Abstract
Direct‐acting antivirals approved for use in patients with end‐stage renal disease (ESRD) now exist. HCV‐positive (HCV+) ESRD patients have the opportunity to decrease the waiting times for transplantation by accepting HCV‐infected kidneys. The optimal timing for HCV treatment (pre‐ vs posttransplant) among kidney transplant candidates is unknown. Monte Carlo microsimulation of 100 000 candidates was used to examine the cost‐effectiveness of HCV treatment pretransplant vs posttransplant by liver fibrosis stage and waiting time over a lifetime time horizon using 2 regimens approved for ESRD patients. Treatment pretransplant yielded higher quality‐adjusted life years (QALYs) compared with posttransplant treatment in all subgroups except those with Meta‐analysis of Histological Data in Viral Hepatitis stage F0 (pretransplant: 5.7 QALYs vs posttransplant: 5.8 QALYs). However, treatment posttransplant was cost‐saving due to decreased dialysis duration with the use of HCV‐infected kidneys (pretransplant: $735 700 vs posttransplant: $682 400). Using a willingness‐to‐pay threshold of $100 000, treatment pretransplant was not cost‐effective except for those with Meta‐analysis of Histological Data in Viral Hepatitis stage F3 whose fibrosis progression was halted. If HCV+ candidates had access to HCV‐infected donors and were transplanted ≥9 months sooner than HCV‐negative candidates, treatment pretransplant was no longer cost‐effective (incremental cost‐effectiveness ratio [ICER]: $107 100). In conclusion, optimal timing of treatment depends on fibrosis stage and access to HCV+ kidneys but generally favors posttransplant HCV eradication.
Keywords: economics, health services and outcomes research, infection and infectious agents—viral: hepatitis C, kidney disease, kidney transplantation/nephrology, quality of life (QoL)
1. INTRODUCTION
The landscape of hepatitis C infection (HCV+) has changed dramatically since the introduction of direct‐acting antivirals (DAAs). Although some earlier regimens were contraindicated in patients with advanced chronic kidney disease (CKD), there are now treatment options available for patients with end‐stage renal disease (ESRD) regardless of genotype.1,2 However, there are considerable costs associated with DAAs,3 and a substantial mortality burden on dialysis,4 creating true equipoise as to whether benefits derived from early HCV treatment outweigh the risk of prolonged dialysis duration. Thus the question for physicians and patients is no longer how to treat HCV infection but rather the optimal timing with respect to kidney transplantation.
Cohort studies have demonstrated higher rates of mortality for dialysis patients with HCV both in the United States and world‐wide.5,6 Among ESRD patients who are transplanted, HCV infection has been associated with a 44% increased risk of posttransplant mortality and allograft loss.7 Posttransplant HCV eradication with DAAs has been embraced by the transplant community as a means to improve outcomes among HCV+ recipients, and emerging data suggest posttransplant cure may enhance patient survival.8
As wait‐time for transplantation exceeds 5 years in many parts of the country, some transplant centers have encouraged dialysis patients to forego HCV treatment until after transplantation to preserve the option to receive a kidney from an HCV‐infected donor. Although the use of HCV‐infected organs shortens wait‐time to transplantation and increases transplant rate for HCV+ recipients, these donor organs are not universally accepted by patients and transplant centers.9,10 HCV‐infected kidneys are more likely to be discarded and HCV has a deleterious effect on allograft outcomes in studies conducted prior to the availability of DAAs11,12; when calculating the Kidney Donor Profile Index (KDPI), HCV infection leads to a lower quality score.13 In an era in which HCV cure is possible in nearly all patients, postponing therapy may expedite transplant and avoid the excess mortality associated with prolonged dialysis exposure.
The number of HCV‐viremic donors is projected to increase, and therefore understanding the implications of HCV treatment decisions on access to transplantation is crucial. To provide guidance to patients and physicians, we undertook this Monte Carlo microsimulation to explore the relative costs and quality‐adjusted life years (QALYs) associated with HCV treatment pretransplant vs posttransplant. This type of decision analysis replicates real‐world experiences of actual patients, modeling an individual‐level multiple disease states, including DAA treatment failure and fibrosis progression. We examined 2 regimens approved for patients with ESRD and specifically considered issues surrounding variation in local waiting time and liver fibrosis stage.
2. METHODS
2.1. Analytic overview
We developed a Monte Carlo microsimulation model of HCV infection and kidney transplantation among HCV+ kidney‐only transplant candidates to estimate the cost‐effectiveness of 2 strategies: (1) treating pretransplant and (2) treating posttransplant. The simulated patient demographics, specifically age, gender, and Meta‐analysis of Histological Data in Viral Hepatitis (METAVIR) stage, were reflective of an observational cohort study of kidney‐only candidates listed as willing to accept an HCV‐infected organ.10 These competing strategies were selected given our hypothesis that pretransplant treatment of HCV may mitigate risk of liver‐related mortality but increase dialysis duration. In contrast, candidates treated posttransplant would have access to a pool of HCV‐viremic donors, reducing dialysis duration, while risking HCV disease progression.9 The model was programmed in TreeAge Pro version 2017 (Williamstown, MA).
Outcomes simulated by our model included the following: deaths on the waitlist, QALYs, and life expectancy. We projected lifetime medical costs assuming a health sector perspective with a 3% annual discount rate to both costs and benefits. We projected 3‐ and 5‐year time horizon, nondiscounted, healthcare sector costs. The incremental cost‐effectiveness ratio (ICER) was calculated as the additional cost divided by QALYs gained compared to the next least‐expensive strategy. We interpreted ICERs using a willingness‐to‐pay threshold (WTP) of $100 000/QALY.14 We defined as “dominated” and eliminated from consideration strategies that either: (1) increased cost but resulted in lower QALY (strong dominance), or (2) resulted in fewer QALYs at a higher cost/QALY gained (extended dominance). The model was simulated with cohorts of 100 000 individuals. We ran these simulations 1000 times to generate 1000 ICERs for treatment pretransplant as compared to posttransplant, which were plotted on the cost‐effectiveness plane. Ninety‐five percent confidence ellipses and the WTP threshold were included to assess the robustness of results. Figures are presented in the Supplemental Materials (Figures S2‐S15). We projected 3‐and 5‐year nondiscounted budgetary impact from the health sector perspective expressed as the undiscounted cost/1000 patients on the transplant waiting‐list. Data for parameter values were obtained from national databases, clinical trials, and observational cohort studies (Table 1).
TABLE 1.
