Abstract
Objective.
Maintenance of lymphatic permeability is essential for normal lymphatic function during adulthood, but the precise signaling pathways that control lymphatic junctions during development are not fully elucidated. The Gs-coupled adrenomedullin signaling pathway is required for embryonic lymphangiogenesis and the maintenance of lymphatic junctions during adulthood. Thus, we sought to elucidate the downstream effectors mediating junctional stabilization in lymphatic endothelial cells.
Approach and Results.
We knocked-down both Rap1A and Rap1B isoforms in human neonatal dermal lymphatic cells (hLECs), and genetically deleted the mRap1 gene in LECs by producing 2 independent, conditional Rap1a/b knockout mouse lines. Rap1A/B knockdown caused disrupted junctional formation with hyperpermeability, and impaired adrenomedullin-induced lymphatic junctional tightening as well as rescue of histamine-induced junctional disruption. Less than 60% of lymphatic-Rap1a/b knockout embryos survived to E13.5 exhibiting interstitial edema, blood-filled lymphatics, disrupted lymphovenous valves and defective lymphangiogenesis. Consistently, inducible lymphatic-Rap1a/b deletion in adult animals prevented adrenomedullin rescue of histamine-induced lymphatic leakage and dilation.
Conclusions.
Rap1 serves as the dominant effector downstream of adrenomedullin to stabilize lymphatic junctions. Rap1 is required for maintaining lymphatic permeability and driving normal lymphatic development.
Keywords: Adrenomedullin, Lymphatics, cell junctions, Rap1, PKA, Endothelium/Vascular Type/Nitric Oxide, Cell Signaling/Signal Transduction
Introduction
Lymphatic vessels are uniquely adapted to continuously drain interstitial fluid, protein and cells, playing a key role in tissue homeostasis, inflammation and other disease conditions involving excessive interstitial fluid1. Lymph fluid is ultimately returned to the blood circulation via lymphovenous valves (LVV) which are formed during the process of venous-lymphatic separation during embryogenesis2, 3, and maintained in adulthood by platelets, thereby preventing the shunting of blood into the lymphatic circulation4. Structural and functional heterogeneity exists within the lymphatic vascular system, as exemplified by different types of endothelial junctions. For example, lymphatic collecting vessels show continuous “zipper-like” junctions, similar in morphology to the junctions of blood capillaries, while lymphatic capillaries show discontinuous, “button-like” junctions5, 6. Appropriate permeability and pumping activity achieved by the different types of junctions in lymphatic vessels ensures normal lymph flow, efficient interstitial fluid uptake and pathogen collection7, 8. Many disease conditions involve dysfunction of the lymphatic barrier and valves, such as lymphangiectasia, lymphedema, and inflammation. Therefore, understanding how lymphatic endothelial cell (LEC) junctions are formed and regulated is of considerable clinical interest and a potential area for therapeutic intervention.
Rap1 is a member of the Ras subfamily of small GTPases that was originally discovered as a mediator of integrin activation and adhesion9, leukocyte arrest and extravasation10, endothelial cell migration, and angiogenesis11. More recent studies have highlighted a critical role for Rap1 in cell-cell junctional regulation12, 13. Like all GTPases, Rap1 is activated by guanine nucleotide exchange factors (GEFs), which promote the transition from an inactive GDP-bound state to an active GTP-bound state. Rap1 exerts its endothelial barrier strengthening effect mainly by promoting cytoskeletal rearrangements, such as increasing circumferential actin bundles and reducing radial stress fibers14–17. Rap1a and Rap1b, two isoforms of Rap1, share 95% sequence homology at the amino acid level. Endothelial-specific deletion of both isoforms using Tie2-Cre causes hemorrhage and embryonic lethality18. At E10.5, tissue degeneration and cranial hemorrhage are found in a significant fraction (50%) of Tie2-double Rap1a/b KO embryos18, and no Tie2-double Rap1a/b KO animals survive to E15.5. Due to the prominent cardiovascular defects and early developmental lethality of EC-Rap1 KO mice, the elucidation of Rap1 function in developmental lymphangiogenesis is precluded. Therefore, whether Rap1 is required for the development of lymphatics or for lymphatic barrier function remains unknown.
A potent lymphatic barrier tightening factor is adrenomedullin (AM=protein, Adm=gene); a multifunctional peptide involved in a variety of physiological processes such as lymphangiogenesis and cardiovascular homeostasis19. Using mouse models, we previously identified an essential role for AM and its receptor complex in lymphatic development during embryogenesis20 and maintenance of normal lymphatic function in adults21. AM signals through the G protein coupled receptor calcitonin receptor-like receptor (CLR=protein, Calcrl=gene) and receptor activity modifying protein 2 (Ramp2) complex22. Calcrl global inducible knockout (KO) mice have lymphatic junctional disruption in numerous organ systems resulting in lymphatic hyperpermeability and lymphatic insufficiency which fully recapitulate the clinical symptoms of lymphangiectasia, characterized as dilation of lymphatic vessels21. Furthermore, previous studies have demonstrated that AM stabilizes the lymphatic endothelial barrier both in vitro and in vivo23, 24. However, the downstream effectors that mediate this stabilization remain elusive. As a Gs-coupled signaling pathway, one of the major downstream effectors of AM stimulation is cAMP, which acts on both PKA (protein kinase A) and Epac1 (exchange protein directly activated by cAMP)15, 25. Given that the AM signaling receptor complex, consisting of Calcrl and Ramp2, is enriched in LECs26, 27, we hypothesized that Epac1-Rap1 is a downstream effector thereby imparting functional effects on LEC junction permeability.
We used human neonatal dermal lymphatic cells (HMVEC-dLyNeo-Der Lym Endo Cells, herein, hLECs) and 2 different LEC-double Rap1a/b KO mouse models to demonstrate that Rap1 plays an important role in the formation and stabilization of LEC junctions and the development of lymphatics. More importantly, we place Rap1 as the key effector downstream of AM responsible for tightening lymphatic junctions under basal conditions and for restoring histamine-induced junctional disruption. Our results give insights into the signaling mechanism underlying AM-stabilization of LEC junctions, which could serve as a potential therapeutic target to modify lymphatic permeability.
Materials and Methods
The authors declare that all supporting data are available within the article [and its online supplementary files]
Other detailed methods are presented in the Online Data Supplement.
Real-time Cellular Impedance Analysis (RTCA)
To quantify barrier function, RTCA assays were performed on the xCELLigence® RTCA DP platform (ACEA Biosciences, Inc.). This method uses electrical impedance to monitor cells grown on micro-electrode coated plates. Changes in impedance reflect changes in barrier function and permeability28. Experiments were essentially performed as previously described29. Briefly, 45,000 hLECs were seeded into each well of an E-Plate 16 (ACEA Biosciences, Inc.) and placed onto the RTCA DP platform in a 37°C/5% CO2 incubator. Monolayer impedance was monitored overnight until a stable cell index was reached, indicating maximal barrier function (usually 16–24 hours after plating). For histamine/AM experiments, upon reaching a stable plateau, histamine or media alone was added for 10 min, then AM or media alone was added for the duration of the experiment. For PKI experiments, PKI or media alone was added for 1 hr, followed by AM or media alone for the remaining time.
hLEC Treatments
Unless noted below, the following treatment conditions were used: hLECs were treated with AM (Bachem, 10 nM) for 30 min, histamine pretreatment (10 μM, Sigma-Aldrich) was for 10 min, and PKI pretreatment (Sigma-Aldrich, 10 μM) was for 1 hour. In Figure 2E XPerT assay, hLECs were treated with AM (10 nM) for 6 hrs. For RTCA assays, treatment of AM (10 nM) was for the remaining duration of the experiment. For junction formation studies, hLECs were transduced with shCtrl or shRap1A/B adenovirus in 10-cm dishes, and 48 hr later were split into E-plate 16 to let new junctions form. For other RTCA assays, hLECs were plated in the E-plate 16 to let the junctions form first before adding the adenovirus to knock down Rap1 and study the effect of AM.
Figure 2. Rap1 is required for AM to linearize and tighten hLEC junctions.
