Abstract
Objective
Actin cytoskeleton assembly and organization, as a result of focal adhesion formation during cell adhesion, are dependent upon reactive oxygen species (ROS) and the cellular redox environment. Polymerase delta interacting protein 2 (Poldip2), a novel regulator of NADPH oxidase 4 (NOX4), plays a significant role in ROS production and cytoskeletal remodeling. Thus, we hypothesized that endogenous ROS derived from Poldip2/NOX4 contribute to redox regulation of actin and cytoskeleton assembly during integrin-mediated cell adhesion.
Approach and Results
Using vascular smooth muscle cells, we verified that H2O2 levels increase during integrin-mediated cell attachment due to activation of NOX4. F-actin was oxidized by sulfenylation during cell attachment, with a peak at 3 hours (0.80±0.04 vs. 0.08±0.13 AU at time zero), which was enhanced by overexpression of Poldip2. Depletion of Poldip2 or NOX4 using siRNA, or scavenging of endogenous H2O2 with catalase, inhibited F-actin oxidation by 78±26, 99±1, and 98±1%, respectively. To determine the consequence of F-actin oxidation, we examined the binding of F-actin to vinculin, a protein involved in focal adhesion complexes that regulates focal adhesion maturation. Binding to vinculin during cell adhesion and migration capacity was inhibited after transfection with actin containing two oxidation-resistant point mutations (C272A and C374A). Silencing of Poldip2 or NOX4 also impaired actin-vinculin interaction, which disturbed maturation of FAs and inhibited cell migration.
Conclusions
These results suggest that integrin engagement during cell attachment activates Poldip2/Nox4 to oxidize actin, which modulates focal adhesion assembly.
Keywords: Cell adhesion molecule, Oxidation, Cytoskeletal Dynamics, NADPH oxidase
TOC category - basic
TOC subcategory - Vascular Biology

Introduction
Actin is the principal component of the cytoskeleton with an indispensable role in maintaining form, internal organization and function of cells, including crucial processes such as adhesion, migration and contraction.1 Actin filaments (F-actin) are dynamic structures made up of G-actin monomers that assemble and disassemble in response to external stimuli into higher-order structures of the cytoskeleton.2 Their association with other cell structures such as the plasma membrane is controlled by interaction with numerous actin-binding proteins.3, 4 An important subgroup of these molecules is focal adhesion (FA) proteins that serve as adaptors linking the actin cytoskeleton via integrins to extracellular matrix (ECM).5 FA assembly, maturation and disassembly have central roles in cellular adhesion and migration.6
Cell adhesion is a complex stepwise process. Formation of local protrusions in association with rapid F-actin assembly7 leads to cell contact with the ECM.8 Subsequently, integrins bind to ECM proteins, thereby activating a signaling mechanism9 that recruits FA adaptor proteins to form nascent FAs.10 Nascent FAs consist of talin, paxillin and focal adhesion kinase.11 In the next step, recruitment of vinculin and other actin binding proteins leads to formation of a flexible bridge between the integrins and the actin cellular network.12 Further strengthening and maturation of FAs is characterized by the increased presence of α-actinin, VASP and zyxin. Mature FAs mechanically couple the actin cytoskeleton to the ECM, allowing transmission of force.13 Once a mechanical link between the actin cytoskeleton and the ECM is established, cell spreading occurs. In addition, further organization of actin filaments provides force for tissue integrity or cell migration.14
Integrin signaling has a crucial role in coordinating complex changes during processes of cell adhesion and migration.15 These transmembrane receptors can act as bidirectional signal transducers. Interaction of their cytoplasmic tails with adaptor proteins such as talin and vinculin increases affinity of integrins for ECM ligands.16 Conversely, binding of ECM ligands triggers a signaling cascade17 leading to FA formation and maturation through integrin clustering, the recruitment of adaptor proteins such as zyxin, and cytoskeletal rearrangement.11
There is growing evidence that reactive oxygen species (ROS) function as important second messengers in integrin-activated signaling pathways during cellular adhesion and migration,18–24 but the source of integrin-triggered ROS production is less clear. Some data suggest mitochondria or 5-lipoxygenase produce ROS during signal transduction initiated by integrin engagement, but NADPH oxidases (NOXs) appear to be a more likely source.18, 23, 25, 26 However, since ROS react promiscuously with numerous biomolecules, they require subcellular compartmentalization for specificity.27
NADPH oxidases of the NOX family are important enzymatic sources of ROS.28 This family of enzymes includes 7 members, NOX1-NOX5, DUOX1 and DUOX2.29, 30 NOX4 is a major source of intracellular hydrogen peroxide (H2O2) in vascular smooth muscle cells (VSMCs)30 and we have previously shown that NOX4 and its regulator Polymerase delta-interacting protein-2 (Poldip2) have an important role in regulating FA turnover and cell migration.26, 31 In addition to altering the function of multiple redox sensitive signaling molecules involved in integrin-signaling,32, 33 ROS can also directly oxidize the actin cytoskeleton 32, 34 or actin-binding proteins.35 A select group of cysteine (Cys) residues on proteins are typical targets of oxidative modification because of their low pKa thiols within certain protein microenvironments.36 Oxidation of critical cysteines in various signaling proteins is considered an important posttranslational regulatory signaling event.37 Among the six cysteine residues of human β-actin,34 Cys272 and Cys374 are of particular interest as targets for oxidation because of their location on the surface of the molecule.34, 38 High, non-physiological concentrations of ROS can cause irreversible cysteine oxidation producing sulfinic and sulfonic acid derivatives, which inhibit actin polymerization and migration.39 However, cysteine oxidation to sulfenic acid, triggered by low physiological levels of cellular oxygen species like H2O2, is a reversible process suitable to function as a redox switch40 and may promote migration, and by inference, adhesion.41–43 In addition to their effect on actin polymerization, oxidation of cysteine residues may affect binding of regulatory proteins to actin.32, 44 Interestingly, it was previously shown that low concentrations of H2O2 promote the formation of stress fibers and vinculin-containing FAs, pointing to an important role of ROS in cell adhesion to the substrate.42
Vinculin is a crucial FA adaptor protein, which in concert with other bridging proteins, such as talin, paxillin and α-actinin, tethers actin filaments to integrins in FAs.45 The role of vinculin in FA formation is multifaceted. In addition to linking integrins and actin, vinculin serves as a scaffold with binding sites for 17 other proteins including talin, α-actinin, actin-related protein (ARP) 2/3, zyxin and paxillin.5, 46 Vinculin in concert with talin activates integrins and promotes actin nucleation and crosslinking. 47 In the present study, we report that activation of integrins during cellular adhesion triggers a Poldip2/NOX4 signaling pathway leading to oxidation of Cys residues in F-actin. Cysteines 272 and 374 are involved in this mechanism, which enhances binding of vinculin to actin and thereby promotes FA maturation.
Materials and methods
Detailed materials and methods can be found in Supplemental Material.
Primary rat aortic smooth muscle cells (RASMs) were prepared locally48 and human aortic smooth muscle cells (HASMs) were purchased from Clonetics.
Intracellular H2O2 was monitored using live confocal microscopy by ratiometric fluorescence of HyPer-3 probe.49 NADPH oxidase activity in membrane fractions was measured by detection of H2O2 using CPH as an ESR spin trap.50, 51 Oxidation of cysteine residues in actin was measured using DCP-Bio1 assays.52, 53
Transmission electron microscopy and immunoperoxidase staining was used to detect Nox4 in vesicles using an antibody given by Dr. Lambeth.54 Some sections were processed using tannic acid to improve images of vesicles.55, 56
Cells were transduced with adenoviruses prepared using the pAdEasy system57 or transfected with plasmids by electroporation. Cells were transfected with siRNA or inactive fluorescent siRNA using lipid reagents.
