Abstract
Background and Purpose
Although perfusion abnormality is an increasingly important therapeutic target, the natural history of tissue at risk without reperfusion treatment is understudied. Our objective was to determine how time affects penumbral salvage and infarct growth in untreated acute ischemic stroke patients and whether collateral status affects this relationship.
Methods
We utilized a prospectively-collected, multicenter acute stroke registry to assess acute stroke patients who were not treated with intravenous thrombolysis or endovascular treatment. We analyzed baseline CT angiogram and CT perfusion within 24 hours of stroke onset along with follow-up imaging, and assessed time from stroke onset to baseline imaging, ASPECTS, vessel occlusion, collaterals, ischemic core and penumbra. Penumbral salvage and infarct growth was calculated. Correlations between time and penumbral salvage and infarct growth were evaluated with Spearman correlation. Penumbral salvage and infarct growth were compared between subjects with good versus poor collateral status using the Wilcoxon rank sum test. Clinical and imaging factors affecting penumbral salvage and infarct growth were evaluated by linear regression.
Results
Among 94 untreated stroke patients eligible for this analysis, the mean age was 65, median NIHSS was 13, and median (range) time from stroke onset to baseline imaging was 2.9 (0.4–23) hours. There was no correlation between time and salvaged penumbra (r= 0.06; p=0.56) or infarct growth (r=−0.05; p=0.61). Infarct growth was higher among those with poor collaterals versus good collaterals (median 52.3 vs 0.9 cc; p<0.01). Penumbral salvage was lower among those with poor collaterals compared to those with good collaterals (poor 0 [0, 0]; good 5.9 cc [0, 29.4]; p<0.01). Multivariable linear regression demonstrated that collaterals, but not time, were significantly associated with infarct growth and penumbral salvage.
Conclusion
In this natural history study, penumbral salvage and infarct growth was less time dependent and more a measure of collateral flow.
Keywords: Collateral circulation, acute stroke, ischemic, time to event outcome, infarction, penumbral salvage, infarct growth
Introduction
The Stroke Imaging Research consortium described mismatch and penumbra as an important treatment-related acute stroke imaging target. 1 Ischemic penumbra is defined as hypoperfused tissue which is at risk for irreversible damage (i.e., infarction), if blood flow is not rapidly restored 2, 3 and can widen the treatment time window for selected patients as seen in recent positive endovascular trials.4, 5 It is believed that without reperfusion, penumbra will progress to infarction, irrespective of the timing of its assessment relative to stroke onset. Studies have demonstrated that penumbra can persist for long time periods of up to 48 hours. 6, 7 One explanation for preservation of penumbra (i.e., delayed progression to infarct) is the presence of robust collaterals that protect the penumbra and thereby increase likelihood of good clinical outcomes. 8–15 Collaterals may be the leading pathophysiological variable differentiating “fast progressors” from “slow progressors”, leading to similar infarct growths at widely varied time points.
Although perfusion imaging is increasingly being used in triage decisions after DAWN and DEFUSE 3 trials and the new 2018 AHA guidelines were released16, the natural history of the ischemic tissue’s fate (tissue at risk) and its interplay of collaterals is understudied. Very limited data for untreated patients are available to serve as controls for patients treated with reperfusion therapies in the setting of penumbra, particularly in delayed time windows. Understanding how time and collaterals influences the natural fate of penumbra becomes especially relevant in the current era where endovascular treatment is offered to patients upto 24 hours based on core and mismatch. 4, 5 The rationale underlying our study is that it is important to understand the natural fate and prognostic significance of penumbra/mismatch and collaterals before using them widely in clinical settings as therapeutic targets.
Our objective was to determine how time from stroke onset affects penumbral salvage and infarct growth in untreated acute ischemic stroke subjects within 24 hours of symptom onset and whether collateral status affects this relationship. We hypothesized that good collaterals will sustain ischemic tissue leading to greater penumbral salvage and slower infarct growth, irrespective of time.