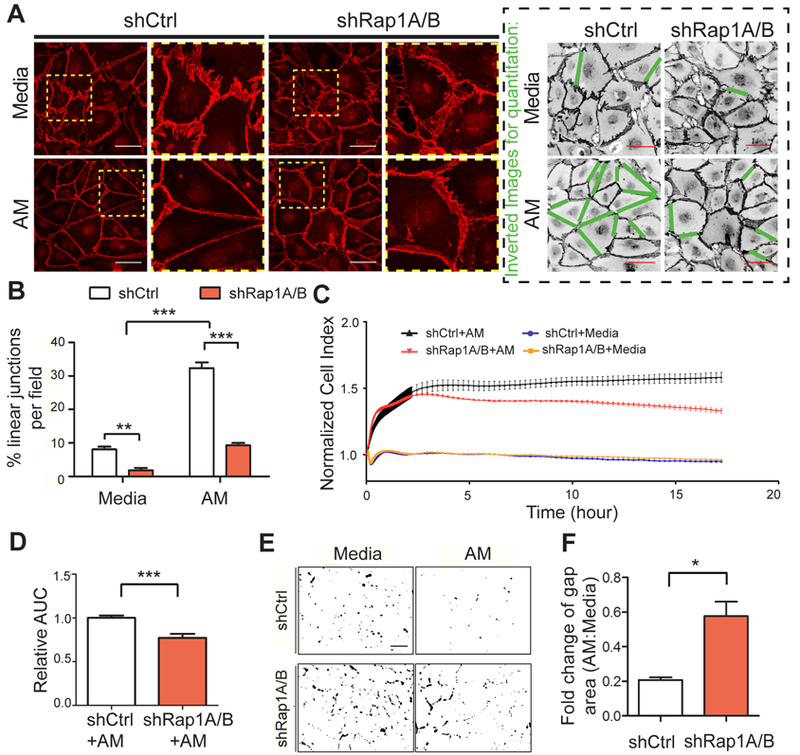
A, Representative images of hLEC junctions visualized by anti-VE-cadherin antibodies (red) in both shCtrl and shRap1A/B hLECs, treated with media alone or AM (10 nM, 30 min). The boxed areas are enlarged to the right of the original images. Inverted black and white images illustrate quantitation method (green lines indicate junctions scored as linear). Scale bar, 50 μm. B, Quantitation of the percentage of linear junctions per field (relative to total number of junctions) for each condition, n=5–6 for each condition. C, Representative RTCA trace for shCtrl vs. shRap1A/B showing the effect of AM (10 nM) or media-alone treatment. Cell index is normalized to time point at which AM treatment started. D, Quantitation of AUC for normalized cell index from each condition; 3–4 replicates for each condition and 3 independent experiments were performed. E, Representative thresholded images of XPerT assay for each condition to demonstrate quantitation method. Black areas indicate regions of high local permeability labeled by fluorescent-avidin binding to exposed biotinylated collagen matrix. Scale bar, 100 μm. F, Quantitation of gap area ratio after AM treatment of shCtrl and shRap1A/B hLEC monolayers. Data plotted as a ratio of gaps in AM-treated/media alone for each group; 5 fields per replicate, 3 replicates per condition and 3 independent experiments were performed. Data are presented as means ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001.
Monolayer Gap Assay
Monolayer gaps were assessed according to previously described methods30. Briefly, knockdown hLECs (GFP-positive) were co-cultured at a 1:4 ratio with uninfected hLECs on coverslips in 24-well plates and allowed to grow until a monolayer was formed. After fixation in 4% PFA, hLECs were immunostained with anti-VE-cadherin antibody (C-19, Santa Cruz) to visualize adherens junctions.
XPerT assay
The “XPerT” assay is another technique to test monolayer barrier function31. For this assay, hLECs were grown on a biotinylated-collagen substrate, then following each given treatment condition, hLEC monolayers were briefly (2 min) incubated with Alexa350-labeled streptavidin (Life Technologies) added directly to culture medium. Under conditions in which monolayer permeability is increased, fluorescent-streptavidin can permeate gaps in the cell monolayer and bind to the exposed biotinylated substrate. Coverslips were immediately washed and fixed with 3.75% formaldehyde and processed for fluorescence microscopy imaging.
Lymphatic Permeability Assay
Ear lymphatic permeability was assessed as previously described21. 7–10 days after the last dose of Tamoxifen, mice were anesthetized with Avertin. We intradermally injected the ears of anesthetized Rap1afl/fl, Rap1bfl/fl; Prox1CreERT2 mice or Rap1afl/fl; Rap1bfl/fl mice with 3 μl of either histamine (10 mM, Sigma-Aldrich) or a combination of histamine (10 mM) and AM (10 μM) in saline with a 10 ul Hamilton syringe fitted with a 30 gauge needle. Three minutes later, the ears were subjected to intradermal injection of 3 μl 0.5% Evans Blue dye (Sigma-Aldrich) in saline. Images of the ears were obtained at 0 min and 10 min after dye injection.
Statistical Analysis
Normality test was performed using Shapiro-Wilk test and equal variance was examined. Unpaired 2-tailed t-test was used for comparisons between two means. If variance was significantly different, Welch’s correction was performed. For multiple comparisons with single factor, one-way ANOVA followed by Bonferroni post hoc test was used. Two-way ANOVA followed by Bonferroni post hoc test was performed for multiple comparisons with two factors. All data are presented as mean ± SEM unless otherwise noted. Statistical significance was set at *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; P > 0.05, not significant (n.s.).
Results
Rap1 is required to form functional LEC junctions in vitro
To determine whether Rap1 plays a role in establishing lymphatic endothelial junctions, we utilized an adenovirally-delivered RNAi approach for efficient knockdown of both Rap1A and Rap1B isoforms in human lymphatic endothelial cells (hLECs)30. mRNA expression of both Rap1A and Rap1B was significantly decreased in hLECs transduced simultaneously with Rap1A and Rap1B shRNA adenovirus, i.e. total Rap1 (“shRap1A/B”) compared with hLECs with a non-targeting negative control adenovirus (“shCtrl”) (Figure 1A). To assess hLEC junctional integrity, knockdown hLECs expressing co-cistronic GFP as a marker for transduction were co-cultured with uninfected hLECs at a ratio of 1:4 and stained for VE-cadherin as a marker for intact junctions. As shown in Figure 1B, continuous VE-cadherin-positive hLEC junctions prominently formed between the GFP-positive shCtrl cells and adjacent uninfected cells (white arrows), while the VE-cadherin staining revealed discontinuous and negative gaps in staining between shRap1A/B Rap1 knockdown cells and adjacent uninfected cells (arrowheads). Quantitation revealed that Rap1A/B knockdown hLECs had a significantly higher percentage of cells with gaps compared to shCtrl hLECs (Figure 1C), demonstrating that expression of Rap1 is required for establishment of hLEC junctions.
Figure 1. Rap1 is required for hLECs to form endothelial cell-cell junctions.
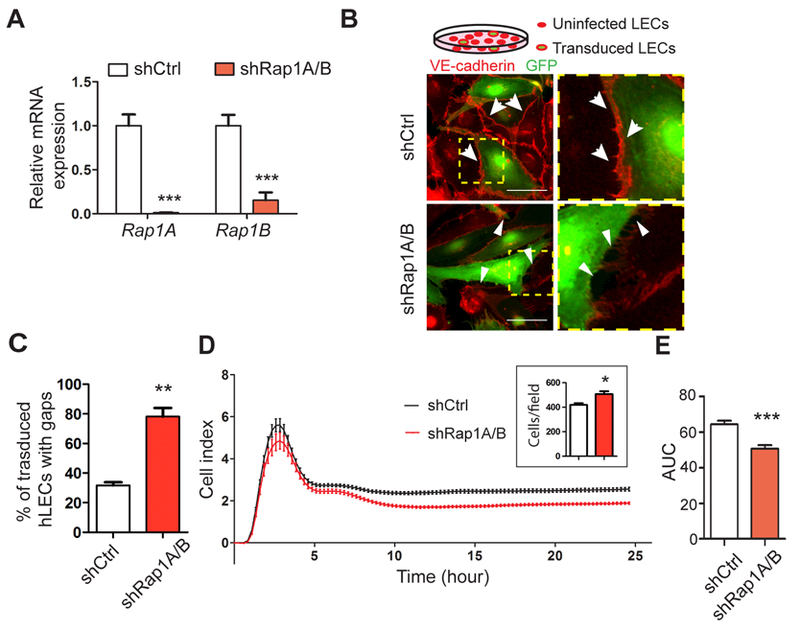
A, Rap1a and Rap1b mRNA levels in negative control knockdown hLECs (shCtrl) compared to hLECs with knockdown of both Rap1 isoforms (shRap1A/B); 3 replicates for each condition and 3 independent experiments were performed. B, Integrity of endothelial cell-cell junctions formed between co-cultured shCtrl or shRap1A/B hLECS (GFP-positive, green), and uninfected hLECs. Cell junctions are visualized using anti-VE-cadherin antibodies (red). The boxed areas are enlarged at right. Arrows indicate VE-cadherin-positive intact junctions; arrowheads point to VE-cadherin-negative gaps between adjacent cells. Scale bar, 50 μm. C, Gap quantitation of control vs. shRap1A/B hLECs; 4–5 fields per condition, 3 replicates for each condition and 3 independent experiments were performed. D, Representative RTCA trace of monolayer impedance (Cell Index) of shCtrl and shRap1A/B hLECs over time. Inset graph shows cell count as confirmation that Rap1-knockdown cells were not seeded at a lower cell density. E, Quantitation of the Area Under the Curve (AUC), n=8. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001.