Assays for mRNA and protein expression were performed using RT-qPCR58 and Western blotting.
Protein subcellular localization was investigated by immunofluorescence staining and confocal microscopy. Focal adhesion assembly was assessed by measuring the zyxin/paxillin ratio.59, 60 Interaction of proteins was measured using proximity ligation assays and confocal microscopy.
Cell migration was investigated in vitro using confocal microscopy in a wound healing assay.
Statistical analysis using one or two-way ANOVA was performed using GraphPad Prism software.
Results
Intracellular H2O2 levels increase during cell adhesion
It was previously shown that production of ROS is an important signaling mechanism in the process of cellular adhesion,21 profoundly affecting FA formation and turnover.26 In order to assess the time course of ROS generation during cellular adhesion, we utilized the cyto-Hyper3 probe, a genetically-encoded, permuted yellow fluorescent protein fused to a modified, H2O2-sensitive, bacterial transcription factor OxyR. Cyto-Hyper3 functions as a ratiometric fluorescent sensor, which allows dynamic, reversible measurement of low physiological concentrations of intracellular H2O2 with high selectivity and sensitivity.49, 61 The pseudo-colored overlay images in Figure 1 represent the ratio of cyto-HyPer3 fluorescent signals, with blue color corresponding to low, green to medium, and red to high level of H2O2 concentrations. We found that during a 6 hour period after seeding, cells expressing cyto-Hyper3 show an initial increase in the fluorescence ratio (Figure 1A, C) peaking at 150 minutes. This change reflects a 55±14% increase in intracellular H2O2 levels compared to time zero. After 150 minutes, the HyPer signal decays, correlating with a decrease in the levels of H2O2, which return to baseline at 6 hours post seeding.
Figure 1. NOX4 produces H2O2 after integrin activation and localizes in vesicles along stress fibers.
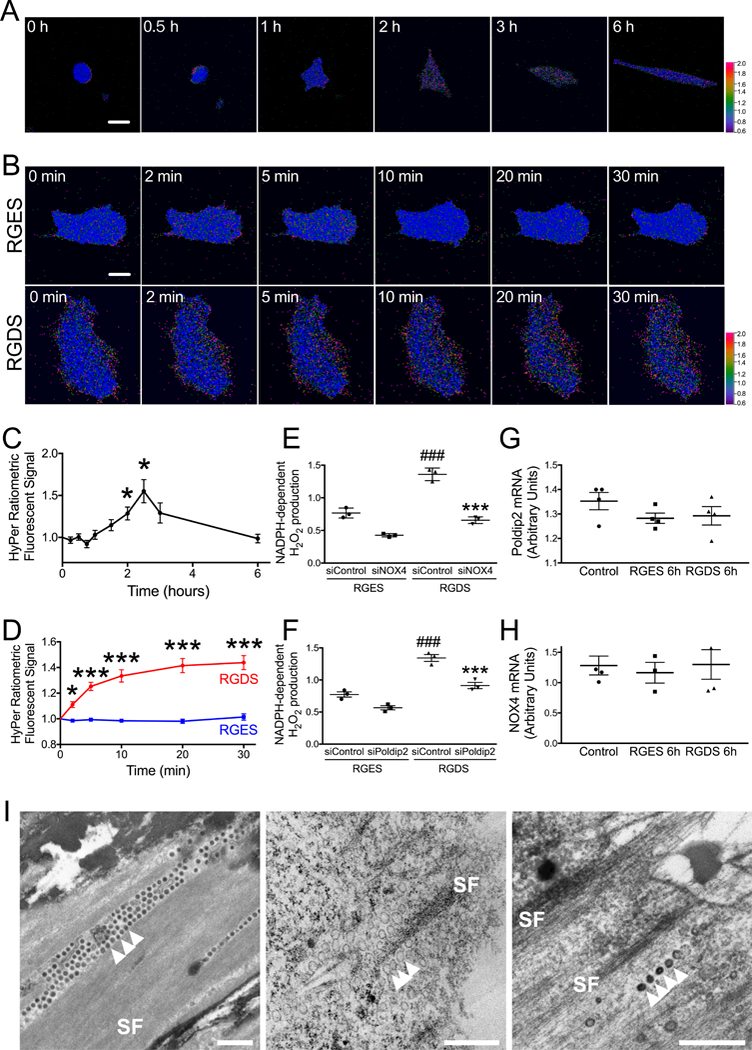
A, C. Representative images (A) and quantified cytoplasmic H2O2 concentration (C) measured in HASMs at indicated times after plating using live fluorescence microscopy in single cells. Following transfection with cyto-HyPer3, the ratio of fluorescence signals is shown in false colors representing cytoplasmic H2O2 concentration. Summary data represent mean ± SEM of single cell measurements from 9 independent experiments. Scale bar: 20 μm, *p<0.05 vs. time zero. B, D. HASMs were exposed to 50 μM of RGDS peptide or its inactive control RGES starting 30 minutes after plating. HyPer3 fluorescence images were acquired at indicated subsequent times. B: representative images. D: mean ± SEM of data of single cell measurements from 16 independent experiments. Scale bar: 15 μm. * p<0.05, *** p<0.001 vs. RGES. E-F. HASMs were transfected with control siRNA (siControl), siNOX4 (E), or siPoldip2 (F). Cells were harvested after 72 hours and incubated in cell culture medium for 30 minutes with agitation before addition of 50 μM RGES or RGDS for 30 min. H2O2 was measured in membrane fractions by ESR using CPH as a spin probe. Bars represent mean ± SEM from 3 independent experiments. ### p<0.001 vs. siControl + RGES, *** p<0.001 vs. siControl + RGDS. G-H. HASMs were treated with 50 μM RGES or RGDS for 6 h. Poldip2 (G) and NOX4 (H) mRNA levels were measured by RT-qPCR and normalized to the housekeeping gene RPL mRNA. Summary data represent mean ± SEM of data from 3 independent experiments. I. Transmission electron micrographs showing vesicles located along stress fibers (SF) in sections parallel to the bottom edge of cultured RASMs. Scale bars: 0.5 μm. Left: The extracellular matrix and vesicles next to the substratum (arrowheads) are densely stained by tannic acid. In contrast, the plastic dish remains white at the top left and bottom right. Middle and Right: Immunoperoxidase staining. While vesicles stain lightly in the absence of primary antibody (Middle, arrowheads), they appear much denser to electrons after incubation with primary antibody against Nox4 (Right, arrowheads).
Previously, it was reported that ROS production during cellular adhesion is controlled by outside-in signaling pathways triggered by integrin engagement of ECM.23 To further explore that possibility, we used the tetrapeptide Arg-Gly-Asp-Ser (RGDS) to activate the integrin-mediated signaling pathways, as this sequence is a part of the integrin binding site of fibronectin. The inactive analog Arg-Gly-Glu-Ser (RGES) was used as a control. VSMCs transfected with cyto-Hyper3 treated with 50 μM RGDS for 30 minutes present a change in fluorescence ratio, which correlates with a 44±5% increase in intracellular H2O2 levels (Figure 1B, D). Control cells treated with 50 μM RGES had no significant change in intracellular H2O2 levels over the same time period (101±2% control).
