mice had fewer litters. Fertility and size of the LH surge were rescued in βERKO mice receiving a WT ovary. These data provided the first experimental evidence that the LH surge is impaired in βERKO females and may be another aspect of their overall reduced fertility. This study shows that ERβ is not necessary within the pituitary and hypothalamus for the generation of a normal LH surge and for normal fertility, but ERβ is essential within the ovary to provide proper hormonal signals for the ovulatory cycle.
Role of ERβ signaling in Granulosa Cells
In the ovary, there is a heterogeneous cell population, and ERβ is specifically expressed in granulosa cells, while ERα is predominately expressed in the theca cells (Binder et al., 2013; Krege et al., 1998b). To isolate pure populations of granulosa cells, Laser Capture Microdissection (LCM) was performed using ovaries from WT and βERKO mice after stimulation with FSH alone or FSH in combination with LH. Use of LCM allowed for targeted isolation of granulosa cells from large antral follicles (FSH) or pre-ovulatory follicles (FSH+LH) in both WT and βERKO mice so that cells at the similar stages of development could be compared. Microarray analysis demonstrated that granulosa cells isolated at similar stages of follicular maturation have altered gene expression in βERKO mice compared to WT mice. While a subset of follicles can grow and respond to FSH and LH in βERKO mice, the transcriptional profile differs in these cells from that observed in WT cells from similar sized follicles, suggesting ERβ-null granulosa cells are not properly differentiated to respond to hormonal stimulation (Binder et al., 2013).
Examination of the genes from large antral follicles after FSH stimulation revealed 414 genes differentially expressed in βERKO granulosa cells compared to WT. These genes included several implicated in E2 biosynthesis, including Adcyap1 and Runx2, which correlates with reduced E2 concentrations in βERKO follicles (Emmen et al., 2005; Rodriguez et al., 2010). While a subset of granulosa cells were able to respond to LH and differentiate into preovulatory granulosa cells in βERKO mice, these cells showed 1,258 genes differentially expressed compared to WT preovulatory granulosa cells. These genes included members of several signaling pathways, including Akap12 and other members of the cAMP/PKA signaling pathway.
These findings indicate that ERβ is necessary for proper differentiation of ovarian granulosa cells in response to gonadotropins during folliculogenesis and provides a novel list of ERβ-dependent estrogen-regulated genes that may contribute to proper follicle maturation and ovulation in vivo.
Ovarian Phenotypes of ERα and ERβ Compound Mutant Mice
Mice that possess neither estrogen receptors alpha nor beta (αβERKO) are anovulatory and infertile similar to the αERKO (Couse et al., 1999b; Dupont et al., 2000). In ovarian sections, there are normal follicular stages found but upon aging to 6–12 months the antrum is underdeveloped, granulosa cell number is small, and the theca is thin and disorganized. Further, there are cystic follicles present and the mice have the same hormonal disruption as seen in the αERKO mice, with even higher LH levels (Couse et al., 1999b; Dupont et al., 2000). The main difference seen in the αβERKO when compared to the single ER KOs is the presence of seminiferous tubule-like structures in the post-pubertal ovary that appear to arise from atretic follicles and have cells with characteristics of Sertoli-like cells found in the male testis (Couse et al., 1999b; Dupont et al., 2000). This phenotype of transdifferentiation in the αβERKO, while absent in each individual KO (αERKO or βERKO), suggests that estrogen signaling involving both ERα and ERβ is necessary for proper ovarian formation and function. There is possible compensation by one or the other ER present in the individual knockout lines or an even more overt effect of the even higher levels of LH in combination with the improper cellular tissue differentiation.