Another technique for assessing endothelial monolayer barrier function is the real-time electrical cell-substrate adhesion assay (RTCA), which measures the electrical impedance across a cell monolayer. Cell impedance over time, shown graphically as area under the curve (AUC), was significantly reduced in shRap1A/B hLEC monolayers compared with shCtrl hLEC monolayers, indicating impaired barrier properties (Figure 1D, representative trace; 1E, quantification). The inset graph in 1D shows cell count as confirmation that Rap1-knockdown cells were not seeded at a lower cell density. Together, these results establish Rap1 as a fundamental GTPase required for the formation of LEC junctions.
Rap1 is required for AM-induced junctional stabilization
AM peptide stimulates a robust reorganization and linearization of LEC junctions both in vitro and in vivo23, resulting in junctional strengthening and stability of lymphatics32, 33. As a Gs-coupled signaling pathway, one of the major downstream effectors of AM stimulation is cAMP. Since cAMP is also a known regulator of Rap1 activity, we hypothesized that AM could exert its LEC junctional stabilization effects by signaling through Rap1. As expected, AM treatment of control (shCtrl) hLECs caused VE-cadherin-positive junction morphology to change from a jagged pattern to a continuous linear morphology (Figure 2A). In contrast, knockdown of Rap1 (shRap1A/B) prevented this AM-induced reorganization of junctional VE-cadherin. Quantification of junctional linearity in shCtrl vs. shRap1A/B hLECs monolayers confirmed that Rap1 is required for junctional linearization (Figure 2B). Functional RTCA assays further showed that AM could not fully exert its function in increasing the impedance of hLEC monolayer in shRap1A/B hLECs (Figure 2C, representative trace; 2D, quantification) compared to shCtrl cells.
Direct measurement of local permeability changes in response to AM treatment was also performed using the “express micromolecule permeability testing” assay (XPerT)31. This technique uses image analysis thresholding to detect and quantify regions of local junction permeability by the binding of a fluorescent-streptavidin tracer to any exposed biotinylated collagen substrate, i.e. to intercellular gaps. Representative threshold images showing gap area fluorescence in shCtrl and shRap1aA/B monolayers with or without AM treatment suggested that AM reduction of the gap area was greater in shCtrl hLECs, and compromised in hLECs with knockdown of Rap1 (Figure 2E). Indeed, quantitation demonstrated that upon AM treatment, both shRap1A/B-treated and shCtrl-treated hLEC monolayers had decreased permeability, depicted graphically as less than 1 when normalized to the gap area of the media-treated groups. However, shRap1A/B-treated hLEC monolayers had increased normalized permeability as indicated by a significantly greater ratio of fluorescent-streptavidin-positive puncta areas compared to shCtrl (Figure 2F). Together, these results consistently establish a critical role for Rap1 in promoting AM-induced stabilization of LEC junctions.
Rap1 Deficiency Prevents AM from Restoring Histamine-stimulated Junction Disruption
Histamine is rapidly released from mast cells and basophils upon allergic inflammation and strongly increases vascular permeability, contributing to edema and inflammation in many conditions34. We treated hLECs with histamine (10 μM) for 10 min and found that cell-cell junctions shown by VE-cadherin staining become more jagged with markedly discernible gaps between cells, indicative of junction disruption. Additionally, cytoplasmic F-actin phalloidin staining was upregulated and reorganized into more prominent stress fibers. However, if hLECs were treated with AM (10 nM) for 30 min following histamine application, these cell-cell junctions became more “zipper-like” and less disrupted, with fewer cytoplasmic stress fibers (Figure 3A, representative images; 3B, quantification). Likewise, RTCA experiments showed that monolayer impedance dropped after histamine was added, indicating a loss of barrier properties (Figure 3C), yet AM treatment restored the impedance much more rapidly than the self-recovery of histamine alone group (Figure 3C). Quantification of cell index at the time point 30 min after AM treatment revealed a similar increase in normalized impedance for both histamine and media treated hLECs (Figure 3D). Together, these results indicate that AM is capable of potently restoring both the disrupted morphology of cell-cell junctions and the decreased barrier function induced by histamine.
Figure 3. AM rescues histamine-induced hLEC junction disruption, and this requires Rap1.
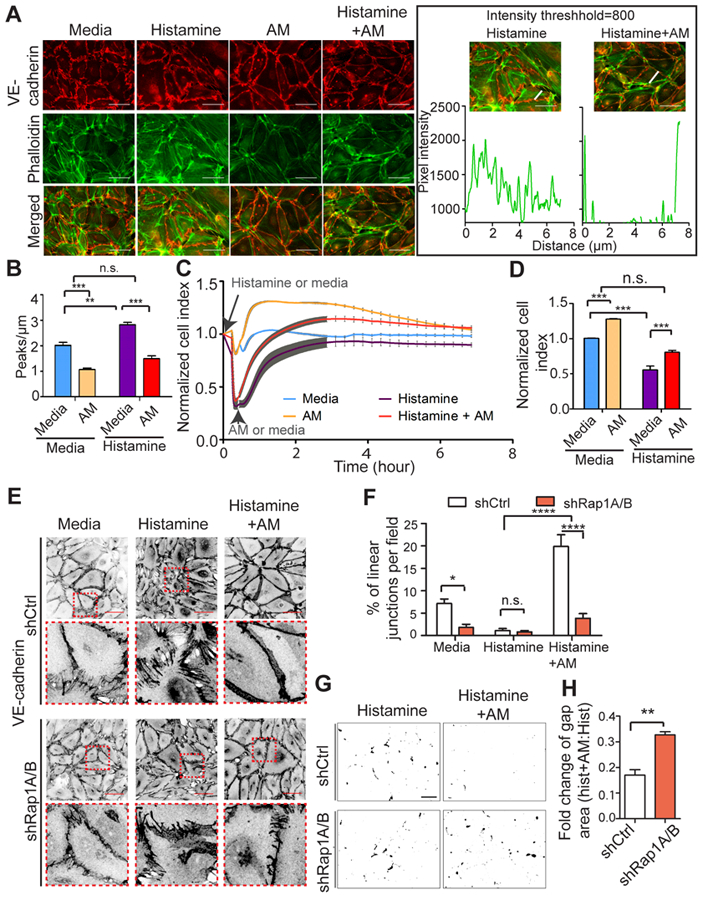
A, Cell junctions were visualized using anti-VE-cadherin antibodies (red); F-actin was visualized using phalloidin (green). The boxed area shows the method for quantification of F-actin using Histamine and Histamine+AM groups as examples. Graph represents fluorescent pixel intensity measured using image J, along the drawn line. Scale bar, 50 μm. B, Quantitation of F-actin. Graph shows number of peaks per micron above a threshold value set at 800. n=5–6. C, Representative RTCA trace of hLEC monolayers pretreated with histamine (10 μM) or media for 10 min followed by +/− AM addition. Cell index is normalized to point at which histamine or media was added, and was assessed for media-, AM-, histamine-, and histamine +AM combination-treated group. D, Quantitation of normalized cell index (30 min after AM treatment) from 3 independent experiments at the 30 min AM time point (10 nM); 3–4 replicates for each conditions and 3 independent experiments were performed. E, Inverted representative images and quantitation of cell junctions visualized using anti-VE-cadherin antibodies for both shCtrl and shRap1A/B hLECs in media, histamine, and histamine +AM combination treatment. The boxed areas are enlarged at the bottom side of the original images. Scale bar, 50 μm. F, Quantitation of the percentage of linear junctions per field (relative to total number of junctions) for each condition; n=6. G, Representative thresholded images of XPerT assay for each condition. Black areas indicate regions of high local permeability labeled by fluorescent-avidin binding to exposed biotinylated collagen matrix. Scale bar, 100 μm. H, XPerT assay quantitation of gap area ratio after AM treatment (10 nM, 30 min) of shCtrl and shRap1A/B hLEC monolayers pretreated with histamine (10 μM, 10 min). Data plotted as ratio of gaps in AM +histamine/histamine alone for each group; 5 fields per condition and 3 independent experiments were performed. Data are presented as means ± SEM. n.s., not significant, *p < 0.05, **p < 0.01, and ***p < 0.001.