NOX4 and Poldip2 promote H2O2 generation during integrin activation and NOX4 localizes in vesicles along stress fibers
NOX4 is a major source of intracellular H2O2 in VSMCs.30 In order to investigate the role of NOX4 in the production of H2O2 during integrin engagement, we measured NADPH-dependent H2O2 production using electron spin resonance (ESR) and a spin probe/HRP detection system as described in Methods. VSMCs transfected with control siRNA and treated with RGDS showed significantly increased NADPH-dependent H2O2 production compared to cells treated with RGES (1.36±0.09 vs. 0.77±0.08 AU). This effect was attenuated in cells transfected with siNOX4 (0.65±0.05 vs. 0.43±0.03 AU) (Figure 1E). We have previously shown that Poldip2 can act as a regulator of NOX4, stimulating ROS production.31 Thus, we hypothesized that Poldip2 may also participate in regulation of NADPH-dependent H2O2 production upon integrin activation. As shown in Figure 1F, RGDS-induced NADPH-dependent H2O2 production was suppressed after transfection of siPoldip2 (0.91±0.09 vs. 0.57±0.05 AU), similar to the effect of siNOX4. Neither RGES nor RGDS had any effect on the expression of Poldip2 and NOX4 (Figure 1G, H).
Interestingly, transmission electron microscopy (TEM) analysis of VSMCs demonstrated abundant vesicles alongside the stress fibers at the bottom of the cell (evident from the electron-dense material which represents extracellular matrix after staining with tannic acid) (Figure 1I left). Some vesicles of this type are positive for NOX4 (Figure 1I, right), suggesting that ROS from NOX4 can target the cytoskeleton. Taken together, these results suggest that integrin activation induced-H2O2 production is dependent on Poldip2 and NOX4 and occurs adjacent to cytoskeletal elements. The mechanism is, however, not based on increased expression of these molecules, but rather results from activation of the oxidase complex.
Integrin activation stimulates F-actin oxidization
Cysteine residues on proteins are typical targets of oxidative modification because of their low pKa thiols in particular protein microenvironments. Oxidation of protein cysteine residues by physiological levels of cellular oxygen species such as H2O2 yields sulfenic acid, which is reversible.40, 62, 63 To determine if actin is a target of H2O2 induced by integrin activation, we used the dimedone-based chemical probe DCP-Bio1 to evaluate actin sulfenylation.52, 53 This reagent covalently traps sulfenic acids but not disulfide bonds (which can be generated subsequent to sulfenic acid formation in proteins, or through thiol-disulfide exchange processes). Thus, it is ideal for detecting sulfenic acids generated during active oxidation processes (prior to subsequent reactions and generation of alternative products) and those which are stabilized within their protein microenvironment.64 VSMCs were harvested at 0, 1, 3, and 6-hour time points after seeding, then lysed in the presence of DCP-Bio1, and F-actin and G-actin were separated by centrifugation. Western blot analysis of streptavidin-agarose pull-down samples revealed an increase in the F-actin oxidation with a peak at 3 hours. In contrast, G-actin was not oxidized during cell attachment (Figure 2A). We next examined F-actin oxidation following integrin engagement. Western blot analysis of VSMCs treated with RGDS for 30 minutes showed an increase in F-actin oxidation compared to RGES treated cells. Of importance, integrin-mediated oxidation of F-actin was suppressed by PEG-catalase, which depletes intracellular H2O2 (Figure 2B). Similar to the cell adhesion experiment, G-actin was not oxidized by integrin activation (Figure 2B).
Figure 2. Integrin activation stimulates F-actin oxidation by H2O2.
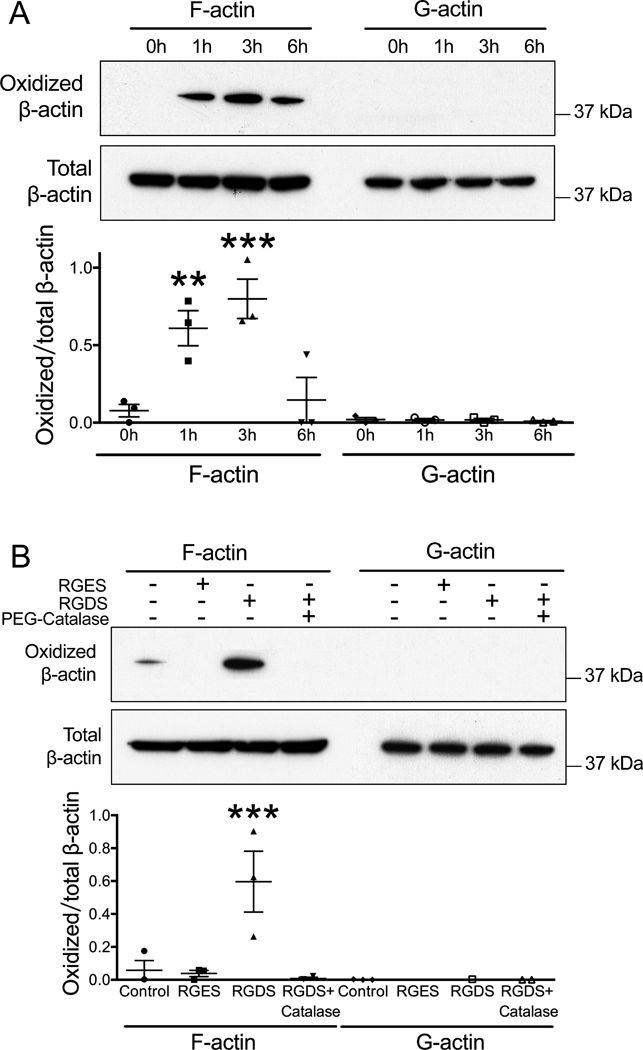
A. RASMs were harvested 0, 1, 3 and 6 hours after plating. DCP-Bio-1 probe was used to label oxidized cysteine residues. F-actin (pellet) and G-actin (supernatant) were separated by centrifugation. Samples were analyzed by Western blot. Data represent mean ± SEM of 3 independent experiments. ** p<0.01, *** p<0.001 vs. 0 h. B. RASMs were treated for 30 minutes with 50 μM of RGES, RGDS alone or RGDS after pre-treatment for 30 minutes with PEG-Catalase (200 U/ml). Data represent mean ± SEM from 3 independent experiments. *** p<0.001 vs. Control.
Integrin mediated F-actin oxidation requires NOX4/Poldip2
To explore the role of NOX4 in F-actin oxidation during cell adhesion, we used siRNA. Cells transfected with siControl showed a typical pattern of F-actin oxidation with a peak at 3 hours post seeding, as previously observed. However, F-actin oxidation in VSMCs transfected with siNOX4 was completely blocked (Figure 3A). Similarly, RGDS-induced F-actin oxidation was suppressed by NOX4 knockdown (Figure 3B), indicating a role for NOX4 in integrin-mediated actin oxidation.
Figure 3. Integrin mediated F-actin oxidation requires NOX4.