Ovarian Phenotypes in Mice Lacking Estradiol Synthesis
When considering the role of E2 in various body functions, it is helpful to not only look at the estrogen receptor mutants but also mice that lack the ability to synthesize E2. Taking this approach, it is possible to dissect hormone ligand dependent responses from non-ligand dependent. Cyp19-null mice (ArKO) were developed for this purpose. Initially these mice had no noticeable phenotype; however, transition to a soy-free diet resulted in noticeable differences between ArKO and WT mice (Fisher et al., 1998). The discrepancy appears to be due to hormonally active components of the feed. The ovaries of ArKO mice have follicles of all stages of development, but no corpora lutea (Britt et al., 2000; Fisher et al., 1998; Toda et al., 2001a). Additionally, ArKO mice develop hemorrhagic and cystic follicles with age due, in part, to disrupted negative feedback that can be corrected with E2 treatment (Toda et al., 2001a). With carefully timed exogenous hormone treatment, ovulation can be partially rescued, showing that the ovulation defect is estrogen-dependent (Toda et al., 2012). When fed a soy-free diet, ArKO ovaries develop the transdifferentiation phenotype seen in the αβERKO ovaries (Britt et al., 2001). The steroid hormone serum composition of the ArKO female is disrupted (E is undetectable, androgens are elevated) and the mice have elevated serum LH (Britt et al., 2000; Fisher et al., 1998). When data from the ArKO mice (lacking ligand) is compiled with mice lacking ERα or ERβ (lack of receptors), it is clear that estrogen plays a major role in ovarian physiology. This is a compound issue that can be attributed to estrogen signaling not only in the ovary, but also in the hypothalamus and pituitary gland, which are involved in negative feedback controlling the trophic hormone levels.
Estrogen receptor in metabolism
Estrogen regulates multiple physiological functions, including reproduction, bone density and metabolic regulations. As a consequence of pleiotropic effects of estrogen, the decline of endogenous estrogen production by the ovaries at menopause often leads to functional disorders including dyslipidemia, impaired glucose tolerance (IGT) and type 2 diabetes mellitus, which increase cardiovascular disease risk in postmenopausal women and directly affect quality of life (Munoz et al., 2002). Several animal models have been developed to further explore the clinical findings of estrogen-dependent metabolic regulation. Indeed, ovexed mice lacking intact estrogen signaling display obesity and IGT; these effects are reversible with the reintroduction of estrogen (E2) (Zhu et al., 2013). Similar results have been seen in Cyp19 (aromatase) knockout (KO) mice, which unable to synthesize E2 from testosterone. Treatment of Cyp19KO mice with exogenous E2 restores the E2 protective effect against the development of metabolic syndrome in both male and female mice (Hewitt et al., 2004; Jones et al., 2000). Studies using the estrogen receptor (ERα and ERβ) knockout mice have demonstrated that ERα plays the essential role in estrogen-mediated metabolic regulation, whereas ERβ does not (Bryzgalova et al., 2006).
Metabolic Phenotype of ERα knockout mice
Metabolic phenotypes of αERKO have been described previously. αERKO females present with obesity, IGT and insulin resistance (Heine et al., 2000). Fat deposition of parametrial and inguinal white adipose tissues (WAT) were higher in regular diet fed 3-month-old αERKO females than in wild-type (WT) littermates. No difference in WT vs. αERKO perirenal WAT or brown adipose tissue (BAT) was observed. Increased adipocyte volume in parametrial and inguinal WAT was accompanied by increased adipocyte number (Heine et al., 2000). These observations suggested that ERα is involved in the adipogenesis; however, the mechanisms responsible for ERα-dependent regulation of adipogenesis remain unclear. Energy intake of WT and αERKO was equal, indicating that obesity was not induced by hyperphagia. In contrast, energy expenditure was reduced in αERKO compared with WT, indicating that altered energy expenditure may contribute to the observed obesity (Heine et al., 2000). Recent reports suggest that decreased locomotion is a cause of reduction of energy expenditure in αERKO mice (Park et al., 2011; Xu et al., 2011).
Physiological role of ERα transactivation domains in metabolism
As described previously, ERα has two transcription activation domains, named AF-1 and AF-2. Physiological roles of ERα AF-1 and AF-2 have been reported using the mouse models, which deleted ERα AF-1 (ERaAF-1°) or ERα AF-2 (ERaAF-2°) (Handgraaf et al., 2013). ERaAF-2° females present with obesity, IGT and insulin resistance, that mimics that seen in αERKO females. In striking contrast, metabolic phenotypes were lacking in ERaAF-1° mice being identical to WT. HFD-induced metabolic disturbances in ovexed ERaAF-1° and WT mice were prevented by E2 administration, whereas an E2-mediated protective effect was totally abrogated in ERaAF-2° and αERKO mice. Thus, the report concluded that the protective effect of E2 towards obesity and insulin resistance is ERα AF-2 dependent but does not require AF-1 (Handgraaf et al., 2013). The molecular mechanism of AF-1 or AF-2 activation or cooperative regulation of ERα AF-1 by AF-2 is still unresolved (Arao et al., 2015). Although the ERα AF-2 mutations (ERaAF-2° and AF2ER) disrupt E2-mediated physiological responses, antagonistic ligands such as fulvestrant and tamoxifen activate AF-1 mediated physiological functions in these mutant mice (Arao et al., 2012; Arao et al., 2011a; Moverare-Skrtic et al., 2014). AF2ER females present with disrupted metabolic phenotypes similar to ERaAF-2° and αERKO mice. Treatment with Tamoxifen to AF2ER females rescued the metabolic phenotypes (Arao A, 2016). This result suggested that ERα AF-1 is able to modulate metabolic regulation, even though it is in contrast to the previous report using a different model system (ERaAF-1°) (Handgraaf et al., 2013). Understanding the mechanism of ligand dependent ERα AF-1 and AF-2 cooperative regulation will be necessary to delineate new therapeutic options for selective modulation of ERα mediated metabolic regulation.