Methods
Study Design and Dataset
The data that support the findings of this study are available from the corresponding author upon reasonable request. This was a retrospective, multicenter study and we used data from prospective stroke registries at four participating institutions: the University of Virginia, Charlottesville, VA; the Center Hospitalier Universitaire Vaudois, Lausanne, Switzerland; the University of Pittsburgh Medical Center, Pittsburgh, PA and Sunnybrook Health Sciences Center, Toronto, Canada. All data were collected and analyzed in compliance with respective institutional review boards, and any identifying information was removed as per Health Insurance Portability and Accountability Act (HIPAA) regulations. We retrospectively identified all consecutive patients admitted to these institutions with suspected acute ischemic stroke from January 2003 to June 2011. During this time period, randomized evidence supported use of IV rtPA up to three hours from onset until 2008 and then up to 4.5 hours from onset, and for intra-arterial thrombolysis up to 6 hours in IV rtPA ineligible patients. Randomized evidence to support use of endovascular therapy as an adjunct to IV rtPA was lacking. The clinical and imaging data in this study belongs to a repository created from the data collected as part of the standard clinical stroke care at the participating institutions. The management of the patients was at the discretion of treating physicians. Some of the clinical variables such as blood pressure and glycemic index were not available in the dataset.
Subjects with acute ischemic stroke and the following inclusion criteria qualified for this study: (1) completion of baseline stroke workup including noncontrast head CT, CT angiogram (CTA) and CT perfusion (CTP) within 24 hours of symptom onset; (2) follow-up CTA within 24–48 hours for recanalization status; (3) follow-up CT or MRI within 5 days for final infarct volume; and (4) lack of reperfusion treatment (patients did not receive intravenous thrombolysis or endovascular treatment). We collected baseline clinical data including age, sex, baseline NIHSS, and time from symptom onset to imaging acquisition.
Image acquisition and analysis
The CTA and CTP imaging studies were performed on 16 and 64 slice CT scanners. The CTA studies included the cervical and intracranial arteries and were obtained with an acquisition protocol as follows: helical mode with 0.5 to 0.8 second gantry rotation; pitch of 1 to 1.375:1; slice thickness of 0.625 to 1.25 mm; reconstruction interval of 0.5 to 1 mm; and acquisition parameters of 120 kVp/200 to 300 mAs. The CTP involved successive gantry rotations in cine mode during intravenous administration of 1 or 2 boluses of 40 to 50 mL of iodinated contrast material at an injection rate of 4 to 5 mL/s. The CTP acquisition ranged from 50 to 70 seconds, with a sampling interval of either 1 or 2 seconds, total coverage range of 20 to 80 mm. CTP acquisition parameters were 80 kVp and 100 to 200 mAs.
An experienced neuroradiologist, with 10 years of experience (A.V.) and blinded to clinical and outcome data, analyzed all imaging. Baseline non contrast head CT imaging features included ASPECT score for ischemic core. We reviewed baseline CTA for site and severity of arterial occlusion and degree of collateral circulation. The occlusion was characterized by the Interventional Management of Stroke (IMS) III trial CTA scoring system for vascular stenosis/occlusion as following: grade 1- complete occlusion; 2- hairline lumen; 3- >50% stenosis; 4- <50% stenosis and 5- normal.17 Grade 1 and 2 was considered as arterial occlusion. Recanalization on the follow up CTA was defined as improvement in the flow to grade 3–5 (non-occlusive) from grade 1–2 (occlusive/near-occlusive). 17 Collateral flow was graded on a scale from 0 to 3 with 0 and 1 (< 50% of ischemic territory that demonstrated cortical pial vessels) considered poor collateral status. 18
For CT perfusion analysis, a brain tissue probability map template (Montreal Neurologic Institute, Montreal, Canada) was registered to the individual baseline unenhanced CT images using SPM8 software (Wellcome Institute), and gray matter and white matter tissue binary masks were generated. Both ipsilateral and contralateral regions of interest were segmented to gray matter and white matter using binary masks. Within ipsilateral regions of interest at baseline imaging, we calculated volumes of perfusion abnormality by using previously published thresholds for gray matter and white matter.19 Tissue with abnormal relative cerebral blood flow <30% was considered ischemic core and tissue with delayed time to maximum (Tmax >6 seconds) was used to identify penumbra. 14, 15 We calculated total volumes of core and penumbra by summing gray matter and white matter volumes.