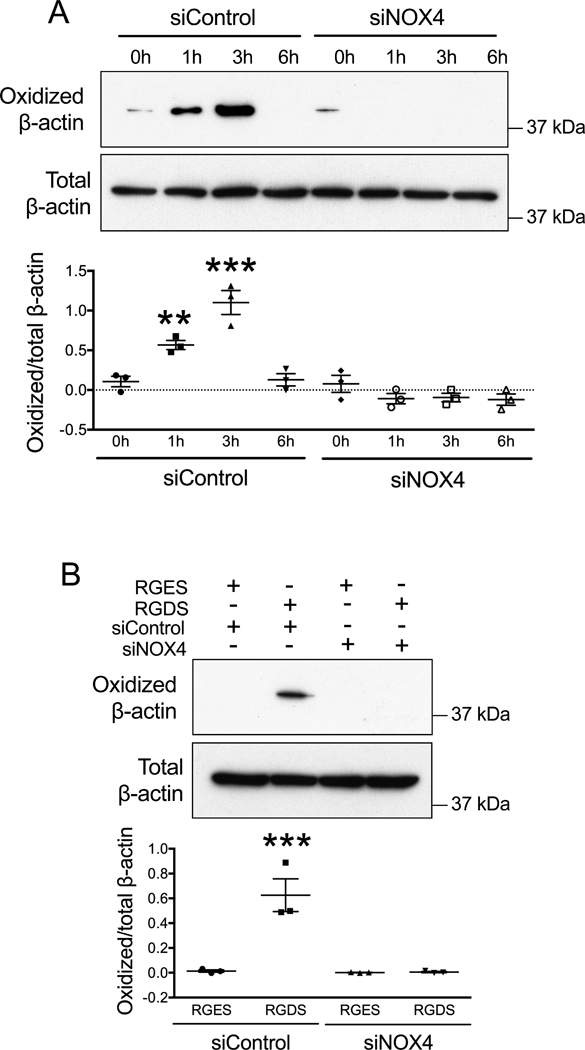
A. RASMs were transfected with control siRNA (siControl) or siNOX4. After 72 hours, cells were re-plated and harvested after 0, 1, 3 and 6 hours before measuring F-actin oxidation as in Figure 2. Data represent mean ± SEM from 3 independent experiments. ** p<0.01, *** p<0.001 vs. siControl at 0 h. B. RASMs were transfected with control siRNA (siControl) or siNOX4. After 72 hours, RGDS or RGES (50 μM) was added for 30 minutes before cell harvesting. F-actin was collected to measure cysteine oxidation, as above. Data represent mean ± SEM from 3 independent experiments. *** p<0.001 vs. RGES.
We have previously shown that Poldip2 can act as a regulator of NOX4, stimulating ROS production.31 Based on our previous results, we hypothesized that Poldip2 may participate in regulation of NOX4 mediated F-actin oxidation. As expected, Poldip2 knockdown in VSMCs inhibited both F-actin oxidation during cell adhesion (Figure 4A) and RGDS induced F-actin oxidation (Figure 4B). The reverse was also true. Adenoviral-mediated overexpression of Poldip2 (AdPoldip2) in VSMCs increased F-actin oxidation compared to cells transfected with control adenovirus (AdGFP) (Figure 4C). These results suggest that the Poldip2/NOX4 signaling pathway may be an effector of F-actin oxidation following integrin activation during cellular adhesion.
Figure 4. Integrin mediated F-actin oxidation requires Poldip2.
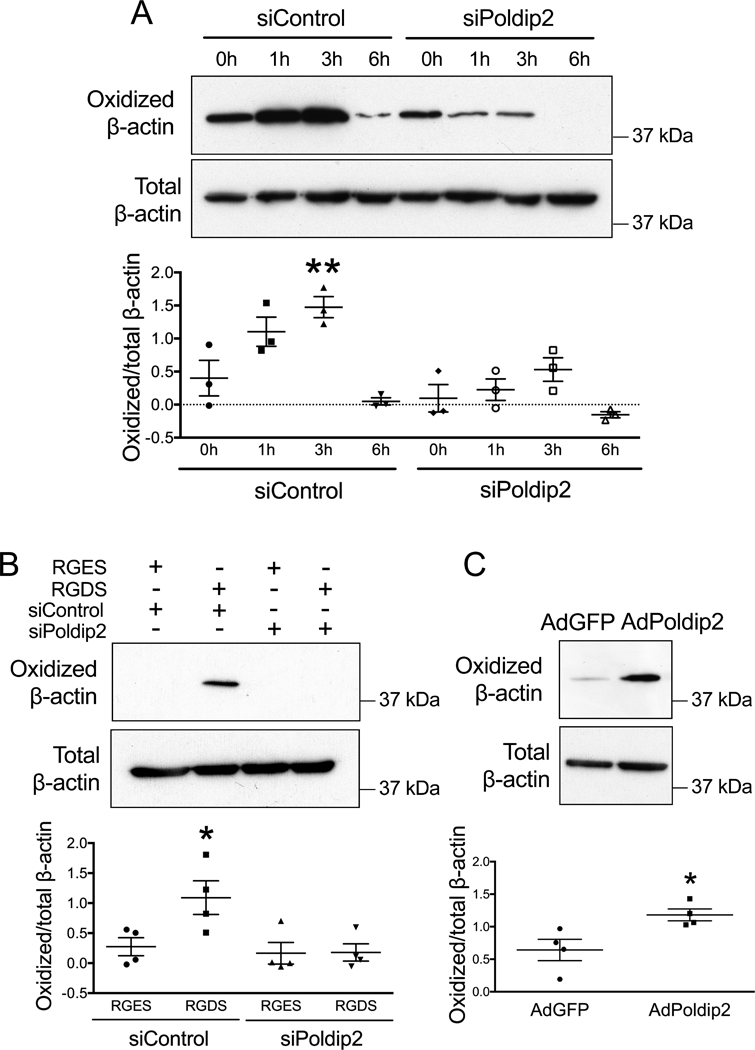
A. RASMs were transfected with control siRNA (siControl) or siPoldip2. After 72 hours, cells were re-plated and harvested after 1, 3 and 6 hours before measuring F-actin oxidation as in Figure 2. Data represent mean ± SEM from 3 independent experiments. ** p<0.01 vs. siControl at 0 h. B. RASMs were transfected with control siRNA (siControl) or siPoldip2. After 72 hours, RGDS or RGES (50 μM) was added for 30 minutes before harvesting. F-actin was collected to measure cysteine oxidation, as above. Data represent mean ± SEM of 4 independent experiments. * p<0.05 vs. siControl. C. Overexpression of Poldip2 induces F-actin oxidation. RASMs were transfected with adenoviruses with no insert (AdGFP) or C-terminal myc-tagged Poldip2 (AdPoldip2) and harvested after 72 hours. Data represent mean ± SEM from 4 independent experiments. * p<0.05 vs. AdGFP.
Actin oxidation on Cysteine 272 and/or 374 is involved in F-actin-vinculin complex assembly
To further analyze the role of F-actin redox regulation during cellular adhesion, we designed two plasmids to express either myc-tagged wild type (WT) human β-actin or an oxidation-resistant mutant of β-actin, with both cysteine 272 and 374 substituted with alanine (C272A and C374A). These residues were selected because of their location on the surface of the molecule.34, 38 We hypothesized that cysteine sulfenylation may affect interactions between F-actin and its binding proteins. As vinculin is a critical actin binding protein involved in FA formation,12, 65 we examined the role of cysteine 272 and 374 oxidation on the interaction between β-actin and vinculin using the proximity ligation assay. VSMCs transfected with either the WT or oxidation-resistant mutant of β-actin were re-plated for 1, 3 and 6 hours. We observed that the F-actin-vinculin complex formation (green) increases between 1 and 3 hours and then begins to return to baseline at 6 hours in cells expressing WT actin. In contrast, in cells expressing the oxidation-resistant C272A, C374A β-actin mutant, complex formation is impaired (Figure 5A-C). This result suggests that actin oxidation on Cys272 and/or Cys374 is important for F-actin-vinculin complex assembly. Note that both recombinant WT and mutant β-actin (green) were incorporated to the same degree into cellular F-actin labeled by phalloidin (red) (Figure 5D), indicating that the C-terminal myc tag and the cysteine mutations do not prevent actin polymerization.
Figure 5. Mutation of cysteines 272 and 374 inhibits F-actin-vinculin interaction and impairs cell migration.