Phenotype of ERα DNA binding domain mutant mice in metabolism
ERα DNA binding domain mutant mice (KIKO) were analyzed to characterize the role for non-genomic and indirect DNA binding transcription (nonclassical ERα signaling) towards mediating metabolic regulation (Park et al., 2011). KIKO mice restored metabolic parameters dysregulated in αERKO mice to normal values, suggesting that the nonclassical ERα signaling rescues body weight and metabolic function. The normalization of energy expenditure, including voluntary locomotor activity leads to nonclassical ERα signaling-mediated normalization of metabolic regulation (Park et al., 2011). The phenotype of KIKO mice suggested that the nonclassical ERα signaling is a potential target for selective modulation of ERα-mediated metabolic regulation. Based on the aberrant DNA binding activity of the KIKO mouse model, further consideration of the metabolic phenotype of the EAAE mouse model with no DNA binding activity will provide a more accurate assessment of the signaling mechanisms involved in metabolic regulation. Additionally, development of other knock-in mutation mouse models will facilitate further evaluation of non-genomic extra-nuclear ERα action. The H2NES ERα mutation which is a cytosol-only form of ERα mutant, even in the presence of hormone, might be useful for such purposes (Burns et al., 2014). We have currently developed such a mouse model and are characterizing the phenotypes to assess the role of non-genomic ERα signaling.
As described above, various functional domains of ERα contribute to differential estrogen mediated metabolic regulations. Development of ligands that selectively regulate specific ERα functional domains and ER cellular signaling mechanisms may be useful for developing more effective targeted therapies for postmenopausal women without undesirable side effects.
References
- Aagaard MM, Siersbaek R, Mandrup S, 2011. Molecular basis for gene-specific transactivation by nuclear receptors. Biochimica et biophysica acta 1812, 824–835. [DOI] [PubMed] [Google Scholar]
- Abot A, Fontaine C, Raymond-Letron I, Flouriot G, Adlanmerini M, Buscato M, Otto C, Berges H, Laurell H, Gourdy P, Lenfant F, Arnal JF, 2013. The AF-1 activation function of estrogen receptor alpha is necessary and sufficient for uterine epithelial cell proliferation in vivo. Endocrinology 154, 2222–2233. [DOI] [PubMed] [Google Scholar]
- Adesanya OO, Zhou J, Samathanam C, Powell-Braxton L, Bondy CA, 1999. Insulin-like growth factor 1 is required for G2 progression in the estradiol-induced mitotic cycle. Proceedings of the National Academy of Sciences of the United States of America 96, 3287–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlanmerini M, Solinhac R, Abot A, Fabre A, Raymond-Letron I, Guihot AL, Boudou F, Sautier L, Vessieres E, Kim SH, Liere P, Fontaine C, Krust A, Chambon P, Katzenellenbogen JA, Gourdy P, Shaul PW, Henrion D, Arnal JF, Lenfant F, 2014. Mutation of the palmitoylation site of estrogen receptor alpha in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proceedings of the National Academy of Sciences of the United States of America 111, E283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlbory-Dieker DL, Stride BD, Leder G, Schkoldow J, Trolenberg S, Seidel H, Otto C, Sommer A, Parker MG, Schutz G, Wintermantel TM, 2009. DNA binding by estrogen receptor-alpha is essential for the transcriptional response to estrogen in the liver and the uterus. Molecular Endocrinology 23, 1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal MC, Krust A, Chambon P, Mark M, 2008a. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ER beta-null mutant. Proceedings of the National Academy of Sciences of the United States of America 105, 2433–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal MC, Krust A, Chambon P, Mark M, 2008b. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proceedings of the National Academy of Sciences of the United States of America 105, 2433–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonson P, Omoto Y, Humire P, Gustafsson JA, 2012a. Generation of ERalpha-floxed and knockout mice using the Cre/LoxP system. Biochemical and biophysical research communications 424, 710–716. [DOI] [PubMed] [Google Scholar]
- Antonson P, Omoto Y, Humire P, Gustafsson JA, 2012b. Generation of ERalpha-floxed and knockout mice using the Cre/LoxP system. Biochemical and biophysical research communications 424, 710–716. [DOI] [PubMed] [Google Scholar]
- Arao A, H.K., Lierz SL, Korach KS, 2016. Differential Function of Estrogen Receptor α in Fat Accumulation of Intra-Abdominal and Inguinal Adipose Tissues. [Google Scholar]