Using this methodology, we next used control and knockdown Rap1 LECs to determine whether Rap1 might be a critical downstream effector of the AM restoration of histamine-stimulated junction disruption. Cell-cell junctions visualized by VE-cadherin staining demonstrated similar histamine-induced junction disruption in both shCtrl and shRap1A/B hLECs: jagged junctions and discernible gaps between cells. However, upon AM treatment, markedly more linearized junctions were observed in the histamine-pretreated shCtrl hLECs compared with shRap1A/B hLECs (Figure 3E, representative images; 3F, quantification). Gap area was quantified by XPerT assay in shCtrl and shRap1A/B monolayers treated with histamine only, or with histamine and AM. Representative threshold images showing gap area fluorescence in shCtrl and shRap1A/B monolayers treated with histamine only or histamine and AM combined suggested that AM-induced reduction of gap area is greater in shCtrl hLECs (Figure 3G). Quantification showed that AM reduced fluorescence gap area in both shRap1A/B and shCtrl hLECs pretreated with histamine, which was indicated by showing normalized gap area (a ratio of the gap area in histamine and AM-treated to that in histamine-treated hLECs) as less than 1. However, shRap1A/B hLECs treated with histamine and AM combined had increased normalized gap area compared with shCtrl, demonstrating that AM was less effective in reducing gap area caused by histamine in the absence of Rap1 (Figure 3H). Overall, these results demonstrate that loss of Rap1 hinders AM from ameliorating histamine-induced lymphatic junction disruption and hyperpermeability.
PKA is an auxiliary effector of AM signaling
We have so far shown that Rap1 plays a key role in LEC junction formation, AM-induced junctional stabilization and barrier function, and the restorative effect of AM on histamine-induced LEC junctional disruption and barrier permeability. However, we noticed that AM still elicited a slight improvement of junction disruption even in Rap1-deficient hLECs (shRap1A/B), both with or without histamine (Figure 2A-D and Figure 3E-F). This small improvement may be due to the small amount of residual Rap1 in shRap1A/B knockdown hLECs. However, another possibility is that other effectors may signal downstream of AM when Rap1 is depleted. The PKA-RhoA signaling pathway, also downstream of cAMP, parallels the Epac1-Rap1 pathway (Figure 4A). Since PKA can inhibit RhoA in a cAMP-dependent manner35, activation of the PKA pathway may also lead to junctional enhancement via RhoA inhibition35 and subsequent reduction in actomyosin contractility (Figure 4A). To explore whether this pathway is downstream of AM signaling, we measured RhoA activity in hLECs treated with AM (10 nM) for 30 min and found that AM significantly decreased RhoA activity (Figure 4B). Phosphorylation of regulatory myosin light chain II (MLC) is another read-out for activation of the RhoA/ROCK signaling pathway; this phosphorylation is rapidly induced in response to histamine34. AM ameliorated histamine-induced stress fibers and p-MLC levels in hLECs (Figure 4C-D). However, similar to the LEC junction formation experiments, the decrease in p-MLC caused by AM treatment was not fully blunted by loss of Rap1 under either basal or histamine-stimulated conditions (Online Figure I A, B). Together, these results suggest that under certain conditions, AM may regulate the RhoA-ROCK-MLC pathway partially independent of Rap1.
Figure 4. AM can signal through the PKA-RhoA-MLC axis, but effect on hLEC junctions is reduced if Rap1 is deficient.
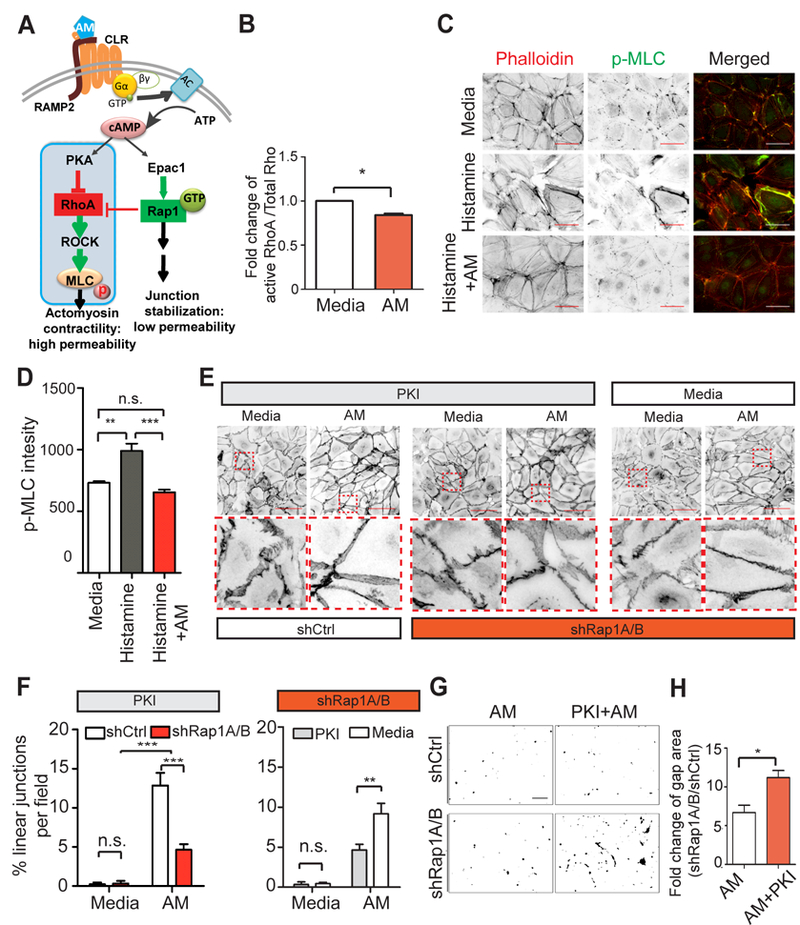
A, Schematic diagram showing PKA-RhoA-MLC pathway parallel with Epac1-Rap1 pathway downstream of AM. B, Quantitation of fold change of RhoA activity in hLECs after AM treatment; 3 replicates per condition, and 3 independent experments were performed. C, F-actin and pMLC were visualized by phalloidin and anti-pMLC antibodies in hLECs under the following conditions: media only, histamine only (10 μM, 10 min), and histamine (10 μM, 10 min) +AM (10 nM, 30 min) combination treatment. Scale bar, 50 μm. D, Quantification of p-MLC2; n=5–6. E, hLEC junctions were visualized using anti-VE-cadherin antibodies in shCtrl and shRap1A/B hLECs with PKI treatment (20 μM) for 1 hour, and shRap1A/B hLECs with media only treatment. Representative inverted images are shown. The boxed areas are enlarged at the bottom side of the original images. Scale bar, 100 μm. F, Quantification of linear junctions percentage in each field to assess whether AM requires Rap1 to restore PKI induced junction disruption (PKI group, left) and whether AM requires PKA pathway to improve disrupted junctions due to Rap1 knockdown (shRap1A/B group, right); n=3–4. G, Representative thresholded images of XPerT assay for each condition. Black areas indicate regions of high local permeability labeled by fluorescent-streptavidin binding to exposed biotinylated collagen matrix. Scale bar, 100 μm. H, XPerT assay quantitation of fold change in percent gap area induced by knockdown of Rap1 after AM or PKI +AM treatment of shCtrl and shRap1A/B hLEC monolayers. Data plotted as a ratio of gap area in shRap1/shCtrl for each treatment group; 5 fields per replicate, 3 replicates per condition, and 3 independent experiments were performed. Data are presented as means ± SEM. n.s., not significant, *p < 0.05.