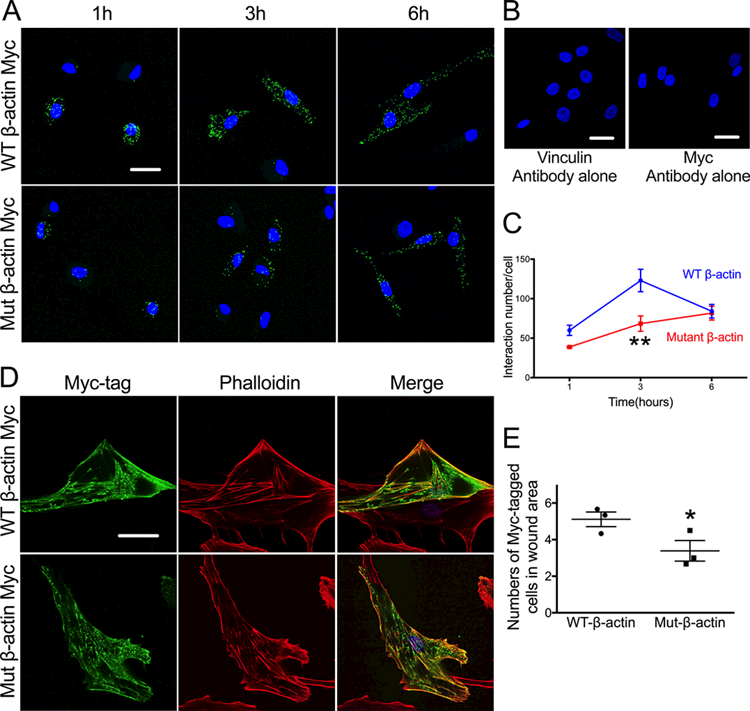
A-C. HASMs were transfected with myc-tagged wild type (WT) or mutant (C272A and C374A) β- actin. After 72 hours, cells were re-plated and fixed during attachment at 1, 3 or 6 hours. Interaction between vinculin and myc-tagged β-actin was measured using a proximity ligation assay and confocal microscopy. A. Maximal intensity projection micrographs of consecutive Z-sections showing myc-tagged β-actin-vinculin interaction in green and nuclear staining with DAPI in blue. Scale bar: 20 μm. B. Negative controls for vinculin and myc-tag antibodies alone. Scale bars: 20 μm. C. Summary of image quantification representing mean ± SEM of data from 3–4 independent experiments. ** p<0.01 vs. WT at 3 h. D. Transfected β-actin was successfully incorporated into stress fibers in HASMs, as shown by confocal microscopy. Myc-tagged β-actin (green) and total actin, labeled by phalloidin (red), showed significant colocalization (yellow) in merged images. Scale bar: 20 μm. E. HASMs were transfected with myc-tagged wild type (WT) or mutant (C272A and C374A) β-actin. After 72 hours, a straight scratch wound was made and 10 ng/ml PDGF was added to stimulate cell migration. Cells were fixed after 6 hours and analyzed by confocal microscopy. The graph presents mean ± SEM of image quantification from 3 independent experiments showing numbers of myc-tagged cells in wound area. * p<0.05 vs. WT.
Mutant cysteine 272A and/or 374A on β-actin impairs VSMCs migration
To investigate the effect of Cysteine 272A and 374A mutation of β-actin on VSMC migration, we performed a wound healing assay. VSMCs transfected with either the WT or the oxidation-resistant mutant of β-actin (C272A/374A) were grown for 72 hours to confluence. Subsequently, a wound was introduced with a sterile pipette. Upon stimulation with 10 ng/ml PDGF for 6 hours, VSMCs transfected with the oxidation-resistant mutant of β-actin migrated less into the wound area than did WT β-actin transfected cells (Figure 5E).
Poldip2 and NOX4 promote F-actin-vinculin complex assembly
Additionally, we were interested in determining the role of Polidp2/NOX4 in F-actin-vinculin complex formation during cell attachment. We depleted Poldip2 or NOX4 in VSMCs by transfecting cells with the corresponding siRNA. The efficiency of knockdown was confirmed by qPCR and western blot (Figure 6A, B). Results showed that β-actin-vinculin interactions were only 47±7% and 76±4% of the control signal upon knockdown of NOX4 and Polidp2, respectively (Figure 6C,D). These results suggest that both NOX4 and Poldip2 depletion can impair F-actin-vinculin complex assembly during cellular adhesion. To further explore interdependence and causality of Poldip2 and NOX4, we simultaneously performed knockdown of NOX4 and overexpression of Poldip2. Overexpression of Poldip2 in cells transfected with control siRNA significantly enhanced β-actin-vinculin interactions. However, this effect was attenuated in Poldip2 overexpressing cells transfected with siNOX4 (Figure 6E-F). This result indicates that the effect of Poldip2 on the formation of β-actin-vinculin complexes depends on enhanced NOX4 activity.
Figure 6. Knockdown of Poldip2 or NOX4 impairs F-actin-vinculin interaction and focal adhesion assembly.

A-D. HASMs were transfected with control siRNA (siControl), siPoldip2 or siNOX4 and co- transfected with inactive fluorescent siGLO. After 72 hours, cells were re-plated and fixed during cell attachment after 1, 3 or 6 hours. A. The knockdown of NOX4 mRNA by siNOX4 was confirmed by RT-qPCR and presented as mean±SEM of data from 4 independent experiments. * p<0.05. B. The knockdown of Poldip2 protein by siPoldip2 (siP), compared to siControl (siC) was confirmed by western blotting. These blots are representative of data from 4 experiments. C. Representative micrographs of proximity ligation assays performed as in Figure 5 showing interaction (green) between vinculin and β-actin, siGLO (red) and nuclear staining with DAPI (blue). Scale bars: 20 μm. D. Summary of image quantification representing mean ± SEM of data from 3–4 independent experiments. Only transfected cells (positive for siGLO) were included. * p<0.05, ** p<0.01, *** p<0.001 vs. siControl. E- F. HASMs were transfected with control siRNA (siControl) or siNOX4 and co-transfected with inactive fluorescent siGLO on day 1. On day 2, cells were infected with adenoviruses expressing GFP and either no insert (AdGFP) or C-terminal myc-tagged Poldip2 (AdPoldip2). After 48 hours, cells were re- plated and fixed during cell attachment at 3 hours. E. Representative micrographs of proximity ligation assays showing interaction (white) between vinculin and β-actin, siGLO (red), GFP (green) and nuclear staining with DAPI (blue). Scale bar: 20 μm. F. Summary of image quantification representing mean ± SEM of data from 3 indepedent experiments. Only transfected cells (positive for siGLO and GFP) were included. ** p<0.01, *** p<0.001, ### p<0.001 vs. siControl+AdGFP. G-H. HASMs were transfected with control siRNA (siControl), siPoldip2 or siNOX4 and co-transfected with inactive fluorescent siGLO. After 72 hours, cells were re-plated and fixed during cell attachment after 1, 3 or 6 hours. G. Representative micrographs showing zyxin(green), paxillin (red), siGLO (purple) and nuclear staining with DAPI (blue). Scale bar: 20 μm. H. Summary of image quantification showing zyxin-paxillin colocalization in FA during cell attachment, including mean ± SEM of data from 3 independent experiments. * p<0.05, *** p<0.001 vs. siControl.
Poldip2 and NOX4 promote FA maturation
We have previously reported that overexpression of Poldip2 inhibits migration by strengthening FAs and consequently impairing FAs turnover. However, knockdown of Poldip2 or NOX4 can also inhibit migration by inducing a loss of FAs. 26, 31 As noted above, nascent FAs consist of talin, paxillin and focal adhesion kinase,11 while mature FAs are characterized by increased presence of α-actinin, VASP and zyxin. Therefore, we examined maturation of FAs during cell attachment by measuring zyxin-paxillin co-localization 1, 3, and 6 hours after seeding.59, 60 In comparison to siControl transfected cells, depletion of Poldip2 or NOX4 significantly inhibited recruitment of zyxin in FAs (Figure 6G-H), indicating disturbed maturation of FAs. Consistent with our previous results, knockdown of either Poldip2 or NOX4 impairs FA maturation.