- Arao Y, Coons LA, Zuercher WJ, Korach KS, 2015. Transactivation Function-2 of Estrogen Receptor alpha Contains Transactivation Function-1-regulating Element. The Journal of biological chemistry 290, 17611–17627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arao Y, Hamilton KJ, Coons LA, Korach KS, 2013. Estrogen receptor alpha L543A, L544A mutation changes antagonists to agonists, correlating with the ligand binding domain dimerization associated with DNA binding activity. The Journal of biological chemistry 288, 21105–21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arao Y, Hamilton KJ, Goulding EH, Janardhan KS, Eddy EM, Korach KS, 2012. Transactivating function (AF) 2-mediated AF-1 activity of estrogen receptor alpha is crucial to maintain male reproductive tract function. Proceedings of the National Academy of Sciences of the United States of America 109, 21140–21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arao Y, Hamilton KJ, Ray MK, Scott G, Mishina Y, Korach KS, 2011a. Estrogen receptor alpha AF-2 mutation results in antagonist reversal and reveals tissue selective function of estrogen receptor modulators. Proc Natl Acad Sci USA 108, 14986–14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arao Y, Hamilton KJ, Ray MK, Scott G, Mishina Y, Korach KS, 2011b. Estrogen receptor alpha AF-2 mutation results in antagonist reversal and reveals tissue selective function of estrogen receptor modulators. Proceedings of the National Academy of Sciences of the United States of America 108, 14986–14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon-Gales A, Fontaine C, Filipe C, Douin-Echinard V, Fouque MJ, Flouriot G, Gourdy P, Lenfant F, Laurell H, Krust A, Chambon P, Arnal JF, 2009. The transactivating function 1 of estrogen receptor alpha is dispensable for the vasculoprotective actions of 17 beta-estradiol. Proceedings of the National Academy of Sciences of the United States of America 106, 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon-Gales A, Krust A, Fontaine C, Abot A, Flouriot G, Toutain C, Berges H, Gadeau AP, Lenfant F, Gourdy P, Chambon P, Arnal JF, 2011. Activation function 2 (AF2) of estrogen receptor-alpha is required for the atheroprotective action of estradiol but not to accelerate endothelial healing. Proceedings of the National Academy of Sciences of the United States of America 108, 13311–13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder AK, Rodriguez KF, Hamilton KJ, Stockton PS, Reed CE, Korach KS, 2013. The absence of ER-beta results in altered gene expression in ovarian granulosa cells isolated from in vivo preovulatory follicles. Endocrinology 154, 2174–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder AK, Rodriguez KF, Stockton PS, Hamilton KJ, Reed CE, Korach KS, In Reivew. The absence of ERβ results in altered gene expression in ovarian granulosa cells from in vivo preovulatory follicles. Endocrinology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguet W, Germain P, Gronemeyer H, 2000. Nuclear receptor ligand-binding domains: three-dimensional structures, molecular interactions and pharmacological implications. Trends in pharmacological sciences 21, 381–388. [DOI] [PubMed] [Google Scholar]
- Bridges PJ, Koo Y, Kang D-W, Hudgins-Spivey S, Lan Z-J, Xu X, DeMayo F, Cooney A, Ko C, 2008. Generation of Cyp17iCre transgenic mice and their application to conditionally delete estrogen receptor alpha (Esr1) from the ovary and testis. Genesis 46, 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt KL, Drummond AE, Cox VA, Dyson M, Wreford NG, Jones ME, Simpson ER, Findlay JK, 2000. An age-related ovarian phenotype in mice with targeted disruption of the Cyp 19 (aromatase) gene. Endocrinology 141, 2614–2623. [DOI] [PubMed] [Google Scholar]
- Britt KL, Drummond AE, Dyson M, Wreford NG, Jones MEE, Simpson ER, Findlay JK, 2001. The ovarian phenotype of the aromatase knockout (ArKO) mouse. Journal of Steroid Biochemistry and Molecular Biology 79, 181–185. [DOI] [PubMed] [Google Scholar]
- Britt KL, Kerr J, O’Donnell L, Jones ME, Drummond AE, Davis SR, Simpson ER, Findlay JK, 2002. Estrogen regulates development of the somatic cell phenotype in the eutherian ovary. Faseb J 16, 1389–1397. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, Khan A, 2006. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 49, 588–597. [DOI] [PubMed] [Google Scholar]
- Burns KA, Korach KS, 2012. Estrogen receptors and human disease: an update. Archives of toxicology 86, 1491–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KA, Li Y, Liu L, Korach KS, 2014. Research resource: comparison of gene profiles from wild-type ERalpha and ERalpha hinge region mutants. Mol Endocrinol 28, 1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Brown M, 2006. Estrogen receptor target gene: an evolving concept. Mol Endocrinol 20, 1707–1714. [DOI] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M, 2005. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122, 33–43. [DOI] [PubMed] [Google Scholar]