To further determine the relationship of these two parallel pathways upon AM stimulation, we inhibited PKA activity by pretreating hLECs with PKI (10 µM), a well-known PKA inhibitor, for one hour before adding AM. PKI induced junction disruption and gap formation between cells; however, AM still could linearize and strengthen hLEC junctions after PKI treatment (Online Figure II A). PKI also decreased the impedance of hLEC monolayer, but again, this effect could be fully restored by AM treatment (Online Figure II B). The increase of cell impedance induced by AM treatment showed no significant difference regardless of PKA activity (Online Figure II C). Thus, inhibition of PKA did not affect AM-induced LEC junctional stabilization under basal conditions. We next wanted to determine whether PKA pathway inhibition could be rescued by AM to restore junction linearity if Rap1 was absent. However, AM could not fully restore the junctional linearity in PKI-treated shRap1A/B LECs (Figure 4E, representative images in PKI group; 4F, left graph), which emphasizes the requirement of Rap1 in the AM-induced junctional restoration process. Interestingly, we also noted that when PKA was inhibited, AM failed to elicit even the minor improvement of junction linearity that we had previously observed in shRap1A/B hLECs (Figure 4E, representative images in shRap1A/B group; 4F, right graph). Furthermore, the slight reduction in gap area in response to AM in shRap1A/B hLECs (compared to shCtrl) was abolished in PKI pretreated cells (Figure 4G, representative images; 4H, quantitation). Thus, AM may still modestly affect hLEC junction stability via the PKA pathway, but only if Rap1 is deficient, indicating that PKA may play a supporting role in mediating the signal from AM under certain conditions.
Lymphatic Abnormalities in LEC-Rap1a/b deficient embryos
Because AM signaling is required for developmental lymphangiogenesis, we reasoned that loss of its critical downstream effector from lymphatic endothelium may result in similarly defective lymphangiogenesis. Thus, to assess the role of Rap1 in lymphatic development and function in vivo, we inactivated both Rap1a and Rap1b isoforms in LECs by crossing a Rap1a/b floxed mouse to two mouse lines with either a constitutively-expressed Lyve1-Cre36 or an inducible Prox1CreERT2 transgene37. The resulting crosses cause the recombination of the loxP sites flanking exons 2 and 3 of Rap1a and that flanking exon1 of Rap1b in a Rap1a/b floxed mouse model38. We examined the embryos at E13.5 and found that some Rap1a/bfl/fl; Lyve1Cre+/− embryos survived until E13.5 but at a much lower than expected Mendelian ratio, while single LEC-Rap1a (Rap1afl/fl; Rap1bfl/+; Lyve1Cre+/−) and LEC-Rap1b KO mice (Rap1afl/+; Rap1bfl/fl; Lyve1Cre+/−) exhibited normal Mendelian ratios at E13.5 (Table 1, online data supplement). Consistent with their reduced numbers, the Rap1a/bfl/fl; Lyve1Cre+/− embryos showed developmental defects including interstitial edema (asterisk in Figure 5A and red arrow in Figure 5B), associated with areas of severe interstitial hemorrhage (white arrowheads in Figure 5A). We observed similar phenotypes with edema and interstitial hemorrhage in the Rap1a/bfl/fl; Prox1CreERT2 embryos (Figure 5A). Histological sectioning revealed appropriately lumenized jugular lymph sacs that were blood-filled (black arrows in Figure 5B) in both Rap1a/bfl/fl; Lyve1Cre+/− and Rap1a/bfl/fl; Prox1CreERT2 embryos compared to control littermates. Moreover, we noted disrupted morphology of LVV with red blood cells passing through into the lymph sac (black arrowhead in Figure 5B). In addition, we found a significant reduction in the density and patterning of dorsal dermal lymphatic capillaries in Rap1a/bfl/fl; Lyve1Cre+/− embryos at E13.5 compared to controls (Figure 5D). We were able to clearly identify organized VE-cadherin positive cell-cell junctions between the lateral borders of adjoining LECs lining the jugular lymph sacs of control Rap1a/bfl/fl embryos. However, this VE-cadherin staining pattern was effaced and compressed to the apical surface of luminal LECs in both Rap1a/bfl/fl; Lyve1Cre+/− and Rap1a/bfl/fl; Prox1CreERT2 embryos (Figure 5E). Collectively, these observations establish an essential role for Rap1 in lymphatic developmental lymphangiogenesis, the formation of LEC junctions and the appropriate establishment of LVVs.
Figure 5. Rap1 is required for normal lymphatic development and function.
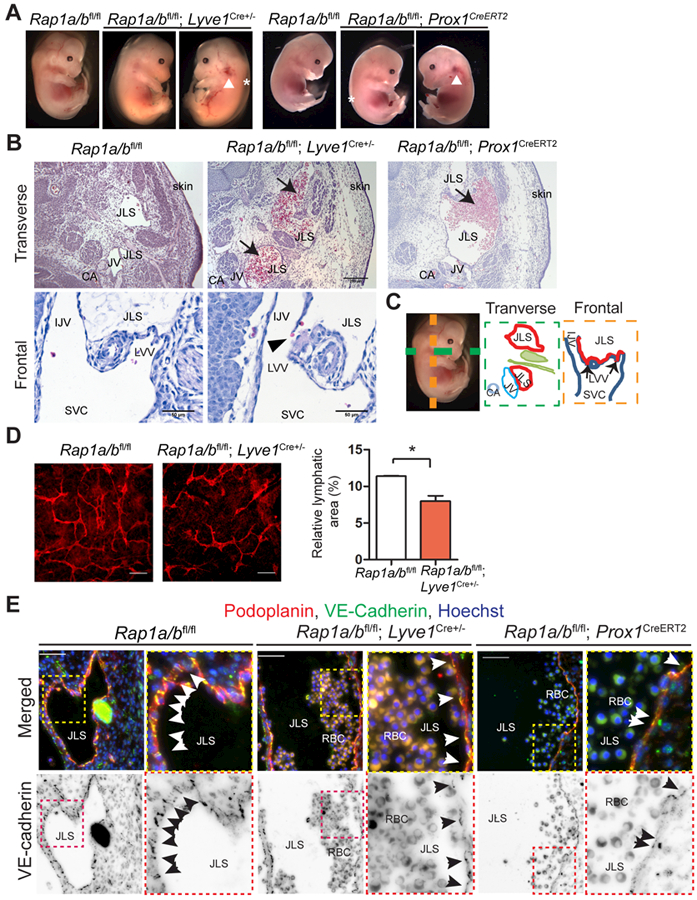
A, Rap1a/bfl/fl; Lyve1Cre+/− and Rap1a/bfl/fl; Prox1CreERT2embryos showed edema (asterisk) and hemorrhage in the jugular region (white arrowhead) and on the side of body at E13.5. B, Representative H&E images showing enlarged, blood-filled jugular lymph sacs (JLS) (black arrows) in transverse sections of both Rap1a/bfl/fl; Lyve1Cre+/− and Rap1a/bfl/fl; Prox1CreERT2embryos and disrupted morphology of LVV in frontal sections (black arrowhead) of Rap1a/bfl/fl; Lyve1Cre+/− embryos. Scale bar, 200 μm for transvers sections and 50 μm for frontal sections. C, Diagram of normal embryo anatomy in transverse and frontal planes (black arrows points to LVV). D, Representative images of dermal lymphatics using anti-podoplanin antibodies (red); quantitation of relative lymphatic area; n=3. Scale bar, 100 μm. E, Representative images of the jugular lymph sacs (JLS). LECs visualized using anti-podoplanin antibodies (red), and LEC junctions (black arrows) labeled by anti-VE-cadherin staining (green). Nuclei were visualized using Hoechst (blue). At right, inverted images of VE-cadherin staining. Boxed areas are enlarged to the right of each original image. JLS, jugular lymph sac; CV, cardinal vein; CA, carotid artery; LVV, lymphovenous valves; IJV, internal jugular vein; SVC, superior vena cava. RBC, red blood cells. Scale bar, 50 μm. Data are presented as means ± SEM. *p < 0.05.