Discussion
In this study, we examined the effect of integrin-mediated actin oxidation on actin-vinculin complex assembly during cell adhesion. Our principal findings are as follows. First, integrin engagement leads to a Poldip2/NOX4-mediated transient increase in H2O2 production during cell adhesion. Second, the increase in H2O2 production during cell adhesion causes oxidation of redox-sensitive cysteine residues on F-actin. Lastly, oxidation of Cys-272 and Cys-374 on F-actin promotes actin-vinculin complex assembly during cellular adhesion and cell migration. Our results suggest that actin oxidation via activation of Poldip2/NOX4 is an important step in cytoskeletal organization during cell adhesion. In addition to altering the function of multiple redox-sensitive signaling molecules involved in integrin-signaling,32, 33, ROS can also directly oxidize actin-binding proteins35 or the actin cytoskeleton itself.32, 34 Our study provides evidence that ROS can regulate processes of cellular adhesion and migration via direct oxidation of actin, leading to increased binding of vinculin.
One of the most interesting findings in our study is that both integrin- and adhesion-mediated oxidation of actin predominantly affects F-actin, but not G-actin. This specificity of freely diffusible ROS is intriguing. Interestingly, a recent study examining the role of actin oxidation and subsequent S-glutathionylation in integrin-mediated cell adhesion reported that protein disulfide isomerase (PDI) binds to glutathionylated G-actin. This is followed by reversal of glutathionylation and increased polymerization of actin, leading to an increase in the F-actin content.44 A similar effect of deglutathionylation is observed in cells stimulated by epidermal growth factor (EGF).66 Reversible protein S-glutathionylation is an important post-translational modification in which thiol groups of cysteine residues oxidized to sulfenic acid form a mixed disulfide with glutathione. In this way, glutathionylation of β-actin can occur selectively by a thiol-exchange mechanism.67 However, as glutathionylation of cysteine will prevent binding of the dimedone-based DCP-Bio1 probe we used in our experiment, the fact that we observed oxidation of F-actin, but not G-actin, may indicate that in our system, cysteine residues in F-actin are oxidized, but do not undergo glutathionylation (at least rapidly enough to out-compete DCP-Bio1 trapping). This is in accordance with findings that glutathionylation leads to instability and breakdown of F-actin. Alternatively, Cys272 and Cys374 may have different fates after exposure to H2O2. Our results also suggest additional regulatory effects of ROS during cellular adhesion, as we have shown that actin oxidation leads to an increase in vinculin binding.
Vinculin is one of the actin-binding proteins that can bind to the actin filament near Cys374.47 This complex protein has a unique structure consisting of a head linked by a proline rich sequence to the tail.68 In the inactive form of the molecule, interaction between the head and tail masks numerous binding sites for ligands.69 Upon recruitment to FAs, vinculin undergoes activation, characterized by a conformational change leading to the head separating from the tail and unmasking cryptic ligand binding sites. Interestingly, vinculin binding to F-actin is proposed to induce a conformational change, leading to its activation and dimerization.70 The binding of vinculin to actin is mediated by two regions located in the tail.71 In the inactive form of vinculin, one of the actin binding regions is available for interaction, while the access to second site is blocked by the head region.70 After activation of vinculin, the second site becomes available, and its binding strength to actin consequently increases, followed by dimerization of the vinculin tail. Our experimental data indicate that actin oxidation on Cys272 and/or Cys374 correlates with an increase in F-actin-vinculin complex assembly, most likely by inducing a conformational change that promotes vinculin binding and activation. It is likely that oxidation of additional cysteines or methionines is also involved, given that some interaction between F-actin and vinculin occurs even in Cys 272/274 mutant expressing cells (Figure 5A). An interesting possibility arising from this observation is that oxidized actin could induce a vinculin conformational change that activates its dimerization. Vinculin dimerization was shown previously to promote actin bundling. Consequently, vinculin dimerization would result in an increase in the vinculin-actin complex assembly as we observed with the proximity ligation assay, and would ultimately lead to FA strengthening and maturation. We have confirmed this effect by observing decreased recruitment of zyxin in FAs, as marker of FA maturation, in cells transfected with siPoldip2 or siNOX4. These results are in concordance with our previous findings that Poldip2/NOX4 regulate FA turnover during cell migration,26 as in our current study actin oxidation and vinculin binding are also controlled by a Poldip2/NOX4-mediated increase in H2O2 production. Furthermore, it appears that actin oxidation is an important component of Poldip2/NOX4 regulation of cellular migration as cells expressing the oxidation-resistant mutant of β-actin (Cys272A/374A) show impaired migration.
Of note, one study suggested that mitochondria and 5-lipoxygenase may be sources of ROS in integrin-mediated signaling.23 However, in our study we have shown that integrin mediated production of H2O2 during cellular adhesion depends on Poldip2 and NOX4. We have demonstrated that NOX4-containing vesicles are located along stress fibers, providing the compartmentalization necessary for controlled and specific action of H2O2 on actin. We have also shown that either Poldip2 or NOX4 knockdown can impair integrin-mediated production of H2O2 and consequent actin oxidation and vinculin binding. This effect of integrin activation is not dependent on increased expression of NOX4 or Poldip2. However, as Poldip2 knockdown prevents the integrin mediated increase in both NADPH oxidase-mediated H2O2 production and actin oxidation in a similar manner to NOX4 knockdown (Figure 1 E, F), it appears that Poldip2 primarily exerts its effect through regulation of NOX4 production of H2O2, although we cannot rule out the possibility that Poldip2 might also regulate trafficking of NOX4 to actin. Conversely, overexpression of Poldip2 leads to an increase in actin oxidation. However, if we simultaneously perform knockdown of NOX4 this effect is lost, indicating that the effects of Poldip2 depend fully on NOX4 activity. Thus, our previously published observation showing that a sustained increase in H2O2 levels due to overexpression of Poldip2 correlates with stabilization and increased strength of FAs may be in part due to an increase in the vinculin-actin complex assembly triggered by actin oxidation.
In summary, these results indicate that Poldip2/NOX4-mediated oxidation of Cys272 and/or Cys374 on F-actin may not just regulate F-actin-vinculin interaction during cellular adhesion leading to formation and strengthening of FAs, but also during cell migration. Because Poldip2/NOX4-mediated ROS signaling regulates both physiological cell migration in wound healing and angiogenesis72, 73 as well as pathological migration in neointimal proliferation and atherosclerosis,74 further work is warranted to fully elucidate the contribution of actin oxidation to these processes.
Supplementary Material
Highlights:
ROS can regulate cellular adhesion via direct oxidation of F-actin.
NOX4-containing vesicles are located along stress fibers, providing the compartmentalization necessary for controlled and specific action of H2O2 on actin.
Actin oxidation on Cys272 and/or Cys374 correlates with an increase in F-actin-vinculin complex assembly during cell adhesion.
These studies uncover a vital role of Poldip2/NOX4 in F actin oxidation and the interaction of F-actin and vinculin that is indispensable in cytoskeleton organization and cell spreading during cell adhesion.