We defined tissue outcome by the final infarct volume (FIV) which was delineated on follow up non contrast head CT or FLAIR imaging by using Medical Image Processing, Analysis and Visualization, Version 4.4.1 (Center for Information Technology, National Institutes of Health, Bethesda, Maryland). Penumbra salvage was calculated by subtracting the FIV from the baseline penumbral volume. Infarct growth was calculated by subtracting the baseline core volume from the FIV.
Statistical analysis
Correlation between time from stroke onset to imaging and penumbral salvage and between time and infarct growth were calculated using Spearman correlation coefficient, including all subjects and among a subset of subjects with ICA and/or M1 occlusions. Penumbral salvage and infarct growth were compared between subjects with good versus poor collateral status using the Wilcoxon rank sum test. Similarly, collateral scores, penumbral salvage and infarct growth were compared between subjects with spontaneous recanalization versus those without recanlization using the Wilcoxon rank sum test. Linear regression models were undertaken using the cube root of infarct growth and also the cube root of penumbral salvage as the dependent variables. The cube root transformation of infarct growth and penumbral salvage volume was used to reduce right skewness with the advantage that this transformation can be applied to zero and the cube root of a volume has the units of a length. After the transformation, the variance was close to the mean for both infarct growth and penumbral salvage. Model assumptions were checked, violations of homoscedasticity and normality of the model residual errors, using residual plots and model assumptions appeared valid (Figures S1 and S2 in online supplement). Time from onset to baseline imaging was the independent variable of interest. Baseline clinical and radiologic covariates included age, gender, NIHSS score, recanalization, vessel occlusion, ASPECTS, dichotomized collateral score (good versus poor) and ordinal collateral scores (0–3). For the penumbral model, ischemic core volume and final infarct volume were also evaluated. Baseline NIHSS score was included as a potential confounder of infarct growth. As a measure of the total symptomatic territory of ischemia (penumbra + core infarct), it represents the total amount of infarct growth potential for a given patient. An alternate model without baseline NIHSS score as a covariate was also considered to assess the robustness of the model. Another alternate model adding time to final imaging was also considered.
All calculations were performed by using statistical software (SAS, version 9.4; SAS Institute, Cary, NC).
Results
Subject characteristics
Among 110 patients with acute ischemic stroke that fit our inclusion criteria, 16 (14.5%) subjects were excluded due to non-diagnostic perfusion studies. Among 94 remaining subjects, median age was 67 years (interquartile range (IQR) 53, 79), median NIHSS was 13 (IQR 5, 19), and median (IQR) time from stroke onset to baseline imaging was 2.9 (1.8, 6.1) hours. (Table 1)
Table 1.
Subject characteristics
| Variables | Total n = 94 |
|---|---|
| Age, y, median (min-max) | 67 (24–98) |
| Male, n (%) | 42 (45%) |
| NIHSS, median (min-max) | 13 (0–32) |
| ASPECTS, median (min-max) | 7 (0–10) |
| Time from stroke onset to imaging, hours, median (min-max) | 2.93 (0.37–22.75) |
| Core Volume, cc, median (min-max) | 30.68 (0.40–172.06) |
| Penumbra, cc, median (min-max) | 41.74 (0.45–411.79) |
| Final infarct volume, cc, median (min-max) | 31.94 (0–253.61) |
| Penumbral salvage, cc median (min-max) | 0 (0 – 292.69) |
| Infarct growth, cc median (min-max) | 5.24 (0 – 189.44) |
| Vessel occlusion* | |
| ICA, n (%) | 33 (35%) |
| M1, n (%) | 43 (46%) |
| M2, n (%) | 26 (28%) |
| ACA, n (%) | 0 |
| Basilar/PCA, n (%) | 1 (1%) |
| Recanalization, n(%) | 38 (40%) (n=75) |
| CTA Collateral Status, n (%) | |
| Good (2 – 3) | 57 (61%) |
| Poor ( 0 – 1) | 36 (39%) |
| CTA Collateral Grade, n (%) | |
| 3 | 27 (29%) |
| 2 | 30 (32%) |
| 1 | 26 (28%) |
| 0 | 10 (11%) |
Vessel occlusion categories are not mutually exclusive.