- Chimge NO, Frenkel B, 2013. The RUNX family in breast cancer: relationships with estrogen signaling. Oncogene 32, 2121–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S, Rainville J, Zhao X, Katzenellenbogen BS, Pfaff D, Vasudevan N, 2014. Estrogen receptor-mediated transcription involves the activation of multiple kinase pathways in neuroblastoma cells. Journal of Steroid Biochemistry and Molecular Biology 139, 45–53. [DOI] [PubMed] [Google Scholar]
- Couse J, 1999. Reproductive phenotypes in estrogen receptor knockout mice: Contrasting roles for estrogen receptor-alpha and estrogen receptor-beta. Biol Reprod 60, 88–88. [Google Scholar]
- Couse JF, Bunch DO, Lindzey J, Schomberg DW, Korach KS, 1999a. Prevention of the polycystic ovarian phenotype and characterization of ovulatory capacity in the estrogen receptor-alpha knockout mouse. Endocrinology 140, 5855–5865. [DOI] [PubMed] [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS, 1999b. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science 286, 2328–2331. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS, 1999. Estrogen receptor null mice: what have we learned and where will they lead us? Endocrine reviews 20, 358–417. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS, 2001. Contrasting phenotypes in reproductive tissues of female estrogen receptor null mice. Annals of the New York Academy of Sciences 948, 1–8. [DOI] [PubMed] [Google Scholar]
- Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS, 1997. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology 138, 4613–4621. [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Deroo BJ, Korach KS, 2005. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology 146, 3247–3262. [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Rodriguez KF, Johnson JA, Poirier D, Korach KS, 2006. The intraovarian actions of estrogen receptor-alpha are necessary to repress the formation of morphological and functional Leydig-like cells in the female gonad. Endocrinology 147, 3666–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Walker VR, Korach KS, 2003. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ER alpha but not ER beta. Molecular Endocrinology 17, 1039–1053. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Cooke PS, Kurita T, 2004. Role of stromal-epithelial interactions in hormonal responses. Archives of Histology and Cytology 67, 417–434. [DOI] [PubMed] [Google Scholar]
- Curtis Hewitt S, Goulding EH, Eddy EM, Korach KS, 2002. Studies using the estrogen receptor alpha knockout uterus demonstrate that implantation but not decidualization-associated signaling is estrogen dependent. Biology of Reproduction 67, 1268–1277. [DOI] [PubMed] [Google Scholar]
- Curtis SH, Korach KS, 1999. Steroid receptor knockout models: Phenotypes and responses illustrate interactions between receptor signaling pathways in vivo, in: O’Malley BW (Ed.), Hormones and Signaling. Academic Press, San Diego, pp. 357–380. [DOI] [PubMed] [Google Scholar]
- Curtis SW, Clark J, Myers P, Korach KS, 1999. Disruption of estrogen signaling does not prevent progesterone action in the estrogen receptor or knockout mouse uterus. Proceedings of the National Academy of Sciences of the United States of America 96, 3646–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroo BJ, Korach KS, 2006. Estrogen receptors and human disease. The Journal of clinical investigation 116, 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M, 2000. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 127, 4277–4291. [DOI] [PubMed] [Google Scholar]
- Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS, 2005. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER){alpha} and ER{beta} null mice indicate a role for ER{beta} in follicular maturation. Endocrinology 146, 2817–2826. [DOI] [PubMed] [Google Scholar]
- Eun Kwon H, Taylor HS, 2004. The role of HOX genes in human implantation. Annals of the New York Academy of Sciences 1034, 1–18. [DOI] [PubMed] [Google Scholar]
- Fisher CR, Graves KH, Parlow AF, Simpson ER, 1998. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proceedings of the National Academy of Sciences of the United States of America 95, 6965–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain P, Staels B, Dacquet C, Spedding M, Laudet V, 2006. Overview of nomenclature of nuclear receptors. Pharmacological reviews 58, 685–704. [DOI] [PubMed] [Google Scholar]
- Green KA, Carroll JS, 2007. Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nature reviews. Cancer 7, 713–722. [DOI] [PubMed] [Google Scholar]