Acknowledgements:
a) Acknowledgement: We thank Dr. Vsevolod Belousov for the pC1-HyPer-3 plasmid. The EM study was supported in part by the Robert P. Apkarian Integrated Electron Microscopy Core (RPAIEMC), which is subsidized by the Emory College of Arts and Sciences and the Emory University School of Medicine and is one of the Emory Integrated Core Facilities. An adenoviral shuttle vector for Poldip2 was prepared by the Emory Integrated Genomics Core (EIGC), which is subsidized by the Emory University School of Medicine and is one of the Emory Integrated Core Facilities. Additional support was provided by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. We also acknowledge the help of Hong Yi and Jeannette Taylor in the RPAIEMC.
b) Sources of funding
This work was supported by National Institutes of Health grants HL38206 and HL095070, as well as the IMAT program at NCI (R33 CA177461).
Abbreviations
- RGDS
Arg-Gly-Asp-Ser
- RGES
Arg-Gly-Glu-Ser
- Cys
Cysteine
- ECM
Extracellular matrix
- G-actin
Globular actin
- F-actin
Filamentous actin
- FA
Focal adhesion
- HASMs
Human aortic smooth muscle cells
- H2O2
Hydrogen peroxide
- NOX
NADPH oxidase
- RASMs
Rat aortic smooth muscle cells
- ROS
Reactive oxygen species
- TEM
Transmission electron microscopy
- VSMCs
Vascular smooth muscle cells
Footnotes
c) Disclosures
None.
References:
- 1.Dominguez R and Holmes KC. Actin structure and function. Annual review of biophysics. 2011;40:169–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korn ED, Carlier MF and Pantaloni D. Actin polymerization and ATP hydrolysis. Science. 1987;238:638–44. [DOI] [PubMed] [Google Scholar]
- 3.Helgeson LA, Prendergast JG, Wagner AR, Rodnick-Smith M and Nolen BJ. Interactions with actin monomers, actin filaments, and Arp2/3 complex define the roles of WASP family proteins and cortactin in coordinately regulating branched actin networks. J Biol Chem. 2014;289:28856–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciobanasu C, Faivre B and Le Clainche C. Integrating actin dynamics, mechanotransduction and integrin activation: the multiple functions of actin binding proteins in focal adhesions. Eur J Cell Biol. 2013;92:339–48. [DOI] [PubMed] [Google Scholar]
- 5.Carisey A and Ballestrem C. Vinculin, an adapter protein in control of cell adhesion signalling. Eur J Cell Biol. 2011;90:157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wehrle-Haller B Structure and function of focal adhesions. Curr Opin Cell Biol. 2012;24:116–24. [DOI] [PubMed] [Google Scholar]
- 7.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–22. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez-San Juan GR, Oakes PW and Gardel ML. Contact guidance requires spatial control of leading-edge protrusion. Mol Biol Cell. 2017;28:1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giancotti FG and Ruoslahti E. Integrin signaling. Science. 1999;285:1028–32. [DOI] [PubMed] [Google Scholar]
- 10.Huttenlocher A and Horwitz AR. Integrins in cell migration. Cold Spring Harb Perspect Biol. 2011;3:a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaidel-Bar R, Ballestrem C, Kam Z and Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116:4605–13. [DOI] [PubMed] [Google Scholar]
- 12.Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ and Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol. 2007;179:1043–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roca-Cusachs P, del Rio A, Puklin-Faucher E, Gauthier NC, Biais N and Sheetz MP. Integrin-dependent force transmission to the extracellular matrix by alpha-actinin triggers adhesion maturation. Proc Natl Acad Sci U S A. 2013;110:E1361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowley E and Horwitz AF. Tyrosine phosphorylation and cytoskeletal tension regulate the release of fibroblast adhesions. J Cell Biol. 1995;131:525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. [DOI] [PubMed] [Google Scholar]
- 16.Ohmori T, Kashiwakura Y, Ishiwata A, Madoiwa S, Mimuro J, Honda S, Miyata T and Sakata Y. Vinculin activates inside-out signaling of integrin alphaIIbbeta3 in Chinese hamster ovary cells. Biochem Biophys Res Commun. 2010;400:323–8. [DOI] [PubMed] [Google Scholar]
- 17.Kumar CC. Signaling by integrin receptors. Oncogene. 1998;17:1365–73. [DOI] [PubMed] [Google Scholar]
- 18.Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T and Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003;161:933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiarugi P Reactive oxygen species as mediators of cell adhesion. Ital J Biochem. 2003;52:28–32. [PubMed] [Google Scholar]
- 20.Dib K, Melander F, Axelsson L, Dagher MC, Aspenstrom P and Andersson T. Down-regulation of Rac activity during beta 2 integrin-mediated adhesion of human neutrophils. J Biol Chem. 2003;278:24181–8. [DOI] [PubMed] [Google Scholar]
- 21.Fiaschi T, Cozzi G, Raugei G, Formigli L, Ramponi G and Chiarugi P. Redox regulation of beta-actin during integrin-mediated cell adhesion. J Biol Chem. 2006;281:22983–91. [DOI] [PubMed] [Google Scholar]
- 22.Giannoni E, Buricchi F, Raugei G, Ramponi G and Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. 2005;25:6391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taddei ML, Parri M, Mello T, Catalano A, Levine AD, Raugei G, Ramponi G and Chiarugi P. Integrin-mediated cell adhesion and spreading engage different sources of reactive oxygen species. Antioxid Redox Signal. 2007;9:469–81. [DOI] [PubMed] [Google Scholar]
- 24.Zeller KS, Riaz A, Sarve H, Li J, Tengholm A and Johansson S. The role of mechanical force and ROS in integrin-dependent signals. PLoS One. 2013;8:e64897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heyworth PG, Robinson JM, Ding J, Ellis BA and Badwey JA. Cofilin undergoes rapid dephosphorylation in stimulated neutrophils and translocates to ruffled membranes enriched in products of the NADPH oxidase complex. Evidence for a novel cycle of phosphorylation and dephosphorylation. Histochem Cell Biol. 1997;108:221–33. [DOI] [PubMed] [Google Scholar]
- 26.Datla SR, McGrail DJ, Vukelic S, Huff LP, Lyle AN, Pounkova L, Lee M, Seidel-Rogol B, Khalil MK, Hilenski LL, Terada LS, Dawson MR, Lassegue B and Griendling KK. Poldip2 controls vascular smooth muscle cell migration by regulating focal adhesion turnover and force polarization. Am J Physiol Heart Circ Physiol. 2014;307:H945–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen K, Craige SE and Keaney JF, Jr. Downstream targets and intracellular compartmentalization in Nox signaling. Antioxid Redox Signal. 2009;11:2467–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandes RP, Weissmann N and Schroder K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic Biol Med. 2014;76:208–26. [DOI] [PubMed] [Google Scholar]
- 29.Griendling KK, Sorescu D and Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. [DOI] [PubMed] [Google Scholar]
- 30.Lassegue B and Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B and Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Q, Huff LP, Fujii M and Griendling KK. Redox regulation of the actin cytoskeleton and its role in the vascular system. Free Radic Biol Med. 2017;109:84–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heo J and Campbell SL. Mechanism of redox-mediated guanine nucleotide exchange on redox-active Rho GTPases. J Biol Chem. 2005;280:31003–10. [DOI] [PubMed] [Google Scholar]
- 34.Lassing I, Schmitzberger F, Bjornstedt M, Holmgren A, Nordlund P, Schutt CE and Lindberg U. Molecular and structural basis for redox regulation of beta-actin. J Mol Biol. 2007;370:331–48. [DOI] [PubMed] [Google Scholar]