Transition probabilities and model inputs for the Monte Carlo simulation evaluating the cost‐effectiveness of HCV treatment timing among kidney transplant candidates
| Probability name | Probability | Range | Source |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) | 56 (9) | 35−70 | Shelton 2018)10 |
| Male, % | 73.6 | 60−80 | |
| HCV Metavir stage at baseline, median (IQR) |
2 (1−3) | ||
| Regimen 1 (glecaprevir/pibrentasvir) | |||
| Deatha | 0.00001 | 0.00001−0.01 | Gane (2017)1 |
| Withdrawal | 0.04 | 0.02−0.08 | Gane (2017)1 |
| Toxicity | 0.01 | 0.003−0.03 | Gane (2017)1 |
| SVR | 0.98 | 0.90−1.00 | Gane (2017)1 |
| Regimen 2 grazoprevir/elbasvir) | |||
| Deatha | 0.00001 | 0.00001−0.01 | |
| Withdrawal | 0.05 | 0.02−0.08 | Roth (2015)2 |
| Toxicity | 0.01 | 0.003−0.03 | Roth (2015)2 |
| SVR | 0.99 | 0.90−1.00 | Roth (2015) Kohli 2016)2,38 |
| No treatment | |||
| Delisting | 0.003 | 0.006−0.01 | Hart (2018)4 |
| Death due to cirrhosis, rate per 100 PY |
1.39 | 0.96−1.85 | Bruno (2009)24 |
| Treated | |||
| Transplant | OPO‐specific | SRTR PSR | |
| Delisting | 0.003 | 0.006−0.01 | Hart (2018)4 |
| Death on waitlist, annual | 0.06 | 0.03−0.24 | USRDS 2017)29 |
ESRD, end‐stage renal disease; HCV, hepatitis C; IQR, interquartile range; PSR, program specific report; PY, person‐years; SRTR, Scientific Registry of Transplant Recipients; SVR, sustained viral response; USRDS, United States Renal Data System.
When disaggregated from background mortality, ESRD‐specific mortality, and HCV‐based mortality, the probability of death was less than zero.
3. MODEL STRUCTURE AND INPUTS
3.1. Model structure
The model simulates the lifetime progression of a cohort of individuals as a series of transitions between clinically meaningful health states. The model generates simulated individuals on a person‐by‐person basis and walks each individual through his/her life course from the time of simulation start until death, using a 1‐month time cycle. A set of transition probabilities, based on cohort studies and clinical trials, governs movement through the model. When a simulated person reaches death, the model tabulates person‐level outcomes for that simulated individual, and then begins the next person‐level simulation. When the model has simulated the life course of a cohort of 100 000 people, it calculates mean outcomes for the cohort, including life expectancy, QALYs, and costs. We experimented with varying cohort sizes, running simulations using cohorts of 10 000 and 1 000 000 individuals. Inferences were confirmed.
Because the model is a micro‐simulation, rather than a Markov,15–18 it can track details of clinical history that impact future outcomes, such as prior HCV treatment history, and also allows for heterogeneity in person life courses. For example, whereas a Markov model would advance the entire cohort through liver fibrosis stages in a deterministic manner, the microsimulation allows for person‐level variance in fibrosis progression such that some individuals progress quickly from HCV infection to cirrhosis, whereas others never reach advanced liver disease.
3.2. Natural history
3.2.1. HCV disease progression
HCV infection is characterized by METAVIR fibrosis stages, F0‐F4. At simulation start, the cohort has a distribution of fibrosis stages reflective of real‐world heterogeneity in HCV disease progression. Each month individuals face a probability of fibrosis progression based on the estimates of median time from HCV infection to cirrhosis.19 HCV infection is associated with increased costs and decreased quality of life (QoL); as individuals advance through METAVIR stages of fibrosis, QoL decreases and healthcare costs increase.20–23 Only when individuals reach METAVIR stage F4 (cirrhosis) do they begin to experience mortality from liver‐attributable causes (Tables 1 and 2).24
TABLE 2.
Utility‐related parameters for the Monte Carlo simulation evaluating the cost‐effectiveness of HCV (hepatitis C) treatment timing among kidney transplant candidates
| Utility lement | Utility | Range | Source |
|---|---|---|---|
| HCV | |||
| F0 | 0.89 | 0.75−1 | Chong (2003) |
| F1 | 0.89 | 0.75−1 | Grieve (2006) |
| F2 | 0.89 | 0.75−1 | Stein (2000)21–23 |
| F3 | 0.62 | 0.55−0.75 | |
| F4 | 0.48 | 0.40−0.60 | |
| Toxicity event | −0.16 | 0.09−0.25 | |
| Waitlist | 0.525 | 0.33−1.00 | Hogan 2002)39 |
| SVR | 0.74−0.92 | 0.60−1.00 | Sherman (2004)40 |
| Transplant | 0.85 | 0.50−1.00 | Snyder (2013) |
| Haller, (2011) | |||
| Hirth, (1999) | |||
| Menzin, (2011) | |||
| Painter, (2013) | |||
| Whiting, (1999) | |||
| Barnieh (2011)41–48 |
SVR, Sustained viral response.
3.2.2. Simulation of ESRD and nonliver mortality
Throughout the simulation, individuals experience decreased quality of life, costs, and mortality related to ESRD. The Scientific Registry of Transplant Recipients (SRTR) Annual Data Report provided the probability of mortality on the kidney waitlist.4 The model assumes that the probability of waitlist mortality remains constant through‐out the analysis.
3.2.3. HCV treatment
We model the 2 treatment regimens available to patients with ESRD: glecaprevir/pibrentasvir and grazoprevir/elbasvir. In the base case, patients receive glecaprevir/pibrentasvir as their initial regimen. Duration of treatment is 12 weeks, with outcomes derived from cohort studies and clinical trials. Upon initiation, patients complete 12 weeks of treatment, halting only for withdrawal, major toxicity, or death. Individuals can experience nontreatment ending toxicity, for which they incur additional costs. If an individual fails to achieve sustained viral response (SVR) on glecaprevir/pibrentasvir for any reason other than death, he is started on a 12‐week regimen of grazoprevir/elbasvir (Table 2).
HCV cure halts fibrosis progression and HCV‐attributable costs decrease by 50%.20,25 Following cure, liver‐attributable mortality is reduced by 94% among those with cirrhosis.26 The residual liver mortality after HCV cure among cirrhotics reflects observed rates of hepatocellular carcinoma.
3.2.4. Dialysis and transplant
The simulation begins with all cohort members on the transplant waiting list. While waiting for deceased donor transplantation, individuals experience elevated ESRD mortality as above. The time to transplant is a function of organ procurement organization (OPO) and HCV infection status. Individuals who are HCV infected are eligible to receive an HCV‐infected allograft, whereas those with cured HCV while wait‐listed can receive only HCV− allografts. In the base case, time to transplant for untreated individuals is one year shorter than time to transplant for those treated pretransplant, due to availability of HCV‐infected kidneys (Tables 1 and 2).9,10 The model assumes waitlist mortality and transplant probabilities among candidates who have achieved SVR is equivalent to HCV‐negative candidates.
During the month of kidney transplant, individuals experience increased mortality, decreased quality of life, and elevated costs related to the transplant procedure itself. Following successful kidney transplant, ESRD mortality is decreased by 24%, and transplant costs decrease to reflect costs associated with transplant maintenance.27,28
3.3. Utilities
Quality of life in every month is a function of 3 independent utility functions: (1) liver‐related, (2) ESRD‐related, and (3) age‐and sex‐adjusted comorbidities. These independent functions are integrated using a multiplicative assumption.
3.4. Costs
The model generated costs from the health sector perspective. During each month, individuals accrued age‐and sex‐stratified background costs attributable to non–HCV‐related healthcare, such as dialysis, transplant‐related costs that varied by time posttransplant, and HCV‐specific costs that varied by disease state. We derived HCV treatment costs from the federal supply schedule. HCV‐attributable costs were obtained from published literature and were varied in sensitivity analyses.20 Dialysis and transplant‐related costs were derived from the United States Renal Data System (USRDS) annual data report.29 Transplant costs are increased in the first year posttransplant to reflect the cost of surgery and induction immunosuppression. All costs are assessed from the health sector perspective in 2017 US dollars (Table 3).