Rap1 is required for AM to stabilize LEC junctions in vivo
To further investigate whether Rap1 is a downstream effector of AM in vivo, we generated Rap1a/bfl/fl; Prox1CreERT2 mice for inducible deletion of both Rap1a and Rap1b isoforms specifically in lymphatics. Mice 3–4 months old were injected with tamoxifen at 5 mg/40 g for 5 consecutive days. Histamine or a combination of histamine and AM was injected intradermally at the inner surface of the ears, followed by intradermal injection of Evans blue dye 5 min later to visualize lymphatic vessels (Figure 6A). In this model, Evans blue dye is rapidly taken up by dermal lymphatics, and the dye staining pattern sharply marks the fine lymphatic vessels adjacent to the injection site (Figure 6A, enlarged schematic, 6B, “0 min” panels). If the lymphatic barrier is leaky, subsequent dye extravasation out of lymphatic vessels will appear as a diffuse, spreading “bruise” on the skin adjacent to the injection site (Figure 6A, enlarged schematic; 6B, “10 min” panels: white arrowheads). In histamine-treated WT ears, there was little to no extravasation when combined with AM treatment (green boxed panel). However, the combination histamine +AM-treated ear of Rap1a/bfl/fl; Prox1CreERT2 mice exhibited more dye extravasation from lymphatic vessels as indicated by the diffuse dye pattern surrounding the lymphatics (red boxed panel, arrowheads), compared to WT, suggesting that AM was incapable of restoring histamine-disrupted LEC junctions when Rap1 was deleted. Next, we compared ear dermal lymphatic diameter visualized by immunostaining using anti-podoplanin. AM decreased the diameter of the lymphatics of histamine-injected ears in WT mice, but not in Rap1a/bfl/fl; Prox1CreERT2 mice (Figure 6C, D). Furthermore, the junctions of the collecting lymphatic vessels were more disrupted in Rap1fl/fl; Prox1CreERT2 mice treated with the combination of histamine and AM (red arrow heads in Figure 6E). Together, these results show that Rap1 is required by AM to restore histamine-induced junction disruption and permeability in vivo.
Figure 6. Rap1 is required for AM to restore histamine-stimulated leakage of lymphatics.
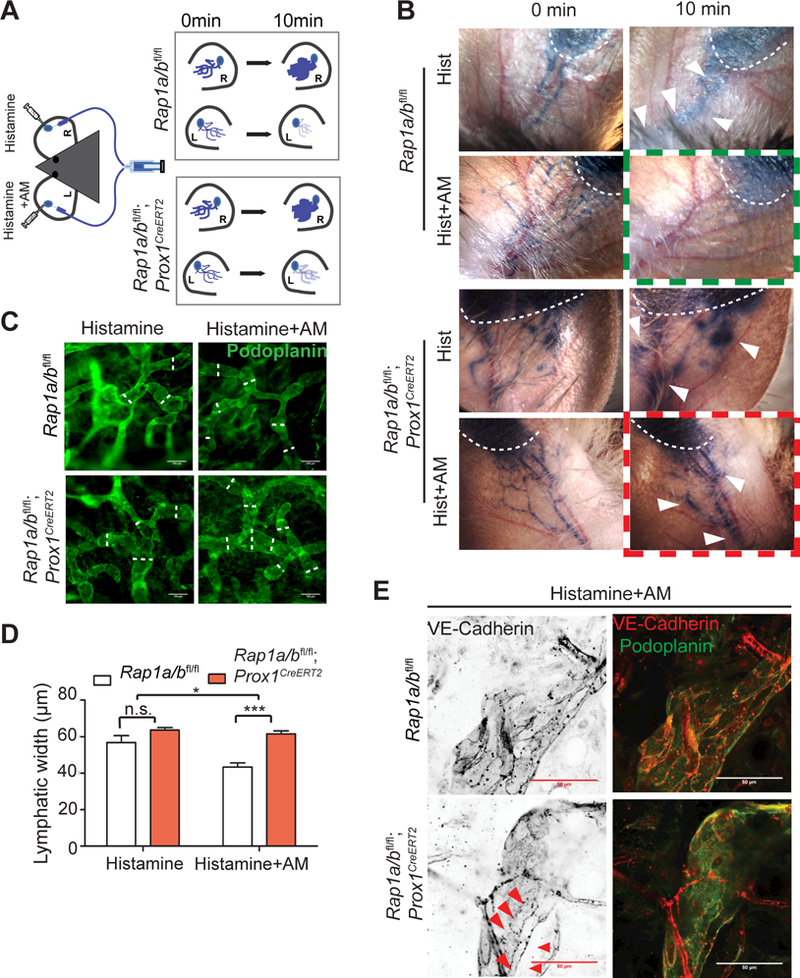
A-B, Diagram of experimental set-up and representative images of Evans Blue dye injection in the ear with histamine (Hist) alone or in combination with AM (Hist+AM) for both Rap1fl/fl and Rap1a/bfl/fl; Prox1CreERT2 mice. AM reduces histamine-induced dye extravasation in WT mice (green boxed panel) but not in LEC-specific Rap1A/B knockout mice (red boxed panel). Evans Blue leakage after 10 min indicated with white arrowheads. C, Ear lymphatics visualized using anti-podoplanin antibodies (green). Scale bar, 100 μm. D, Quantitation of average lymphatic diameter in Rap1a/bfl/fl or Rap1a/bfl/fl; Prox1CreERT2 mice injected with histamine alone (Hist) or in combination with AM (Hist+AM); 40–100 measurements per mouse and 3 mice for each group. E, Lymphatic junctions visualized using anti-VE-cadherin (red). Collecting lymphatics of WT mice exhibit linear “zipper-like” morphology while Rap1a/bfl/fl; Prox1CreERT2 mice exhibit discontinuous VE-cadherin staining (red arrowheads). Scale bar, 50 μm. Data are presented as means ± SEM. n.s., not significant, *p < 0.05, ***p<0.001.
Discussion
In contrast to the blood circulation, the lymphatic vasculature features loose junctions which facilitate the entry of fluid, protein and cells1. However, dysregulation of the lymphatic barrier may lead to the development of edema, obesity, altered lymphocyte circulation, inflammation and depressed immune system39, and may even favor the spread of cancer cells40. In the present study, we report for the first time that Rap1 plays an important role in the formation and integrity of LEC junctions and lymphatic development, and is the dominant effector mediating the tightening effect of AM, a prominent lymphangiogenic factor, under basal conditions and in restoring histamine-induced junctional disruption both in vitro and in vivo.
AM stabilizes BEC junctions by elevating cAMP levels41–45. In BECs, Rap1 can be activated by a cAMP-responsive GEF Epac1 and this results in enhanced barrier function14. However, whether Rap1 is the dominant molecular effector mediating the junction tightening effect of AM, particularly in LECs, remains unclear. Our results demonstrate for the first time that Rap1 is essential for AM to exert its junction tightening effect in LECs. Consistent with our results, Garcia Ponce, et al. showed that cortactin was critical for adrenomedullin secretion and subsequent inhibition of ROCK for maintenance of BEC barrier function46.
In parallel with Epac1-Rap1, PKA is another downstream effector of cAMP (Figure 4A), which can regulate endothelial barrier function by inactivating RhoA40. In BECs, under conditions of high RhoA activity, radial stress fibers and enhanced contractility increase vascular permeability47. We found that RhoA activity in LECs was reduced by AM treatment, which is consistent with the junctional enhancement we see upon AM treatment. The junctional stabilizing effects of AM therefore could be attributable to the Epac1-Rap1 arm of the pathway48, 49, inhibition of RhoA via the PKA pathway, or both. To tease out whether the PKA and Epac1-Rap1 pathways coordinate to regulate endothelial junctions upon AM stimulation, we used PKI, an inhibitor of PKA50, 51. PKA signaling was not required for AM-induced stabilization of hLEC junctions and reduction of monolayer gap area in normal hLECs (Online Figure II), suggesting a dominant role for Rap1. However, when PKA was inhibited in shRap1A/B knockdown hLECs, AM treatment no longer rescued junctional linearity or reduced monolayer gap area (Figure 4E-H). Together, these results emphasize the importance of Rap1 as the dominant effector pathway downstream of AM for lymphatic junctional tightening, whereas PKA may function as an auxiliary pathway that cannot fully effectuate the effects of AM. Whether Rap1 exerts its barrier stabilization function in LECs via effects on the actin cytoskeleton, including decreasing radial stress fibers and increasing cortical actin bundles52, or by feeding into the RhoA inhibitory pathway in LECs remains to be determined. ArhGAP29, a RhoA GTPase-specific inhibitor, is known to be activated by Rap148, 49, and can help control endothelial barrier function by subsequent reduction of tension on radial stress fibers. It is possible that both mechanisms contribute to barrier function53
Numerous studies have examined the role of histamine in BEC junction hyperpermeability caused by pathological conditions including allergic diseases, asthma, and anaphylactic shock34. However, limited research has focused on the effect of histamine in lymphatic vasculature, especially on the LEC junctions. Breslin54 showed that histamine can decrease TER in LECs by a pathway involving RhoA-ROCK. In our study, we also consistently observed that histamine decreased the cellular impedance of a hLEC monolayer. Surprisingly, we demonstrate for the first time that AM can restore this decreased cell impedance and rescue the histamine-induced cell-cell junctional disruption and increased stress fiber formation within 30 min following AM treatment. Furthermore, we studied the role of Rap1 in AM-rescue of histamine-induced hyperpermeability of LEC junction using an inducible lymphatic-specific KO mouse model, Rap1a/bfl/fl; Prox1CreERT2. In this model, using histamine treatment to induce permeability, AM failed to restore lymphatic barrier function in the absence of Rap1. This was also consistent with the dilation of lymphatics in Rap1a/bfl/fl; Prox1CreERT2 ears even upon AM stimulation (Figure 6). These results support the notion that expression of AM and its activation of the Rap1 pathway in adults is required for regulation of lymphatic junctions, thereby facilitating the maintenance of interstitial fluid dynamics. In the context of interstitial edema, induced by histamine release, inflammation or other pathophysiological challenges, this signaling pathway becomes even more critical, implying that AM-based therapies could have tremendous value for the attenuation of interstitial edema, as exemplified by histamine-induced pathological conditions.