Time and tissue fate
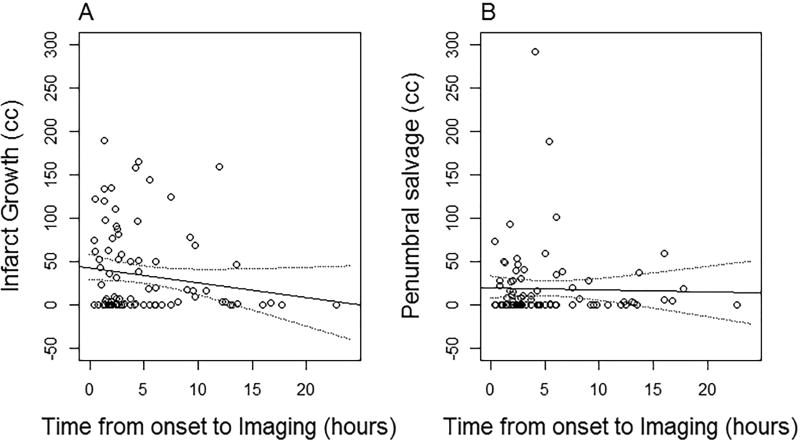
No correlation was observed between time of imaging from stroke onset and salvaged penumbra (r= 0.06; p=0.57), nor between time and infarct growth (r=−0.11; p=0.29). (Figure 1)
Figure 1.
Relationship of infarct growth (Panel A) and penumbral salvage (Panel B) with time
Collateral status and tissue fate
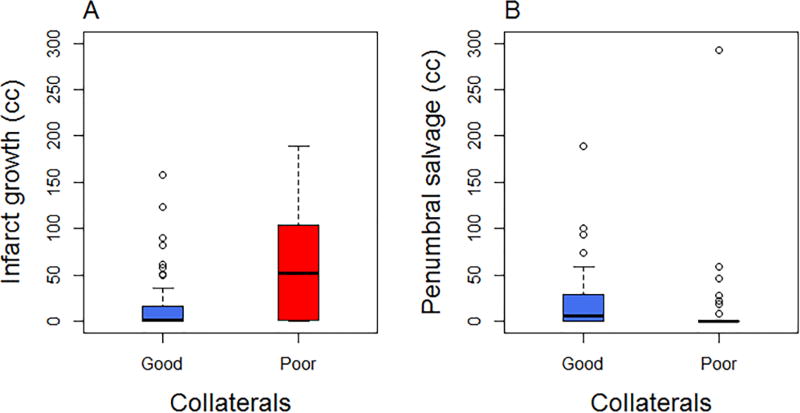
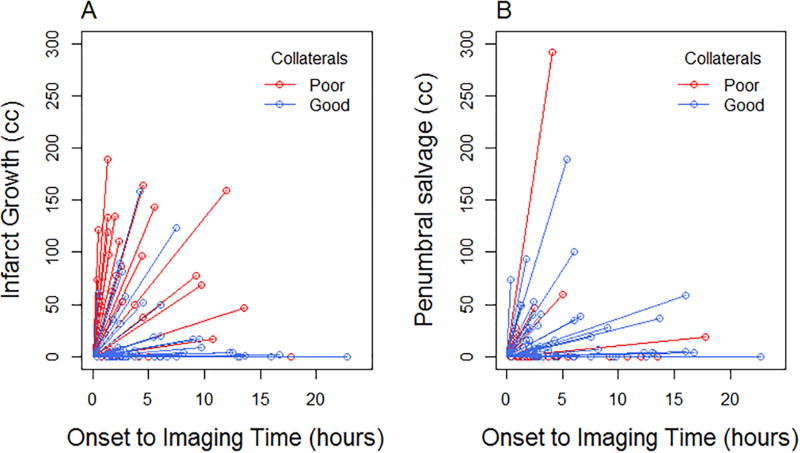
Of the total 94 subjects, 57 subjects (61%) had good CTA collateral status (30 with grade 2, 27 with grade 3) and 36 (39%) had poor collateral status (10 with grade 0 and 26 with grade 1). Infarct growth was higher among those with poor collaterals compared to those with good collaterals (median [interquartile range]: poor 52.3 cc [1.7, 104.2]; good 0.9 cc [0, 16.2]; p<0.01). (Figures 2 and 3, panel A) Penumbral salvage was lower among those with poor collaterals compared to those with good collaterals (poor 0 [0, 0]; good 5.9 cc [0, 29.4]; p<0.01). (Figures 2 and 3, panel B) Additional table and figures of infarct growth and penumbral salvage stratified by collateral grades 0–3 are provided in the online supplement (Table S1, Figures S3 and S4). We did not find an interaction between collateral status and time in the association with infarct growth (interaction p=0.84) or penumbral salvage (interaction p=0.50).