TABLE 3.
Cost‐related parameters for the Monte Carlo simulation evaluating the cost‐effectiveness of HCV (hepatitis C) treatment timing among kidney transplant Candidates
| Cost element | Costa | Range | Source |
|---|---|---|---|
| HCV | |||
| F0 | 245 | 185–305 | Davis (2011)20 |
| F1 | 245 | 185–305 | |
| F2 | 245 | 185–305 | |
| F3 | 440 | 315–550 | |
| F4 | 830 | 620–1050 | |
| Waitlist | 87 597 | 60 000–100 000 | USRDS (2017)29 |
| SVR | 0.5 × HCV | Davis (2011)20 | |
| Regimen 1 (glecaprevir/ pibrentasvir), 4 weeks |
9830 | 8000–13 200 | Federal Supply Schedule Price |
| Regimen 2 (grazoprevir/ elbasvir), 4 weeks |
15 840 | 10 920–17 377 | Federal Supply Schedule Price, Truven Health Analytics |
| Toxicity event | 241 | 120–360 | Ferenci (2014) Chaiwat (2009) Gao (2012)49–51 |
| Transplant, first year | 130 545 | 100 000–180 000 | USRDS (2017)29 |
| Transplant, after first year |
24 282 | 15 000–40 000 | USRDS (2017)29 |
SVR, sustained viral response.
USD, 2017.
3.5. Model inputs
The simulated HCV+ waitlist cohort has a median age of 57 (interquartile range [IQR] 52‐62), is 73.6% male and 52.8% African American with a median METAVIR fibrosis score of 2 (IQR 1‐3) (Figure S1).10 Time to transplant among candidates treated pretransplant was informed by SRTR Program Specific Reports, which provide median time to deceased donor transplant and annual deceased donor transplant rate for each OPO.30 Kucirka et al reported that HCV+ candidates accepting HCV‐infected organs were transplanted one year more quickly than HCV− candidates who were in the pre‐DAA era, supporting shorter waiting times for untreated HCV+ candidates.9 Mortality with cirrhosis was informed by a cohort study of HCV+ cirrhotic individuals in the pre‐DAA era and by multivariable adjusted time‐to‐event analyses.24 Reduction in cirrhosis mortality post‐SVR was reported in a cohort study using multivariable Cox proportional hazards.26 Roth et al conducted a randomized study of grazoprevir‐elbasvir among adults with stage 4 or 5 CKD, informing the treatment efficacy parameters (SVR, withdrawal, and toxicity).2 Probability of SVR, withdrawal, and toxicity of glecaprevir‐pibren‐tasvir were derived from a 2017 randomized control trial for adults with stage 4 or 5 CKD.1
3.6. Analyses
In the base case analysis we populated the model with best estimates for every parameter and then simulated the lifetime out‐comes of the cohort assuming: (1) all patients were treated prior to kidney transplant, (2) all patients were treated posttransplant, and all patients were treatmentnaive. We conducted stratified analyses in which we considered the decision for cohorts by liver fibrosis stage. Sensitivity analyses were conducted and our inferences were confirmed (described in greater detail in the Supplemental Materials).
4. RESULTS
4.1. Clinical outcomes
In the pretransplant treatment strategy, 97.9% achieved cure of HCV with the primary regimen. Overall 28.0% died on the waitlist and 58.2% achieved transplantation in the lifetime horizon model. Life expectancy was 164.5 months and treatment afforded an additional 65.6 quality‐adjusted life month (QALM). The total cost associated with treatment pretransplant was $735 600 (Table 4).
TABLE 4.
Cost‐effectiveness of treatment strategies for hepatitis C virus infection among kidney waitlist candidates by fibrosis stage at waitlist addition
| Treatment strategy | cost, $ | Incremental cost, $ |
QALM | Incremental QALM |
ICER, $/ QALY |
Undiscounted life months |
5 y budget impact, $ |
|---|---|---|---|---|---|---|---|
| Overall | |||||||
| Treat posttransplant | 682 400 | 61.8 | 164.8 | 391 560 600 | |||
| Treat pretransplant | 735 700 | 53 300 | 65.6 | 3.9 | 162 800 | 164.5 | 419 087 100 |
| F0 | |||||||
| Treat posttransplant | 696 200 | 69.5 | 167.8 | 393 551 000 | |||
| Treat pretransplant | 736 000 | 39 800 | 68.5 | −1.1 | Dom | 164.0 | 418 573 200 |
| F1 | |||||||
| Treat posttransplant | 689 400 | 67.9 | 165.7 | 395 221 600 | |||
| Treat pretransplant | 732 200 | 42 800 | 68.6 | 0.7 | 722 800 | 164.3 | 419 620 700 |
| F2 | |||||||
| Treat posttransplant | 690 500 | 65.5 | 167.2 | 393 873 200 | |||
| Treat pretransplant | 741 000 | 50 500 | 69.1 | 3.6 | 167 200 | 166.1 | 421 481 700 |
| F3 | |||||||
| Treat posttransplant | 676 600 | 58.7 | 163.4 | 391 384 200 | |||
| Treat pretransplant | 736 500 | 59 900 | 67.5 | 8.8 | 82 000 | 164.3 | 418 446 000 |
| F4 | |||||||
| Treat posttransplant | 663 000 | 49.3 | 160.5 | 383 726 900 | |||
| Treat pretransplant | 731 500 | 68 500 | 54.1 | 4.7 | 173 800 | 163.2 | 416 710 350 |
Dom, dominated (more costly, and less effective); QALM, quality adjusted life month; ICER, incremental cost‐effectiveness ratio; QALY, quality adjusted life years.
In the posttransplant treatment strategy, 98.5% of those transplanted achieved HCV cure with the primary regimen, whereas 26.0% died on the waiting list and 60.8% achieved transplantation. Life expectancy was greater than with treatment pretransplant (164.8 months vs 164.5 months), but treatment posttransplant was associated with lower quality‐adjusted life expectancy (61.8 QALM vs 65.6 QALM). Posttransplant treatment improved mortality but resulted in worse quality of life than treatment pretransplant, likely because individuals treated posttransplant lived with HCV‐attributable morbidity for longer periods. The total cost associated with this strategy was $682 400 (Table 4).
4.2. Overall cost‐effectiveness
Overall, treating pretransplant was associated with increased QALMs (65.6, SD 0.44 vs 61.8, SD 0.45), and increased costs ($735 700, SD $4546 vs $682 400, SD $4249), yielding an ICER of $162 800/QALY gained. On a cost per life‐year gained basis, however, the treatment pretransplant strategy was dominated—it provided worse outcomes at higher cost than treating posttransplant. Among the entire cohort, the 5‐year budgetary impact of treating pretransplant was $27 526 500 more expensive per 1000 HCV+ kidney transplant candidates vs treating posttransplant (pretransplant: $419 087 100 vs posttransplant: $391 560 600) (Table 4).