- Hah N, Kraus WL, 2014. Hormone-regulated transcriptomes: lessons learned from estrogen signaling pathways in breast cancer cells. Molecular and cellular endocrinology 382, 652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handgraaf S, Riant E, Fabre A, Waget A, Burcelin R, Liere P, Krust A, Chambon P, Arnal JF, Gourdy P, 2013. Prevention of obesity and insulin resistance by estrogens requires ERalpha activation function-2 (ERalphaAF-2), whereas ERalphaAF-1 is dispensable. Diabetes 62, 4098–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS, 2000. Increased adipose tissue in male and female estrogen receptor- alpha knockout mice. Proceedings of the National Academy of Sciences of the United States of America 97, 12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt KN, Pratis K, Jones ME, Simpson ER, 2004. Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology 145, 1842–1848. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS, 2003. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen Molecular endocrinology (Baltimore, Md: 17, 2070–2083. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Kissling GE, Fieselman KE, Jayes FL, Gerrish KE, Korach KS, 2010a. Biological and biochemical consequences of global deletion of exon 3 from the ER alpha gene. Faseb J 24, 4660–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Li L, Grimm SA, Chen Y, Liu L, Li Y, Bushel PR, Fargo D, Korach KS, 2012. Research Resource: Whole-Genome Estrogen Receptor alpha Binding in Mouse Uterine Tissue Revealed by ChIP-Seq Molecular endocrinology (Baltimore, Md: 26, 887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Li L, Grimm SA, Winuthayanon W, Hamilton KJ, Pockette B, Rubel CA, Pedersen LC, Fargo D, Lanz RB, DeMayo FJ, Schutz G, Korach KS, 2014. Novel DNA Motif Binding Activity Observed In Vivo With an Estrogen Receptor alpha Mutant Mouse. Molecular Endocrinology 28, 899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Li Y, Li L, Korach KS, 2010b. Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor alpha to estrogen-responsive elements. The Journal of biological chemistry 285, 2676–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, O’Brien JE, Jameson JL, Kissling GE, Korach KS, 2009. Selective Disruption of ER alpha DNA-Binding Activity Alters Uterine Responsiveness to Estradiol. Molecular Endocrinology 23, 2111–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Winuthayanon W, Korach KS, 2016. What’s new in estrogen receptor action in the female reproductive tract. Journal of molecular endocrinology 56, R55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Harada N, Ito S, Takagi Y, Maeda S, 1998. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochemical and biophysical research communications 252, 445–449. [DOI] [PubMed] [Google Scholar]
- Huang CC, Orvis GD, Wang Y, Behringer RR, 2012. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS One 7, e44285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL, 2002a. An estrogen receptor (ER)alpha deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol 16, 2188–2201. [DOI] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL, 2002b. An estrogen receptor (ER)alpha deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol. Endocrinol. 16, 2188–2201. [DOI] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL, 2001. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. Journal of Biological Chemistry 276, 13615–13621. [DOI] [PubMed] [Google Scholar]
- Jayes FL, Burns KA, Rodriguez KF, Kissling GE, Korach KS, 2014. The naturally occurring luteinizing hormone surge is diminished in mice lacking estrogen receptor Beta in the ovary. Biol Reprod 90, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EV, 1962. On the mechanism of estrogen action. Perspectives in biology and medicine 6, 47–59. [DOI] [PubMed] [Google Scholar]
- Jensen EV, Jacobson HI, 1960. Fate of steroidal estorgen in target tissues, in: Pincus G, Vollmer EP (Eds.), Biological Activities of Steroids in Relation to Cancer. Academic Press, New York, pp. 161–174. [Google Scholar]
- Jensen EV, Jacobson HI, 1962. Basic guides to the mechanism of estrogen action., Recent Prog Horm Res, pp. 387–414. [Google Scholar]
- Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER, 2000. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proceedings of the National Academy of Sciences of the United States of America 97, 12735–12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Levin ER, 2001. Rapid actions of plasma membrane estrogen receptors. Trends in endocrinology and metabolism: TEM 12, 152–156. [DOI] [PubMed] [Google Scholar]