Figure 2.
Boxplot of collaterals and infarct growth (Panel A) and penumbral salvage (Panel B)
Figure 3.
Collateral clock incorporating individual subject data for infarct growth (Panel A) and penumbral salvage (Panel B)
Vessel occlusion and recanalization
Of 72 subjects with single or multisegment vessel occlusions (17 intracranial ICA, 17 M1, 11 ICA +M1, 1 ICA + M2, 4 ICA + M1 +M2, 10 M2, 11 M1 + M2, 0 ACA, 1 PCA), 61 subjects had large vessel occlusions (intracranial ICA and/or M1). Among those with large vessel occlusions, the same lack of correlation between time and penumbral salvage (r=0.09; p=0.49) and infarct growth (r=−0.09; p=0.50) persisted.
We further compared collateral scores, penumbral salvage, and infarct growth according to recanalization status among 75 subjects with followup CTA imaging. Spontaneous recanalization was seen in 38/75 (51%). Comparing subjects with and without recanalization, there was no statistically significant difference in collateral scores (median (IQR): 1 (1, 2) vs. 2 (1, 2), p= 0.54), penumbral salvage (median (IQR): 0 (0, 19.0) vs. 0 (0, 34.9), p=0.35), and infarct growth (median (IQR): 27.1 (0, 81.6) vs 6.1 (0, 62.8), p = 0.33).
Factors associated with infarct growth and penumbral salvage
In the individual analysis of associations with infarct growth, NIHSS, baseline vessel occlusion, and collateral score were associated with infarct growth, but there was no evidence of an association between time and infarct growth. Only the association between collateral score and infarct growth remained significant in the multivariable model. In the individual and multivariable analyses of associations with penumbra salvage, collateral score was associated with penumbra salvage, but there was no evidence of an association between time and penumbra salvage. (Table 2) Adding variable of time from baseline imaging to final infarct imaging in the regression model did not change these associations. (Online supplement, Table S2) Removing baseline NIHSS as a covariate from the model did not change the results. The significant association of collaterals with both infarct growth and penumbral salvage also persisted using an ordinal score instead of a dichotomized collateral grading. (Online supplement, Table 1)
Table 2.
Regression coefficients (with standard errors) on the individual and multivariable associations with the cube root infarct growth and cube root penumbra salvage
| Infarct growth Estimate (SE) |
Penumbra Salvage Estimate (SE) |
|||
|---|---|---|---|---|
| Variable |
Individual effect |
Multivariable effect |
Individual effect |
Multivariable effect |
| Age (per year) | 0.01 (0.01) | 0.001 (0.01) | −0.002 (0.01) | −0.004 (0.01) |
| Gender (female) | 0.70 (0.40) | 0.80 (0.44) | −0.12 (0.35) | −0.58 (0.41) |
| NIHSS | 0.11 (0.02)* | 0.08 (0.03)* | −0.04 (0.02) | −0.01 (0.03) |
| Recanalization | 0.47 (0.45) | −0.13 (0.44) | −0.28 (0.42) | −0.10 (0.41) |
| Large vessel occlusion | 1.21 (0.40)* | −0.03 (0.54) | 0.51 (0.36) | 1.32 (0.51)* |
| Poor collateral score (poor vs good) | 1.75 (0.37)* | 1.29 (0.48)* | −1.10 (0.34)* | −1.74 (0.45)* |
| Time from onset to baseline imaging | −0.07 (0.04) | −0.01 (0.05) | 0.01 (0.04) | −0.02 (0.05) |
p-value <0.05
Discussion
The main finding of our multicenter study of untreated acute stroke patients is that collateral flow strongly dictates the natural evolution of ischemic injury irrespective of the timing of assessment of the penumbra. Better collaterals were associated with larger penumbral salvage and decreased infarct growth in untreated stroke patients within 24 hours of stroke onset. By contrast, time from stroke onset was neither associated with penumbral salvage nor infarct growth. Patients with spontaneous recanalization trended towards greater penumbral salvage and decreased infarct growth, although did not demonstrate a statistically significant difference. This could be related to delayed timing of the spontaneous recanalization.