4.3. Cost‐effectiveness by fibrosis stage
When stratified by fibrosis stage, the optimal strategy differed for cirrhotic vs noncirrhotic individuals. Among those who entered the simulation with earlier stage fibrosis (F0‐F2), treating posttransplant improved life expectancy compared to treating pretransplant. Among those with advanced fibrosis or cirrhosis (F3‐F4), treating pretransplant improved survival. When considering quality‐adjusted survival, however, the outcomes were reversed. Among patients with no fibrosis (F0), treatment post‐transplant resulted in 1.1 more QALM (mean 68.5, SD 0.44 vs mean 69.6, SD 0.46) than treatment pretransplant. However, for every other METAVIR stage, pretransplant treatment was associated with increased QALM (F1: 68.6, SD: 0.46 vs 67.9, SD: 0.43; F4: 54.1, SD: 0.36 vs 49.3, SD: 0.35). Treating posttransplant was consistently cost‐saving (F0: $736 000, SD: $4561 vs $682 400, SD: $4465; F4: $731 500, SD: $4459 vs $663 000, SD: $4377). Moreover, the incremental cost of treating pretransplant increased with each progressive METAVIR stage such that the incremental cost among those with F0 at simulation start was $39 800 compared to $68 500 among those with stage F4. When considering cost‐effectiveness, treatment pretransplant was dominated (more costly and less effective) among those who were F0 at study entry (ICER: −$442 100) and had an ICER > $100 000/QALY for those with F1, F2, and F4 disease. When assessing the 5‐year budgetary impact of the 2 strategies, the undiscounted 5‐year cost of treating posttransplant was consistently lower than the equivalent cost of treating pretransplant across all METAVIR stages (Table 4, Figures 1 and 2). The cost‐effectiveness results were consistent, even when assuming a 1.1‐fold increased risk of death among HCV‐infected candidates (Table S1).
FIGURE 1.
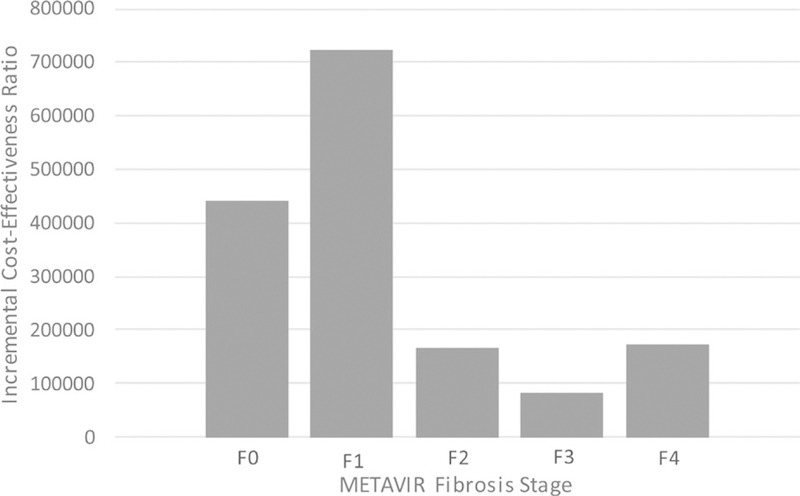
Incremental cost‐effectiveness ratios for treatment pretransplant as compared to treatment posttransplant by METAVIR fibrosis stage
FIGURE 2.
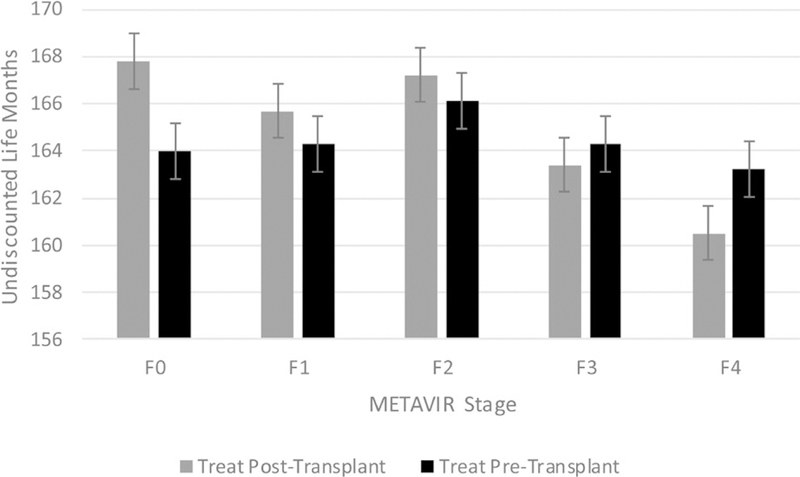
Undiscounted 5 months by METAVIR fibrosis stage and treatment strategy
4.4. Cost‐effectiveness by wait time
Treating pretransplant yielded more QALM until HCV+ candidates were likely to achieve transplantation 24 months more quickly with HCV‐infected donors (6 months difference: 65.2, SD: 0.43 vs 59.3, SD: 0.45; 24 months difference: 66.1, SD: 0.42 vs 66.5, SD: 0.42). However, treating posttransplant was consistently cost‐saving across the different times to transplant (6 months difference: $736 000, SD: $4809 vs $700 600, SD: $4469; 24 months difference: $725 600, SD: $4432 vs $634 000, SD: $4154). When the difference in waiting time was <6 months, treating pretransplant was cost‐effective (ICER: $71 500). However, once the difference in waiting time was ≥9 months, treatment pretransplant ceased to be cost‐effective (ICER: $107 100) (Table 5). When the difference in time to transplant was ≥24 months, treatment pretransplant was dominated (ICER: $−3 330 200). When assuming HCV‐infected candidates experience 1.1‐fold increased risk of death on dialysis, inferences were consistent (Table S2).
TABLE 5.
Cost‐effectiveness of treatment strategies for hepatitis C virus infection among kidney waitlist by availability of HCV+ organs (HCV+ candidates transplanted more quickly)
| Treatment strategy | Cost, $ | Incremental cost, $ |
QALM | Incremental QALM |
ICER, $/ QALY |
Undiscounted life months |
5 y budget impact, $ |
|---|---|---|---|---|---|---|---|
| 6 mo diff. | |||||||
| Treat posttransplant | 700 700 | 59.3 | 162.5 | 412 996 200 | |||
| Treat pretransplant | 736 000 | 35 300 | 65.2 | 5.9 | 71 500 | 164.2 | 423 081 000 |
| 9 mo diff. | |||||||
| Treat posttransplant | 690 700 | 60.4 | 163.5 | 407 400 300 | |||
| Treat pretransplant | 734 900 | 44 200 | 65.3 | 5.0 | 107 100 | 164.3 | 421 939 000 |
| 12 mo diff. | |||||||
| Treat posttransplant | 682 400 | 61.8 | 164.8 | 383 726 900 | |||
| Treat pretransplant | 735 700 | 53 300 | 65.6 | 3.9 | 162 800 | 164.5 | 416 710 300 |
| 18 mo diff. | |||||||
| Treat posttransplant | 658 300 | 63.9 | 167.1 | 386 909 800 | |||
| Treat pretransplant | 730 900 | 72 500 | 65.7 | 1.8 | 489 000 | 164.8 | 413 829 900 |
| 24 mo diff. | |||||||
| Treat posttransplant | 634 000 | 66.5 | 169.4 | 374 017 500 | |||
| Treat pretransplant | 725 600 | 91 600 | 66.11 | −0.3 | Dom | 165.3 | 407 602 500 |
Dom, dominated (more costly and less effective); QALM, quality‐adjusted life month; ICER, incremental cost‐effectiveness ratio; QALY, quality‐adjusted life year.