- Klinge CM, 2000. Estrogen receptor interaction with co-activators and co- repressors. Steroids 65, 227–251. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O, 1998a. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proceedings of the National Academy of Sciences of the United States of America 95, 15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O, 1998b. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proceedings of the National Academy of Sciences of the United States of America 95, 15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA, 1996. Cloning of a novel receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Sciences of the United States of America 93, 5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P, 2000. Estrogen receptor pathways to AP-1. Journal of Steroid Biochemistry and Molecular Biology 74, 311–317. [DOI] [PubMed] [Google Scholar]
- Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ, 2006. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nature genetics 38, 1204–1209. [DOI] [PubMed] [Google Scholar]
- Lee S, Kang DW, Hudgins-Spivey S, Krust A, Lee EY, Koo Y, Cheon Y, Gye MC, Chambon P, Ko C, 2009. Theca-specific estrogen receptor-alpha knockout mice lose fertility prematurely. Endocrinology 150, 3855–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER, 2015. Extranuclear Steroid Receptors Are Essential for Steroid Hormone Actions. Annual Review of Medicine, Vol 66 66, 271–280. [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC, 2011. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 331, 912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O, 1993a. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proceedings of the National Academy of Sciences of the United States of America 90, 11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O, 1993b. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90, 11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. , 1995. The nuclear receptor superfamily: the second decade. Cell 83, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moverare-Skrtic S, Borjesson AE, Farman HH, Sjogren K, Windahl SH, Lagerquist MK, Andersson A, Stubelius A, Carlsten H, Gustafsson JA, Ohlsson C, 2014. The estrogen receptor antagonist ICI 182,780 can act both as an agonist and an inverse agonist when estrogen receptor alpha AF-2 is modified. Proceedings of the National Academy of Sciences of the United States of America 111, 1180–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J, Derstine A, Gower BA, 2002. Fat distribution and insulin sensitivity in postmenopausal women: influence of hormone replacement. Obes Res 10, 424–431. [DOI] [PubMed] [Google Scholar]
- O’Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL, 2006. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. The Journal of biological chemistry 281, 26683–26692. [DOI] [PubMed] [Google Scholar]
- O’Lone R, Frith MC, Karlsson EK, Hansen U, 2004. Genomic targets of nuclear estrogen receptors Molecular endocrinology (Baltimore, Md: 18, 1859–1875. [DOI] [PubMed] [Google Scholar]
- Park CJ, Zhao Z, Glidewell-Kenney C, Lazic M, Chambon P, Krust A, Weiss J, Clegg DJ, Dunaif A, Jameson JL, Levine JE, 2011. Genetic rescue of nonclassical ERalpha signaling normalizes energy balance in obese Eralpha-null mutant mice. The Journal of clinical investigation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar S, Laws MJ, Bagchi IC, Bagchi MK, 2015. Uterine Epithelial Estrogen Receptor-alpha Controls Decidualization via a Paracrine Mechanism Molecular endocrinology (Baltimore, Md: 29, 1362–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Kim JK, O’Mahony F, Lee EYHP, Luderer U, Levin ER, 2009. Developmental Phenotype of a Membrane Only Estrogen Receptor alpha(MOER) Mouse. Journal of Biological Chemistry 284, 3488–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Lewis M, Hammes S, Levin ER, 2014. Membrane-Localized Estrogen Receptor alpha Is Required for Normal Organ Development and Function. Dev Cell 29, 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Hathaway HJ, 2015. What have we learned about GPER function in physiology and disease from knockout mice? Journal of Steroid Biochemistry and Molecular Biology 153, 114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby VE, Korach KS, 1984. The influence of 17 beta-estradiol on patterns of cell division in the uterus. Endocrinology 114, 694–702. [DOI] [PubMed] [Google Scholar]
- Rodriguez KF, Couse JF, Jayes FL, Hamilton KJ, Burns KA, Taniguchi F, Korach KS, 2010. Insufficient luteinizing hormone-induced intracellular signaling disrupts ovulation in preovulatory follicles lacking estrogen receptor-{beta}. Endocrinology 151, 2826–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper RJ, Griffith JS, Lyttle CR, Doerge RW, McNabb AW, Broadbent RE, Teuscher C, 1999. Interacting quantitative trait loci control phenotypic variation in murine estradiol-regulated responses. Endocrinology 140, 556–561. [DOI] [PubMed] [Google Scholar]