Total Rap1a/b KO mice die at E10.5, and Tie2-Rap1a/b endothelial-KO mice do not survive past E15.518, 55. Our observation that Rap1a/bfl/fl; Lyve1Cre+/− mice displayed increased embryonic lethality around E13.5 (Table 1 in the online data supplement) is in line with these other studies, but also emphasizes the importance of Rap1 in lymphatics. Surviving Rap1a/bfl/fl; Lyve1Cre+/− embryos at E13.5 showed hemorrhage on the side of body and jugular region, accompanied by severe edema. Histological examination revealed disrupted morphology of LVVs, disorganized LEC junctions and impaired lymphangiogenesis, along with blood-filled jugular lymph sacs (Figure 5). Dysregulation of blood-lymphatic separation has been shown to contribute to blood-filled jugular lymph sacs56, 57. Lymphatic-specific deletion of the Zinc finger transcription factor Gata2, which can transactivate Prox1, caused pronounced edema, pooling of blood in the jugular region, enlarged jugular lymph sacs and blood-filled lymphatics58, similar to our observations in Rap1a/bfl/fl; Lyve1Cre+/− mice. RapGEF2, also known as PDZ-GEF1, is an activator of Rap1. RapGEF2−/− yolk sac cells showed significant downregulation of Gata2 at both mRNA and protein levels, which may be attributable to inactivation of B-raf/ERK, an effector downstream of Rap1 and responsible for activation of transcription factor SCL and subsequent transcription of Gata259. Together, these results emphasize that LEC Rap1 signaling is critical for normal LVV development and function.
Overall, our findings define a critical role for Rap1 in lymphatic development including junction formation, organization and barrier function. Moreover, we show that Rap1 is indispensable for AM to stabilize LEC junctions, whereas PKA is not required but may mediate AM signaling as an auxiliary effector in Rap1-deficient hLECs. Our study sheds light on how PKA and Epac1-Rap1 pathways may coordinate to mediate AM signaling, and provides therapeutic implications for lymphatic diseases and other pathological conditions accompanied by lymphatic hyperpermeability.
Supplementary Material
Highlights.
Rap1 is essential for the formation of lymphatic endothelial junctions.
Rap1 is critical for lymphangiogenesis and lymphatic barrier function during embryogenesis.
Rap1 acts as the dominant effector mediating the tightening effect of adrenomedullin on lymphatics.
Acknowledgements
We thank Dr. Wolfgang Bergmeier, UNC-CH for advice on our paper and Dr. Reema Davis, UNC-CH for advice on whole-mount immunofluorescence.
Sources of Funding
The work was supported by NIH grants DK099156 and HL1290086 (K.M.C) and GM029860 and HL114388 to (K.B).
Abbreviations
- LEC
Lymphatic endothelial cell
- LVV
Lymphovenous valves
- AM
Adrenomedullin
- Ramp2
Receptor activity modifying protein 2
- KO
Knockout
- PKA
Protein kinase A
- Epac
Exchange protein directly activated by cAMP
- Rap
Ras-related protein
- BEC
Blood endothelial cell
- GEF
Guanine nucleotide exchange factor
- RTCA
Real-time electrical cell-substrate adhesion assay
- XPerT
Express micromolecule permeability testing
- AUC
Area under the curve
Footnotes
Disclosures
The authors have declared that no conflict of interest exists.
References
- 1.Aspelund A, Robciuc MR, Karaman S, Makinen T and Alitalo K. Lymphatic System in Cardiovascular Medicine. Circ Res 2016;118:515–30. [DOI] [PubMed] [Google Scholar]
- 2.van der Putte SC. The early development of the lymphatic system in mouse embryos. Acta Morphol Neerl Scand 1975;13:245–86. [PubMed] [Google Scholar]
- 3.Srinivasan RS and Oliver G. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev 2011;25:2187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess PR, Rawnsley DR, Jakus Z, Yang Y, Sweet DT, Fu J, Herzog B, Lu M, Nieswandt B, Oliver G, Makinen T, Xia L and Kahn ML. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J Clin Invest 2014;124:273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dejana E and Orsenigo F. Endothelial adherens junctions at a glance. Journal of Cell Science 2013;126:2545–2549. [DOI] [PubMed] [Google Scholar]
- 6.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E and McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 2007;204:2349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zolla V, Nizamutdinova IT, Scharf B, Clement CC, Maejima D, Akl T, Nagai T, Luciani P, Leroux JC, Halin C, Stukes S, Tiwari S, Casadevall A, Jacobs WR Jr., Entenberg D, Zawieja DC, Condeelis J, Fooksman DR, Gashev AA and Santambrogio L. Aging-related anatomical and biochemical changes in lymphatic collectors impair lymph transport, fluid homeostasis, and pathogen clearance. Aging Cell 2015;14:582–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scallan JP, Zawieja SD, Castorena-Gonzalez JA and Davis MJ. Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol 2016;594:5749–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bos JL, de Bruyn K, Enserink J, Kuiperij B, Rangarajan S, Rehmann H, Riedl J, de Rooij J, van Mansfeld F and Zwartkruis F. The role of Rap1 in integrin-mediated cell adhesion. Biochem Soc Trans 2003;31:83–6. [DOI] [PubMed] [Google Scholar]
- 10.Lagarrigue F, Kim C and Ginsberg MH. The Rap1-RIAM-talin axis of integrin activation and blood cell function. Blood 2016;128:479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmona G, Gottig S, Orlandi A, Scheele J, Bauerle T, Jugold M, Kiessling F, Henschler R, Zeiher AM, Dimmeler S and Chavakis E. Role of the small GTPase Rap1 for integrin activity regulation in endothelial cells and angiogenesis. Blood 2009;113:488–97. [DOI] [PubMed] [Google Scholar]
- 12.Boettner B and Van Aelst L. Control of cell adhesion dynamics by Rap1 signaling. Curr Opin Cell Biol 2009;21:684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pannekoek WJ, Kooistra MR, Zwartkruis FJ and Bos JL. Cell-cell junction formation: the role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochim Biophys Acta 2009;1788:790–6. [DOI] [PubMed] [Google Scholar]
- 14.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW and Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 2005;105:1950–5. [DOI] [PubMed] [Google Scholar]
- 15.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K and Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 2005;25:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kooistra MR, Corada M, Dejana E and Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett 2005;579:4966–72. [DOI] [PubMed] [Google Scholar]
- 17.Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA and Burridge K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem 2005;280:11675–82. [DOI] [PubMed] [Google Scholar]
- 18.Chrzanowska-Wodnicka M, White GC 2nd, Quilliam LA and Whitehead KJ. Small GTPase Rap1 Is Essential for Mouse Development and Formation of Functional Vasculature. PLoS One 2015;10:e0145689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbons C, Dackor R, Dunworth W, Fritz-Six K and Caron KM. Receptor activity-modifying proteins: RAMPing up adrenomedullin signaling. Mol Endocrinol 2007;21:783–96. [DOI] [PubMed] [Google Scholar]
- 20.Fritz-Six KL, Dunworth WP, Li M and Caron KM. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest 2008;118:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoopes SL, Willcockson HH and Caron KM. Characteristics of multi-organ lymphangiectasia resulting from temporal deletion of calcitonin receptor-like receptor in adult mice. PLoS One 2012;7:e45261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG and Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998;393:333–9. [DOI] [PubMed] [Google Scholar]