The model was further stratified by both time to transplant and fibrosis stage, showing that among patients with earlier stage disease (F1 or less), treating posttransplant was always preferred. Among those with F2 or F3, treating posttransplant was preferred unless expected time to transplant exceeded 71 months. Treating pretransplant yielded more QALM and LM for those with severe liver disease (F4) but did not meet the threshold to be considered cost‐effective (Table 6, Figure 3).
TABLE 6.
Cost‐effectiveness of treatment strategies for hepatitis C virus infection among kidney waitlist candidates by fibrosis stage at waitlist addition and time to transplant (HCV+ candidates transplanted a year more quickly than HCV− candidates)
| Treatment strategy | Cost, $ | Incremental cost, $ | QALM | Incremental QALM | ICER, $/QALY | Undiscounted life months |
|---|---|---|---|---|---|---|
| F0 < 18 mo | ||||||
| Treat posttransplant | 489 800 | 92.5 | 203.0 | |||
| Treat pretransplant | 551 200 | 61 400 | 87.2 | −5.3 | Dom | 196.3 |
| F0 18−71 mo | ||||||
| Treat posttransplant | 623 100 | 78.9 | 187.7 | |||
| Treat pretransplant | 682 100 | 59 000 | 76.6 | −2.3 | Dom | 184.6 |
| F0 > 71 mo | ||||||
| Treat posttransplant | 660 900 | 76.0 | 185.1 | |||
| Treat pretransplant | 709 900 | 49 100 | 74.4 | −1.6 | Dom | 181.8 |
| F1 < 18 mo | ||||||
| Treat posttransplant | 489 800 | 92.5 | 203.1 | |||
| Treat pretransplant | 550 700 | 60 900 | 87.2 | −5.3 | Dom | 196.2 |
| F1 18−71 mo | ||||||
| Treat posttransplant | 622 000 | 78.2 | 187.5 | |||
| Treat pretransplant | 682 400 | 60 400 | 76.6 | −1.5 | Dom | 184.6 |
| F1 > 71 mo | ||||||
| Treat posttransplant | 764 500 | 65.3 | 174.9 | |||
| Treat pretransplant | 800 000 | 35 500 | 67.2 | 1.9 | 224 381 | 173.3 |
| F2 <18 mo | ||||||
| Treat posttransplant | 489 600 | 91.9 | 202.9 | |||
| Treat pretransplant | 551 100 | 61 500 | 87.2 | −4.7 | Dom | 196.3 |
| F2 18−71 mo | ||||||
| Treat posttransplant | 618 400 | 75.6 | 186.6 | |||
| Treat pretransplant | 682 300 | 63 900 | 76.6 | 1.0 | 790 292 | 184.6 |
| F2>71 mo | ||||||
| Treat posttransplant | 756 500 | 61.0 | 173.0 | |||
| Treat pretransplant | 799 900 | 43 400 | 67.1 | 6.1 | 84 953 | 173.2 |
| F3 < 18 mo | ||||||
| Treat posttransplant | 488 700 | 88.4 | 202.4 | |||
| Treat pretransplant | 550 200 | 61 600 | 85.7 | −2.7 | Dom | 196.0 |
| F3 18−71 mo | ||||||
| Treat posttransplant | 613 200 | 69.8 | 185.0 | |||
| Treat pretransplant | 682 500 | 69 400 | 75.6 | 5.8 | 144 760 | 184.6 |
| F3>71 mo | ||||||
| Treat posttransplant | 746 200 | 54.4 | 170.5 | |||
| Treat pretransplant | 799 200 | 53 000 | 66.2 | 11.8 | 53 779 | 173.1 |
| F4 < 18 mo | ||||||
| Treat posttransplant | 481 200 | 71.1 | 198.6 | |||
| Treat pretransplant | 545 300 | 64 100 | 68.4 | −2.7 | Dom | 193.7 |
| F4 18−71 mo | ||||||
| Treat posttransplant | 596 200 | 57.3 | 179.4 | |||
| Treat pretransplant | 675 100 | 79 000 | 60.0 | 2.7 | 348 406 | 181.7 |
| F4 > 71 mo | ||||||
| Treat posttransplant | 718 700 | 45.2 | 163.6 | |||
| Treat pretransplant | 792 600 | 73 900 | 52.6 | 7.4 | 119 335 | 170.9 |
Dom, dominated (more costly and less effective); QALM, quality‐adjusted life month); ICER, incremental cost‐effectiveness ratio; QALY, quality‐adjusted life year.
FIGURE 3.
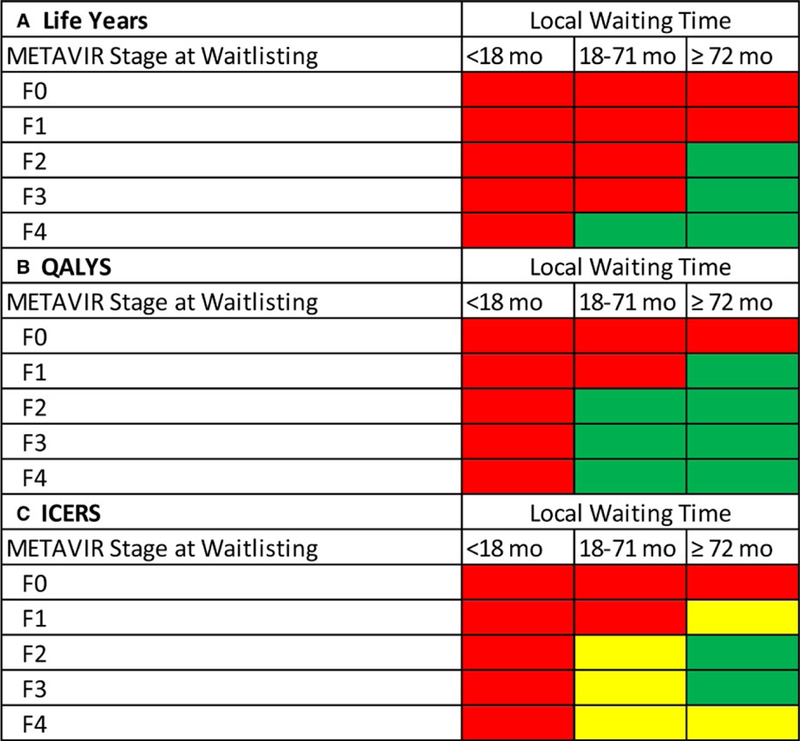
Nomogram for treatment timing (pretransplant vs posttransplant) by METAVIR Stage at waitlist addition and OPO median time to transplantation, assuming a 1‐y decrease in wait‐time with acceptance of a HCV‐infected donor
4.5. Cost‐effectiveness by waitlist mortality
When annual mortality rate on dialysis ranged from 3% to 6%, a trend toward increasing ICERs was noted. As previously noted, treatment post‐transplant was consistently cost‐saving, whereas treatment pretransplant afforded a greater number of QALMs. However, the difference in effectiveness of treatment strategy was not sufficiently substantial to designate treatment pretransplant cost‐effective. In fact, the cost of treating pretransplant per QALY gained increased substantially with greater dialysis mortality (3% ICER: $155 600; 6% ICER: $162 800; 12% ICER: $188 000; 18% ICER: $242 600; 24% ICER: $303 800) (Table 7). These inferences were consistent even when assuming a 1.1‐fold increased risk of death among HCV‐infected candidates (Table S3).