- Safe S, 2001. Transcriptional activation of genes by 17 beta-estradiol through estrogen receptor-Sp1 interactions, in: Litwack G, Begley T (Eds.), Vitamins and Hormones - Advances in Research and Applications, Vol 62 Academic Press, San Diego CA, pp. 231–252. [DOI] [PubMed] [Google Scholar]
- Schomberg DW, Couse JF, Mukherjee A, Lubahn DB, Sar M, Mayo KE, Korach KS, 1999. Targeted disruption of the estrogen receptor-alpha gene in female mice: characterization of ovarian responses and phenotype in the adult. Endocrinology 140, 2733–2744. [DOI] [PubMed] [Google Scholar]
- Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, Sugg SL, Temple KA, Wondisford FE, Korach KS, Woodruff TK, Greene GL, 2008. An estrogen receptor-alpha knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology 149, 2970–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, 1998. Cross-talk between peptide growth factor and estrogen receptor signaling pathways. Biol Reprod 58, 627–632. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ, 1992. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359, 76–79. [DOI] [PubMed] [Google Scholar]
- Toda K, Hayashi Y, Ono M, Saibara T, 2012. Impact of ovarian sex steroids on ovulation and ovulatory gene induction in aromatase-null mice. Endocrinology 153, 386–394. [DOI] [PubMed] [Google Scholar]
- Toda K, Takeda K, Okada T, Akira S, Saibara T, Kaname T, Yamamura K, Onishi S, Shizuta Y, 2001a. Targeted disruption of the aromatase P450 gene (Cyp19) in mice and their ovarian and uterine responses to 17beta-oestradiol. J Endocrinol 170, 99–111. [DOI] [PubMed] [Google Scholar]
- Toda K, Takeda K, Okada T, Akira S, Saibara T, Kaname T, Yamamura K, Onishi S, Shizuta Y, 2001b. Targeted disruption of the aromatase P450 gene (Cyp19) in mice and their ovarian and uterine responses to 17beta-oestradiol. The Journal of endocrinology 170, 99–111. [DOI] [PubMed] [Google Scholar]
- Tong W, Pollard JW, 1999. Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E- and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol Cell Biol 19, 2251–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada-Hiraike O, Hiraike H, Okinaga H, Imamov O, Barros RP, Morani A, Omoto Y, Warner M, Gustafsson JA, 2006a. Role of estrogen receptor beta in uterine stroma and epithelium: Insights from estrogen receptor beta−/− mice. Proceedings of the National Academy of Sciences of the United States of America 103, 18350–18355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada-Hiraike O, Hiraike H, Okinaga H, Imamov O, Barros RP, Morani A, Omoto Y, Warner M, Gustafsson JA, 2006b. Role of estrogen receptor beta in uterine stroma and epithelium: Insights from estrogen receptor beta−/− mice. Proceedings of the National Academy of Sciences of the United States of America 103, 18350–18355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall EH, Case LK, Hewitt SC, Nguyen-Vu T, Candelaria NR, Teuscher C, Lin CY, 2014. Genetic control of ductal morphology, estrogen-induced ductal growth, and gene expression in female mouse mammary gland. Endocrinology 155, 3025–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall EH, Hewitt SC, Liu L, del Rio R, Case LK, Lin CY, Korach KS, Teuscher C, 2013. Genetic control of estrogen-regulated transcriptional and cellular responses in mouse uterus. Faseb J 27, 1874–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Dey SK, 2006. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 7, 185–199. [DOI] [PubMed] [Google Scholar]
- Winuthayanon W, Hewitt SC, Korach KS, 2014. Uterine epithelial cell estrogen receptor alpha-dependent and -independent genomic profiles that underlie estrogen responses in mice. Biol Reprod 91, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS, 2010. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proceedings of the National Academy of Sciences of the United States of America 107, 19272–19277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ, 2011. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14, 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Brown WC, Cai Q, Krust A, Chambon P, McGuinness OP, Stafford JM, 2013. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes 62, 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Pollard JW, 2007. Estradiol-17 beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proceedings of the National Academy of Sciences of the United States of America 104, 15847–15851. [DOI] [PMC free article] [PubMed] [Google Scholar]