Our findings are consistent with the growing body of literature that collaterals are an important determinant of tissue outcome.20–22 In a study of proximal MCA occlusions receiving endovascular treatment, collaterals and reperfusion success were more important in determining penumbral loss than the time from onset to reperfusion.23 Poor collaterals also independently correlated with larger infarct growth in patients following endovascular treatment.24 In a study investigating the relationship of penumbra, collaterals and their interaction over time within 6 hours of stroke onset, there was a divergent effect by collateral status suggesting that the collateral status at initial presentation may define a group with a different natural history.25 We did not find such an interaction and this may be related to the concept of ‘collateral failure’ leading to loss of penumbral tissue over time or perhaps sample size limitations.26 A recent study of proximal vessel occlusions in acute stroke patients upto 6 hours found that core volume decreased while collateral scores increased with time, thus demonstrating that collaterals modulate the time penumbra relationship.4, 5, 27 The strength of our study that we have studied the natural history of evolution of stroke, without the confounding treatment effects of either intravenous thrombolysis or endovascular treatment. Another strength of our study is that we assessed the natural evolution over an extended time window of 24 hours, which is relevant given the new guidelines for endovascular treatment upto 24 hours.
It is important to note that perfusion imaging is a snapshot in time and that it does not capture the dynamic nature of how quickly or slowly the tissue at risk or penumbra expands into irreversible infarct. This pace of infarct growth is variable and depends on the robustness and recruitment of collaterals among other factors such as ischemic preconditioning, cerebral perfusion pressure, level of vessel occlusion, reperfusion and recanalization. It is time to refine the “Time is Brain” concept and to consider that the collateral clock may be more important than the time clock for individual patients.
Our study has important implications including making a strong case for further understanding the variations and determinants of collaterals. With DAWN and DEFUSE 3 results, we now have proven benefit of perfusion based selection for endovascular treatment upto 24 hours, however the role of collaterals in patient selction is still understudied. The updated 2018 AHA recommendations also state that it may be reasonable to incorporate collateral flow status into clinical decision making to determine eligibility for mechanical thrombectomy, with a level B evidence.16 Although additional trials using advanced imaging to extend the treatment window both for intravenous and endovascular therapy are underway and will inform us further on optimal triage 28 we would argue that retrospective studies of the natural history of control, untreated patients are a quick and cost effective way to understand fundamental pathophysiological concepts. We need to understand how the natural evolution of penumbra is modified by the complex interplay of time, vessel occlusion, recanalization status and collateral flow. This can then further support the use of intravenous thrombolysis and endovascular treatment in extended time windows. The use of collaterals as a potential triage for treatment also has practical implications as stroke centers without automated perfusion softwares may not be able to use the perfusion selection criteria in a timely fashion and may have to rely on CTA based information including collateral status. 29 Better understanding of the collateral pathophysiology will also help in separating which patients will be slow versus fast progressors, eventually translating to newer neuroprotective treatments in acute stroke.30
We acknowledge several important limitations to our study, most significantly the retrospective design. There maybe inherent case selection bias as patients in our cohort did not receive any reperfusion treatment and those presenting within the therapeutic time window may have had larger baseline ischemic core. Another limitation is that we utilized cross sectional data; obtaining multiple imaging points is challenging given the constraints of cost, contrast administration and radiation dose, particularly in CT based imaging. We did not consider additional factors determining rate of infarct growth such as hyperglycemia, blood pressure, head position, genetics and comorbidities which in turn may influence the collaterals and ischemic tolerance. Finally, our inability to show some associations may be due to an underpowered sample size rather than an absence of a true relationship.