- 23.Dunworth WP, Fritz-Six KL and Caron KM. Adrenomedullin stabilizes the lymphatic endothelial barrier in vitro and in vivo. Peptides 2008;29:2243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Triacca V, Guc E, Kilarski WW, Pisano M and Swartz MA. Transcellular Pathways in Lymphatic Endothelial Cells Regulate Changes in Solute Transport by Fluid Stress. Circ Res 2017;120:1440–1452. [DOI] [PubMed] [Google Scholar]
- 25.Ishizaka Y, Ishizaka Y, Tanaka M, Kitamura K, Kangawa K, Minamino N, Matsuo H and Eto T. Adrenomedullin stimulates cyclic AMP formation in rat vascular smooth muscle cells. Biochem Biophys Res Commun 1994;200:642–6. [DOI] [PubMed] [Google Scholar]
- 26.Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T and Detmar M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol 2003;162:575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S and Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J 2002;21:4593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atienza JM, Yu N, Kirstein SL, Xi B, Wang X, Xu X and Abassi YA. Dynamic and label-free cell-based assays using the real-time cell electronic sensing system. Assay Drug Dev Technol 2006;4:597–607. [DOI] [PubMed] [Google Scholar]
- 29.Monaghan-Benson E and Wittchen ES. In vitro analyses of endothelial cell permeability. Methods Mol Biol 2011;763:281–90. [DOI] [PubMed] [Google Scholar]
- 30.Wittchen ES, Aghajanian A and Burridge K. Isoform-specific differences between Rap1A and Rap1B GTPases in the formation of endothelial cell junctions. Small GTPases 2011;2:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubrovskyi O, Birukova AA and Birukov KG. Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab Invest 2013;93:254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris TJ and Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol 2010;11:502–14. [DOI] [PubMed] [Google Scholar]
- 33.Huveneers S and de Rooij J. Mechanosensitive systems at the cadherin–F-actin interface. Journal of Cell Science 2013;126:403–413. [DOI] [PubMed] [Google Scholar]
- 34.Mikelis CM, Simaan M, Ando K, Fukuhara S, Sakurai A, Amornphimoltham P, Masedunskas A, Weigert R, Chavakis T, Adams RH, Offermanns S, Mochizuki N, Zheng Y and Gutkind JS. RhoA and ROCK mediate histamine-induced vascular leakage and anaphylactic shock. Nat Commun 2015;6:6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiao J, Huang F and Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2003;284:L972–80. [DOI] [PubMed] [Google Scholar]
- 36.Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR and Cyster JG. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med 2010;207:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM and Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 2007;21:2422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan BX, Vautier F, Ito W, Bolshakov VY and Morozov A. Enhanced cortico-amygdala efficacy and suppressed fear in absence of Rap1. J Neurosci 2008;28:2089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alitalo K The lymphatic vasculature in disease. Nat Med 2011;17:1371–80. [DOI] [PubMed] [Google Scholar]
- 40.Tacconi C, Correale C, Gandelli A, Spinelli A, Dejana E, D’Alessio S and Danese S. Vascular endothelial growth factor C disrupts the endothelial lymphatic barrier to promote colorectal cancer invasion. Gastroenterology 2015;148:1438–51 e8. [DOI] [PubMed] [Google Scholar]
- 41.Temmesfeld-Wollbruck B, Hocke AC, Suttorp N and Hippenstiel S. Adrenomedullin and endothelial barrier function. Thromb Haemost 2007;98:944–51. [DOI] [PubMed] [Google Scholar]
- 42.Hippenstiel S, Witzenrath M, Schmeck B, Hocke A, Krisp M, Krull M, Seybold J, Seeger W, Rascher W, Schutte H and Suttorp N. Adrenomedullin reduces endothelial hyperpermeability. Circ Res 2002;91:618–25. [DOI] [PubMed] [Google Scholar]
- 43.Shimekake Y, Nagata K, Ohta S, Kambayashi Y, Teraoka H, Kitamura K, Eto T, Kangawa K and Matsuo H. Adrenomedullin stimulates two signal transduction pathways, cAMP accumulation and Ca2+ mobilization, in bovine aortic endothelial cells. J Biol Chem 1995;270:4412–7. [DOI] [PubMed] [Google Scholar]
- 44.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H and Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 1993;192:553–60. [DOI] [PubMed] [Google Scholar]
- 45.Isumi Y, Shoji H, Sugo S, Tochimoto T, Yoshioka M, Kangawa K, Matsuo H and Minamino N. Regulation of adrenomedullin production in rat endothelial cells. Endocrinology 1998;139:838–46. [DOI] [PubMed] [Google Scholar]
- 46.Garcia Ponce A, Citalan Madrid AF, Vargas Robles H, Chanez Paredes S, Nava P, Betanzos A, Zarbock A, Rottner K, Vestweber D and Schnoor M. Loss of cortactin causes endothelial barrier dysfunction via disturbed adrenomedullin secretion and actomyosin contractility. Sci Rep 2016;6:29003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Buul JD and Timmerman I. Small Rho GTPase-mediated actin dynamics at endothelial adherens junctions. Small GTPases 2016;7:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Post A, Pannekoek WJ, Ponsioen B, Vliem MJ and Bos JL. Rap1 Spatially Controls ArhGAP29 To Inhibit Rho Signaling during Endothelial Barrier Regulation. Mol Cell Biol 2015;35:2495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Post A, Pannekoek WJ, Ross SH, Verlaan I, Brouwer PM and Bos JL. Rasip1 mediates Rap1 regulation of Rho in endothelial barrier function through ArhGAP29. Proc Natl Acad Sci U S A 2013;110:11427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lum H, Jaffe HA, Schulz IT, Masood A, RayChaudhury A and Green RD. Expression of PKA inhibitor (PKI) gene abolishes cAMP-mediated protection to endothelial barrier dysfunction. Am J Physiol 1999;277:C580–8. [DOI] [PubMed] [Google Scholar]
- 51.Profirovic J, Gorovoy M, Niu J, Pavlovic S and Voyno-Yasenetskaya T. A novel mechanism of G protein-dependent phosphorylation of vasodilator-stimulated phosphoprotein. J Biol Chem 2005;280:32866–76. [DOI] [PubMed] [Google Scholar]
- 52.Ando K, Fukuhara S, Moriya T, Obara Y, Nakahata N and Mochizuki N. Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization. J Cell Biol 2013;202:901–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pannekoek WJ, Vliem MJ and Bos JL. Multiple Rap1 effectors control Epac1-mediated tightening of endothelial junctions. Small GTPases 2018:1–8. [DOI] [PMC free article] [PubMed]
- 54.Breslin JW. ROCK and cAMP promote lymphatic endothelial cell barrier integrity and modulate histamine and thrombin-induced barrier dysfunction. Lymphat Res Biol 2011;9:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH and White GC 2nd. Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest 2005;115:680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, Tybulewicz V, Lowell CA, Lepore JJ, Koretzky GA and Kahn ML. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science 2003;299:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ichise H, Ichise T, Ohtani O and Yoshida N. Phospholipase C gamma 2 is necessary for separation of blood and lymphatic vasculature in mice. Development 2009;136:191–195. [DOI] [PubMed] [Google Scholar]
- 58.Kazenwadel J, Betterman KL, Chong CE, Stokes PH, Lee YK, Seeker GA, Agalarov Y, Demir CS, Lawrence DM, Sutton DL, Tabruyn SP, Miura N, Salminen M, Petrova TV, Matthews JM, Hahn CN, Seott HS and Harvey NL. GATA2 is required for lymphatic vessel valve development and maintenance. Journal of Clinical Investigation 2015;125:2979–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satyanarayana A, Gudmundsson KO, Chen X, Coppola V, Tessarollo L, Keller JR and Hou SX. RapGEF2 is essential for embryonic hematopoiesis but dispensable for adult hematopoiesis. Blood 2010;116:2921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