TABLE 7.
Cost‐effectiveness of treatment strategies for hepatitis C virus infection among kidney waitlist candidates
| Treatment strategy | Cost, $ | Incremental cost, $ |
QALM | Incremental QALM |
ICER, $/QALY | Undiscounted life months |
|---|---|---|---|---|---|---|
| Overall (3% annual dialysis mortality) | ||||||
| Treat posttransplant | 808 200 | 75.4 | 208.3 | |||
| Treat pretransplant | 885 100 | 76 900 | 81.3 | 5.9 | 155 600 | 211.3 |
| Overall (6% annual dialysis mortality) | ||||||
| Treat posttransplant | 682 400 | 61.8 | 164.8 | |||
| Treat pretransplant | 735 700 | 53 300 | 65.6 | 3.9 | 162 800 | 164.5 |
| Overall (12% annual dialysis mortality) | ||||||
| Treat posttransplant | 509 700 | 42.8 | 109.3 | |||
| Treat pretransplant | 541 900 | 32 100 | 44.8 | 2.1 | 188 000 | 107.3 |
| Overall (18% annual dialysis mortality) | ||||||
| Treat posttransplant | 400 700 | 31.1 | 77.0 | |||
| Treat pretransplant | 424 444 | 23 900 | 32.3 | 1.2 | 242 600 | 74.6 |
| Overall (24% annual dialysis mortality) | ||||||
| Treat posttransplant | 325 400 | 23.4 | 56.8 | |||
| Treat pretransplant | 345 700 | 20 300 | 24.2 | 0.8 | 303 800 | 54.9 |
QALM, quality‐adjusted life month; ICER, incremental cost‐effectiveness ratio; QALY, quality‐adjusted life years.
5. DISCUSSION
We used a Monte Carlo simulation model of HCV treatment timing with respect to kidney transplantation to investigate the clinical outcomes, costs, and cost‐effectiveness of pretransplant vs posttransplant treatment. We found the optimal treatment timing, which was modified by liver fibrosis stage and local wait‐times, accounting for the variation in availability of HCV‐viremic donors. For those with minimal fibrosis (F0‐F2), HCV treatment posttransplant was less costly, associated with an increased life expectancy, was often a dominating strategy, and was cost‐effective, assuming a willingness to pay of $100 000/QALY gained. In contrast, for those with advanced disease (F3‐F4), HCV treatment pretransplant was associated with more life‐years gained. Posttransplant treatment was cost‐effective if acceptance of an HCV‐viremic donor would decrease the local wait‐time by as few as 9 months. When considering liver fibrosis stage and local wait‐times together, treatment posttransplant was preferred except in individuals with moderately advanced fibrosis (F2‐3) in long wait‐time areas, who risked liver disease progression while awaiting transplantation; for these individuals the one‐year wait‐time advantage afforded by HCV‐viremic donors was insufficient to offset the increase in liver‐related mortality.
These results are consistent with our understanding of the risks of death while waiting for a kidney transplant. For patients with advanced liver disease, the risk of hepatic decompensation while on dialysis outweighs the risk of dialysis‐related mortality and therefore, treatment pretransplant enables them to remain candidates. In contrast, for patients with minimal fibrosis, dialysis‐related complications drive mortality and earlier access to transplant improves survival. Knowledge of local wait‐times and transplant center acceptance practices for HCV‐viremic organs is critical in treatment decision‐making. If patients were able to accept an HCV‐infected kidney and decrease their wait‐time to transplantation by as few as 9 months then posttransplant treatment was cost‐effective; if wait‐times were reduced by 12 months then posttransplant treatment also led to a longer life expectancy.
The reason to delay HCV therapy is to preserve a patient’s option to accept an HCV‐viremic organ, and thereby accelerate transplantation. HCV‐viremic donors provide more than 800 kidneys per year to the deceased donor pool, and this contribution is projected to increase in light of the opioid epidemic affecting many parts of the United States.31,32 However, the magnitude of that benefit varies, as not all transplant centers utilize HCV‐viremic organs and many potential HCV‐infected donors are not pursued for organ donation. Given the projected increase in availability of HCV‐infected donors, it is imperative that all centers use HCV‐infected organs whenever appropriate. However, for patients listed at centers not utilizing HCV‐infected organs, and therefore without access to them, pretransplant HCV treatment would instead be appropriate.
For patients with advanced liver disease, the calculus is different. Although they are still at risk for dialysis‐related mortality and benefit from prompt transplantation, in our model the relative contribution of mortality risk from liver disease outweighed those associated with dialysis. They should be treated pretransplant in order to preserve their candidacy for a kidney transplant alone, and this is especially true of those in long‐wait areas.
It also bears discussion that this decision analysis may be impacted by the considerable costs of DAAs in the United States. Although health plans and insurers do not pay the publically quoted cost for these medications, and we have used the average wholesale pricing (AWP) to estimate costs in our models, the financial burden associated with HCV cure remains significant. HCV‐infected kidneys shorten dialysis duration and provide an opportunity to mitigate waitlist mortality. If more favorable pricing—in line with the very low generic drug costs available in the developing world—could be obtained, insurance coverage and the cost of treatment would be less influential in the decision, possibly leading to increased rates of transplantation due to greater usage of HCV− infected organs.
Our findings are somewhat in contrast to a recent Markov decision analysis by Kiberd et al,33 which found that pretransplant treatment of HCV was preferred, as it afforded patients more life‐years, except in cases of patients with “low” HCV‐related mortality or with access to HCV‐infected organs. There are several essential methodologic differences in our studies that may account for the variation in findings: (1) Kiberd et al used general dialysis and wait‐list mortality estimates, rather than rates specific to wait‐listed HCV+ candidates;(2) they did not model liver disease by fibrosis stage, despite marked increase in patient mortality with advancing fibrosis stage; (3) they did not account for relative differences in transplantation rates at the OPO level; and (4) they assumed that all patients were cured, which does not accurately reflect real‐world experience.34,35 When we employ similar assumptions to our model, we reach similar conclusions. Our analysis adds substantially to the literature, however, because we demonstrate that the decision surrounding HCV treatment among kidney transplant candidates is critically dependent on disease stage, OPO waiting times, and organ availability. Together the literature suggests that, like most decisions in medicine, the answer to the question is complex and there is no single answer appropriate for all patients.