Conclusion
In this multicenter cohort of untreated acute stroke patients, we found no time dependence of the natural tissue fate, but rather an association with collateral flow. Larger prospective studies are warranted to understand complex relationship of time, collaterals and tissue outcome that can inform future trials and could have treatment implications, particularly as the stroke field keeps extending the treatment time limits.
Supplementary Material
Acknowledgments
None
Sources of funding: This work was supported by Center for Clinical and Translational Science and Training (CCTST) KL2 TR001426-01grant.
Footnotes
Disclosures:
A.V: KL2 TR001426-01.
R.A: Grant funding CIHR, PSI Foundation, Alternate Funding Plan, University of Toronto Medical Imaging, Brain Spine Nerve research axis
H.S: No disclosures
M.R : No disclosures
Q.H: No disclosures
P.M: Significant research grant from ‘Swiss Heart Foundation’, Speaker fees from ‘Medtronic’, advisory board of ‘Medtronic’, renumeration from Penumbra Inc. for ’PROMISE steering committee’.
T.J: No disclosures
T.T: No disclosures
M.W: No disclosures
P.K: The University of Cincinnati Dept of Neurology received funds for PK’s research efforts from Genentech (PRISMS Trial), She has also received consulting fees from Lumosa for DSMB and consultant efforts, from Biogen for DSMB, From Neurospring for effort as Chair of Clinical Program Board, renumeration from Neuravi (Travel support for academic workshop.
References
- 1.Warach SJ, Luby M, Albers GW, Bammer R, Bivard A, Campbell BC, et al. Acute stroke imaging research roadmap iii imaging selection and outcomes in acute stroke reperfusion clinical trials: Consensus recommendations and further research priorities. Stroke. 2016;47:1389–1398. doi: 10.1161/STROKEAHA.115.012364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 3.Heiss WD. Ischemic penumbra: Evidence from functional imaging in man. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20:1276–1293. doi: 10.1097/00004647-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. The New England journal of medicine. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. The New England journal of medicine. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 6.Marchal G, Beaudouin V, Rioux P, de la Sayette V, Le Doze F, Viader F, et al. Prolonged persistence of substantial volumes of potentially viable brain tissue after stroke: A correlative pet-ct study with voxel-based data analysis. Stroke. 1996;27:599–606. doi: 10.1161/01.str.27.4.599. [DOI] [PubMed] [Google Scholar]
- 7.Darby DG, Barber PA, Gerraty RP, Desmond PM, Yang Q, Parsons M, et al. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion mri. Stroke. 1999;30:2043–2052. doi: 10.1161/01.str.30.10.2043. [DOI] [PubMed] [Google Scholar]
- 8.Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain : a journal of neurology. 2009;132:2231–2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Feng LQ, Bi Q, Wang YP. Characteristics and risk factors of cerebrovascular accidents after percutaneous coronary interventions in patients with history of stroke. Chinese medical journal. 2010;123:1515–1519. [PubMed] [Google Scholar]
- 10.Liebeskind DS. Reperfusion for acute ischemic stroke: Arterial revascularization and collateral therapeutics. Current opinion in neurology. 2010;23:36–45. doi: 10.1097/WCO.0b013e328334da32. [DOI] [PubMed] [Google Scholar]
- 11.Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: A potential therapeutic target. The Lancet. Neurology. 2011;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- 12.Liebeskind DS, Tomsick TA, Foster LD, Yeatts SD, Carrozzella J, Demchuk AM, et al. Collaterals at angiography and outcomes in the interventional management of stroke (ims) iii trial. Stroke. 2014;45:759–764. doi: 10.1161/STROKEAHA.113.004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fanou EM, Knight J, Aviv RI, Hojjat SP, Symons SP, Zhang L, et al. Effect of collaterals on clinical presentation, baseline imaging, complications, and outcome in acute stroke. AJNR. American journal of neuroradiology. 2015;36:2285–2291. doi: 10.3174/ajnr.A4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. The New England journal of medicine. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 15.Zhu G, Michel P, Aghaebrahim A, Patrie JT, Xin W, Eskandari A, et al. Prediction of recanalization trumps prediction of tissue fate: The penumbra: A dual-edged sword. Stroke. 2013;44:1014–1019. doi: 10.1161/STROKEAHA.111.000229. [DOI] [PubMed] [Google Scholar]