- 35.Cameron JM, Gabrielsen M, Chim YH, Munro J, McGhee EJ, Sumpton D, Eaton P, Anderson KI, Yin H and Olson MF. Polarized cell motility induces hydrogen peroxide to inhibit cofilin via cysteine oxidation. Curr Biol. 2015;25:1520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forman HJ, Fukuto JM and Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol. 2004;287:C246–56. [DOI] [PubMed] [Google Scholar]
- 37.Waszczak C, Akter S, Jacques S, Huang J, Messens J and Van Breusegem F. Oxidative post-translational modifications of cysteine residues in plant signal transduction. J Exp Bot. 2015;66:2923–34. [DOI] [PubMed] [Google Scholar]
- 38.Terman JR and Kashina A. Post-translational modification and regulation of actin. Curr Opin Cell Biol. 2013;25:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DalleDonne I, Milzani A and Colombo R. H2O2-treated actin: assembly and polymer interactions with cross-linking proteins. Biophys J. 1995;69:2710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lo Conte M and Carroll KS. The redox biochemistry of protein sulfenylation and sulfinylation. J Biol Chem. 2013;288:26480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moldovan L, Moldovan NI, Sohn RH, Parikh SA and Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res. 2000;86:549–57. [DOI] [PubMed] [Google Scholar]
- 42.Pan Q, Qiu WY, Huo YN, Yao YF and Lou MF. Low levels of hydrogen peroxide stimulate corneal epithelial cell adhesion, migration, and wound healing. Invest Ophthalmol Vis Sci. 2011;52:1723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wani R, Bharathi NS, Field J, Tsang AW and Furdui CM. Oxidation of Akt2 kinase promotes cell migration and regulates G1-S transition in the cell cycle. Cell Cycle. 2011;10:3263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sobierajska K, Skurzynski S, Stasiak M, Kryczka J, Cierniewski CS and Swiatkowska M. Protein disulfide isomerase directly interacts with beta-actin Cys374 and regulates cytoskeleton reorganization. J Biol Chem. 2014;289:5758–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T and Schwartz MA. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinhard M, Rudiger M, Jockusch BM and Walter U. VASP interaction with vinculin: a recurring theme of interactions with proline-rich motifs. FEBS Lett. 1996;399:103–7. [DOI] [PubMed] [Google Scholar]
- 47.Wen KK, Rubenstein PA and DeMali KA. Vinculin nucleates actin polymerization and modifies actin filament structure. J Biol Chem. 2009;284:30463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griendling KK, Taubman MB, Akers M, Mendlowitz M and Alexander RW. Characterization of phosphatidylinositol-specific phospholipase C from cultured vascular smooth muscle cells. J Biol Chem. 1991;266:15498–504. [PubMed] [Google Scholar]
- 49.Bilan DS, Pase L, Joosen L, Gorokhovatsky AY, Ermakova YG, Gadella TW, Grabher C, Schultz C, Lukyanov S and Belousov VV. HyPer-3: a genetically encoded H(2)O(2) probe with improved performance for ratiometric and fluorescence lifetime imaging. ACS Chem Biol. 2013;8:535–42. [DOI] [PubMed] [Google Scholar]
- 50.Dikalov S, Griendling KK and Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG and Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klomsiri C, Nelson KJ, Bechtold E, Soito L, Johnson LC, Lowther WT, Ryu SE, King SB, Furdui CM and Poole LB. Use of dimedone-based chemical probes for sulfenic acid detection evaluation of conditions affecting probe incorporation into redox-sensitive proteins. Methods Enzymol. 2010;473:77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nelson KJ, Klomsiri C, Codreanu SG, Soito L, Liebler DC, Rogers LC, Daniel LW and Poole LB. Use of dimedone-based chemical probes for sulfenic acid detection methods to visualize and identify labeled proteins. Methods Enzymol. 2010;473:95–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD and Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–83. [DOI] [PubMed] [Google Scholar]
- 55.Simionescu N and Simionescu M. Galloylglucoses of low molecular weight as mordant in electron microscopy. I. Procedure, and evidence for mordanting effect. Journal of Cell Biology. 1976;70:608–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner RC. The effect of tannic acid on electron images of capillary endothelial cell membranes. Journal of Ultrastructure Research. 1976;57:132. [DOI] [PubMed] [Google Scholar]
- 57.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B and Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sutliff RL, Hilenski LL, Amanso AM, Parastatidis I, Dikalova AE, Hansen L, Datla SR, Long JS, El-Ali AM, Joseph G, Gleason RL Jr, Taylor WR, Hart CM, Griendling KK and Lassegue B. Polymerase delta interacting protein 2 sustains vascular structure and function. Arterioscler Thromb Vasc Biol. 2013;33:2154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malik-Sheriff RS, Imtiaz S, Grecco HE and Zamir E. Diverse patterns of molecular changes in the mechano-responsiveness of focal adhesions. Sci Rep. 2018;8:2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith MA, Blankman E, Deakin NO, Hoffman LM, Jensen CC, Turner CE and Beckerle MC. LIM domains target actin regulators paxillin and zyxin to sites of stress fiber strain. PLoS One. 2013;8:e69378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bilan DS and Belousov VV. In Vivo Imaging of Hydrogen Peroxide with HyPer Probes. Antioxid Redox Signal. 2018;29:569–584. [DOI] [PubMed] [Google Scholar]
- 62.Furdui CM and Poole LB. Chemical approaches to detect and analyze protein sulfenic acids. Mass Spectrom Rev. 2014;33:126–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poole LB and Nelson KJ. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr Opin Chem Biol. 2008;12:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keyes JD, Parsonage D, Yammani RD, Rogers LC, Kesty C, Furdui CM, Nelson KJ and Poole LB. Endogenous, regulatory cysteine sulfenylation of ERK kinases in response to proliferative signals. Free Radic Biol Med. 2017;112:534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Golji J and Mofrad MR. The interaction of vinculin with actin. PLoS Comput Biol. 2013;9:e1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Boja ES, Tan W, Tekle E, Fales HM, English S, Mieyal JJ and Chock PB. Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem. 2001;276:47763–6. [DOI] [PubMed] [Google Scholar]
- 67.Johansson M and Lundberg M. Glutathionylation of beta-actin via a cysteinyl sulfenic acid intermediary. BMC Biochem. 2007;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bakolitsa C, Cohen DM, Bankston LA, Bobkov AA, Cadwell GW, Jennings L, Critchley DR, Craig SW and Liddington RC. Structural basis for vinculin activation at sites of cell adhesion. Nature. 2004;430:583–6. [DOI] [PubMed] [Google Scholar]
- 69.Gilmore AP and Burridge K. Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4–5-bisphosphate. Nature. 1996;381:531–5. [DOI] [PubMed] [Google Scholar]
- 70.Janssen ME, Kim E, Liu H, Fujimoto LM, Bobkov A, Volkmann N and Hanein D. Three-dimensional structure of vinculin bound to actin filaments. Mol Cell. 2006;21:271–81. [DOI] [PubMed] [Google Scholar]
- 71.Huttelmaier S, Bubeck P, Rudiger M and Jockusch BM. Characterization of two F-actin-binding and oligomerization sites in the cell-contact protein vinculin. Eur J Biochem. 1997;247:1136–42. [DOI] [PubMed] [Google Scholar]
- 72.Amanso AM, Lassegue B, Joseph G, Landazuri N, Long JS, Weiss D, Taylor WR and Griendling KK. Polymerase delta-interacting protein 2 promotes postischemic neovascularization of the mouse hindlimb. Arterioscler Thromb Vasc Biol. 2014;34:1548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K and Keaney JF Jr. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu S, Chamseddine AH, Carrell S and Miller FJ Jr., Nox4 NADPH oxidase contributes to smooth muscle cell phenotypes associated with unstable atherosclerotic plaques. Redox Biol. 2014;2:642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