Our study has several strengths. Monte Carlo simulation permits us to model outcomes on the person level, and the model has “memory,” meaning we can model important clinical events including prior treatment failure. Disease states can vary on the individual level, so we can better simulate variation in fibrosis progression than is possible using other techniques. We used data derived from studies of actual HCV+ patients to generate model inputs, rather than relying on summary measures of survival, transplantation or treatment success for the dialysis, and transplant populations as a whole. We derived treatment data from published studies of the only DAAs approved for patients with ESRD, to better reflect the SVR rates they would encounter; we accounted for differences in survival based on fibrosis stage; we accounted for differences in transplant rate based on OPO and local utilization of HCV‐infected organs; and we modeled different scenarios for the wait‐time advantage afforded by acceptance of an HCV‐infected kidney. Our findings were robust and remained consistent in our sensitivity analyses.
There are limitations to our study. This is a simulation of outcomes based on the decision to treat HCV pre‐ or posttransplant; these results do not reflect actual patient outcomes. Although we used published cohort studies and registry data to derive simulation inputs, we were limited by the accuracy and completeness of those sources. HCV serostatus among transplant candidates is not reported in registry data and can only be inferred through other means, such as willingness to accept an HCV‐infected organ; it is possible that published reports of waiting list outcomes for HCV+ patients failed to capture the entire cohort. The availability of HCV‐infected organs modified the expected cost benefit with treatment; we utilized the estimates of a one‐year wait‐time advantage for those accepting an HCV‐infected organ based on published data. If the wait‐time advantage for those accepting a HCV‐infected donor in the current era is different, then our model estimates may not hold true. We are also unable to forecast HCV‐infected donor availability and thus are unable to predict outcomes assuming any changes in the donor pool. In addition, we assumed only HCV+ candidates had access to HCV‐infected organs. Should research protocols providing HCV‐infected organs to HCV−recipients gain wider acceptance,32,36,37 opening the pool of HCV‐infected donors to HCV− recipients, there would no longer be a wait‐time advantage in delaying therapy and treating pretransplant would drive care. Although we modeled elbasvir/grazoprevir as salvage therapy, treatment guidelines do not offer specific recommendations for treatment failure in patients with ESRD; inferences were consistent when we considered sofosbuvir/velpatasvir/voxilaprevir as a salvage regimen instead.
In conclusion, we have demonstrated the optimal timing of HCV treatment, which was posttransplant for the majority of patients. Posttransplant treatment was associated with greater cost‐effectiveness, longer life expectancy, and a lower budgetary impact. Patients with advanced liver disease (F3‐4) may benefit from special consideration and availability of HCV‐infected organs is critical to decision‐making. These findings should be considered when clinicians and patients discuss HCV therapy, and may serve as a foundation for insurer coverage for HCV treatment with DAAs peritransplant.
Supplementary Material
ACKNOWLEDGMENTS
The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for/of the SRTR. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government. The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
Funding information
Minneapolis Medical Research Foundation; National Institute on Drug Abuse, Grant/Award Number: (P30DA040500)
Abbreviations:
- AWP
average wholesale pricing
- CKD
chronic kidney disease
- DAA
direct‐acting antivirals
- ESRD
end‐stage renal disease
- HCV−
hepatitis C negative
- HCV+
hepatitis C infection
- ICER
incremental cost‐effectiveness ratio
- IQR
interquartile range
- KDPI
Kidney Donor Profile Index
- LM
life months
- OPO
organ procurement organization
- QALM
quality‐adjusted life month
- QALY
quality‐adjusted life year
- QoL
quality of life
- SD
standard deviation
- SRTR
Scientific Registry of Transplant Recipients
- SVR
sustained viral response
- UNOS
United Network for Organ Sharing
- USRDS
United States Renal Data System
Footnotes
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr Sawinski has consulted for Merck (advisory board), the manufacturer of elbasvir‐grazoprevir. Dr Reese has received investigator initiated grants from Merck to support trials of using HCV‐infected organs in HCV‐negative transplant recipients. The other authors have no conflicts of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Gane E, Lawitz E, Pugatch D, et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. New England J Med 2017;377(15):1448–1455. [DOI] [PubMed] [Google Scholar]
- 2.Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment‐naive and treatment‐experienced patients with hepatitis C virus genotype 1 infection and stage 4‐5 chronic kidney disease (the C‐SURFER study): a combination phase 3 study. Lancet (London, England) 2015;386(10003):1537–1545. [DOI] [PubMed] [Google Scholar]
- 3.Hoofnagle JH, Sherker AH. Therapy for hepatitis C—The costs of success. N Eng J Med 2014;370(16):1552–1553. [DOI] [PubMed] [Google Scholar]
- 4.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2016 annual data report: kidney. Am J. Transplant 2018;18(suppl 1):18–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalantar‐Zadeh K, Kilpatrick RD, McAllister CJ, et al. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol 2007;18(5):1584–1593. [DOI] [PubMed] [Google Scholar]
- 6.Goodkin DA, Bragg‐Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 2003;14(12):3270–3277. [DOI] [PubMed] [Google Scholar]
- 7.Sawinski D, Forde KA, Eddinger K, et al. Superior outcomes in HIV‐positive kidney transplant patients compared with HCV‐infected or HIV/HCV‐coinfected recipients. Kidney Int 2015;88(2):341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Axelrod DA, Schnitzler MA, Alhamad T, et al. The impact of direct‐acting antiviral agents on liver and kidney transplant costs and out‐comes [published online ahead of print 2018]. Am J Transplant [DOI] [PMC free article] [PubMed]
- 9.Kucirka LM, Singer AL, Ros RL, Montgomery RA, Dagher NN, Segev DL. Underutilization of hepatitis C‐positive kidneys for hepatitis C‐positive recipients. Am J Transplant 2010;10(5):1238–1246. [DOI] [PubMed] [Google Scholar]
- 10.Shelton BA, Sawinski D, Mehta S, Reed RD, MacLennan PA, Locke JE. Kidney transplantation and waitlist mortality rates among candidates registered as willing to accept a hepatitis C infected kidney. Transpl Infect Dis 2017;20:e12829v. [DOI] [PubMed] [Google Scholar]
- 11.Reese PP, Abt PL, Blumberg EA, Goldberg DS. Transplanting hepatitis C‐positive kidneys. New Eng J Med 2015;373(4):303–305. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg DS, Blumberg E, McCauley M, Abt P, Levine M. Improving organ utilization to help overcome the tragedies of the opioid epidemic. Am J Transplant 2016;16(10):2836–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation 2009;88(2):231–236. [DOI] [PubMed] [Google Scholar]
- 14.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost‐effectiveness analyses: second panel on cost‐effectiveness in health and medicine. JAMA 2016;316(10):1093–1103. [DOI] [PubMed] [Google Scholar]
- 15.Linas BP, Barter DM, Morgan JR, et al. The cost‐effectiveness of sofosbuvir‐based regimens for treatment of hepatitis C virus genotype 2 or 3 infection. Ann Intern Med 2015;162(9):619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linas BP, Barter DM, Leff JA, et al. The cost‐effectiveness of improved hepatitis C virus therapies in HIV/hepatitis C virus coinfected patients. AIDS (London, England) 2014;28(3):365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost‐effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Inter Med 2015;162(6):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chhatwal J, Samur S, Kues B, Ayer T, Roberts MS. Optimal timing of hepatitis C treatment for patients on the liver transplant waiting list. Hepatology 2017;65(3):777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage‐specific fibrosis progression rates in chronic hepatitis C virus infection: a meta‐analysis and meta‐regression. Hepatology 2008;48(2):418–431. [DOI] [PubMed] [Google Scholar]
- 20.Davis KL, Mitra D, Medjedovic J, Beam C, Rustgi V. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol 2011;45(2):e17–e24. [DOI] [PubMed] [Google Scholar]
- 21.Chong CA, Gulamhussein A, Heathcote EJ, et al. Health‐state utilities and quality of life in hepatitis C patients. Am J Gastroenterol 2003;98(3):630–638. [DOI] [PubMed] [Google Scholar]
- 22.Grieve R, Roberts J, Wright M, et al. Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut 2006;55(9):1332–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein K, Dalziel K, Walker A, et al. Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice. Health Technol Assess (Winchester, England) 2002;6(31):1–122. [PubMed] [Google Scholar]
- 24.Bruno S, Zuin M, Crosignani A, et al. Predicting mortality risk in patients with compensated HCV‐induced cirrhosis: a long‐term prospective study. Am J Gastroenterol 2009;104(5):1147–1158. [DOI] [PubMed] [Google Scholar]
- 25.Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis 2011;52(7):889–900. [DOI] [PubMed] [Google Scholar]
- 26.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all‐cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012;308(24):2584–2593. [DOI] [PubMed] [Google Scholar]
- 27.Roth D, Gaynor JJ, Reddy KR, et al. Effect of kidney transplantation on outcomes among patients with hepatitis C. J Am Soc Nephrol 2011;22(6):1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbott KC, Lentine KL, Bucci JR, Agodoa LY, Peters TG, Schnitzler MA. The impact of transplantation with deceased donor hepatitis c‐positive kidneys on survival in wait‐listed long‐term dialysis patients. Am J Transplant 2004;4(12):2032–2037. [DOI] [PubMed] [Google Scholar]
- 29.United States Renal Data System. USRDS annual data report: epidemiology of kidney disease in the United States 2017 Bethesda, MD: United States Renal Data System; 2017. [Google Scholar]
- 30.Recipients SRoT. Program‐Specific Reports 2018. https://www.srtr.org/reports-tools/program-specific-reports/. Accessed May 10, 2018.