- 16.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 17.Demchuk AM, Goyal M, Yeatts SD, Carrozzella J, Foster LD, Qazi E, et al. Recanalization and clinical outcome of occlusion sites at baseline ct angiography in the interventional management of stroke iii trial. Radiology. 2014;273:202–210. doi: 10.1148/radiol.14132649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, et al. Ct angiography clot burden score and collateral score: Correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR. American journal of neuroradiology. 2009;30:525–531. doi: 10.3174/ajnr.A1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eilaghi A, Brooks J, d'Esterre C, Zhang L, Swartz RH, Lee TY, et al. Reperfusion is a stronger predictor of good clinical outcome than recanalization in ischemic stroke. Radiology. 2013;269:240–248. doi: 10.1148/radiol.13122327. [DOI] [PubMed] [Google Scholar]
- 20.Liebeskind DS, Jahan R, Nogueira RG, Zaidat OO, Saver JL. Impact of collaterals on successful revascularization in solitaire fr with the intention for thrombectomy. Stroke. 2014;45:2036–2040. doi: 10.1161/STROKEAHA.114.004781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menon BK, Qazi E, Nambiar V, Foster LD, Yeatts SD, Liebeskind D, et al. Differential effect of baseline computed tomographic angiography collaterals on clinical outcome in patients enrolled in the interventional management of stroke iii trial. Stroke. 2015;46:1239–1244. doi: 10.1161/STROKEAHA.115.009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun CH, Connelly K, Nogueira RG, Glenn BA, Zimmermann S, Anda K, et al. Aspects decay during inter-facility transfer predicts patient outcomes in endovascular reperfusion for ischemic stroke: A unique assessment of dynamic physiologic change over time. Journal of neurointerventional surgery. 2015;7:22–26. doi: 10.1136/neurintsurg-2013-011048. [DOI] [PubMed] [Google Scholar]
- 23.Jung S, Gilgen M, Slotboom J, El-Koussy M, Zubler C, Kiefer C, et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain : a journal of neurology. 2013;136:3554–3560. doi: 10.1093/brain/awt246. [DOI] [PubMed] [Google Scholar]
- 24.Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. Journal of neurology, neurosurgery, and psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheripelli BK, Huang X, McVerry F, Muir KW. What is the relationship among penumbra volume, collaterals, and time since onset in the first 6 h after acute ischemic stroke? International journal of stroke : official journal of the International Stroke Society. 2016;11:338–346. doi: 10.1177/1747493015620807. [DOI] [PubMed] [Google Scholar]
- 26.Campbell BC, Christensen S, Tress BM, Churilov L, Desmond PM, Parsons MW, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:1168–1172. doi: 10.1038/jcbfm.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal S, Bivard A, Warburton E, Parsons M, Levi C. Collateral response modulates the time-penumbra relationship in proximal arterial occlusions. Neurology. 2018;90:e316–e322. doi: 10.1212/WNL.0000000000004858. [DOI] [PubMed] [Google Scholar]
- 28.Amiri H, Bluhmki E, Bendszus M, Eschenfelder CC, Donnan GA, Leys D, et al. European cooperative acute stroke study-4: Extending the time for thrombolysis in emergency neurological deficits ecass-4: Extend. International journal of stroke : official journal of the International Stroke Society. 2016;11:260–267. doi: 10.1177/1747493015620805. [DOI] [PubMed] [Google Scholar]
- 29.de Havenon A, Southerland AM. In large vessel occlusive stroke, time is brain… But collaterals are time. Neurology. 2018;90:153–154. doi: 10.1212/WNL.0000000000004870. [DOI] [PubMed] [Google Scholar]
- 30.Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: Clinical and research implications. Stroke. 2017;48:2621–2627. doi: 10.1161/STROKEAHA.117.017673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.