- 31.Anesi JA, Goldberg DS. Maximizing utilization of the donor pool by appropriate classification of hepatitis C antibody‐positive donors. Am J Transplant 2017;17(11):2757–2758. [DOI] [PubMed] [Google Scholar]
- 32.Durand CM, Bowring MG, Brown DM, et al. Direct‐acting antiviral prophylaxis in kidney transplantation from hepatitis C virus‐infected donors to noninfected recipients: an open‐label nonrandomized trial. Ann Intern Med 2018;168(8):533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiberd B, Doucette K, Vinson A, Tennankore K. Hepatitis C virus infected kidney wait list patients: treat now or treat later? [published online ahead of print 2018] Am J Transplant [DOI] [PubMed]
- 34.Saxena V, Koraishy FM, Sise ME, et al. Safety and efficacy of sofosbuvir‐containing regimens in hepatitis C‐infected patients with impaired renal function. Liver Int 2016;36(6):807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saxena V, Khungar V, Verna EC et al. Safety and efficacy of current direct‐acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: results from the HCV‐TARGET study. Hepatology (Baltimore, Md.) 2017;66(4):1090–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCauley M, Mussell A, Goldberg D, et al. Race, risk, and willingness of end‐stage renal disease patients without hepatitis C virus to accept an HCV‐infected kidney transplant. Transplantation 2018;102(4):e163–e170. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg DS, Abt PL, Blumberg EA, et al. Trial of transplantation of HCV‐infected kidneys into uninfected recipients. New Eng J Med 2017;376(24):2394–2395. [DOI] [PubMed] [Google Scholar]
- 38.Kohli A, Alshati A, Georgie F, Manch R, Gish RG. Direct‐acting antivirals for the treatment of chronic hepatitis C in patients with chronic kidney disease. Therapeutic Adv Gastroenterol 2016;9(6):887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogan TJ, Elliott WJ, Seto AH, Bakris GL. Antihypertensive treatment with and without benazepril in patients with chronic renal insufficiency: a US economic evaluation. Pharmaco Economics 2002;20(1):37–47. [DOI] [PubMed] [Google Scholar]
- 40.Sherman KE, Sherman SN, Chenier T, Tsevat J. Health values of patients with chronic hepatitis C infection. Arch Intern Med 2004;164(21):2377–2382. [DOI] [PubMed] [Google Scholar]
- 41.Snyder RA, Moore DR, Moore DE. More donors or more delayed graft function? A cost‐effectiveness analysis of DCD kidney transplantation. Clin Transplant 2013;27(2):289–296. [DOI] [PubMed] [Google Scholar]
- 42.Haller M, Gutjahr G, Kramar R, Harnoncourt F, Oberbauer R. Cost‐effectiveness analysis of renal replacement therapy in Austria. Nephrol Dial Transplant 2011;26(9):2988–2995. [DOI] [PubMed] [Google Scholar]
- 43.Hirth RA, Held PJ, Orzol SM, Dor A. Practice patterns, case mix, Medicare payment policy, and dialysis facility costs. Health Services Res 1999;33(6):1567–1592. [PMC free article] [PubMed] [Google Scholar]
- 44.Menzin J, Lines LM, Weiner DE, et al. A review of the costs and cost effectiveness of interventions in chronic kidney disease: implications for policy. Pharmaco Econom 2011;29(10):839–861. [DOI] [PubMed] [Google Scholar]
- 45.Painter P, Krasnoff JB, Kuskowski M, Frassetto L, Johansen K. Effects of modality change on health‐related quality of life. Hemodial Int 2012;16(3):377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajan M, Lai KC, Tseng CL, et al. Estimating utilities for chronic kidney disease, using SF‐36 and SF‐12‐based measures: challenges in a population of veterans with diabetes. Quality Life 2013;22(1):53–64. [DOI] [PubMed] [Google Scholar]
- 47.Whiting JF, Zavala EY, Alexander JW, First MR. The cost‐effectiveness of transplantation with expanded donor kidneys. Transplant Proc 1999;31(1–2):1320–1321. [DOI] [PubMed] [Google Scholar]
- 48.Barnieh L, Manns BJ, Klarenbach S, McLaughlin K, Yilmaz S, Hemmelgarn BR. A description of the costs of living and standard criteria deceased donor kidney transplantation. Am J Transplant 2011;11(3):478–488. [DOI] [PubMed] [Google Scholar]
- 49.Ferenci P, Bernstein D, Lalezari J, et al. ABT‐450/r‐ombitasvir and dasabuvir with or without ribavirin for HCV. New Eng J Med 2014;370(21):1983–1992. [DOI] [PubMed] [Google Scholar]
- 50.Chaiwat O, Lang JD, Vavilala MS, et al. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology 2009;110(2):351–360. [DOI] [PubMed] [Google Scholar]
- 51.Gao X, Stephens JM, Carter JA, Haider S, Rustgi VK. Impact of adverse events on costs and quality of life in protease inhibitor‐based combination therapy for hepatitis C. Expert Rev Pharmacoeconomics Outcomes Res 2012;12(3):335–343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


