Abstract
Viral infections in patients with post-kidney transplantation are often difficult to diagnose as well as treat. We herein report three cases with severe viral infections after kidney transplantation. All their causative pathogens could be detected promptly by polymerase chain reaction and flow cytometry during the early stages of infection. These examinations would also be of great use to monitor therapeutic responses and disease activity. It is indeed true that no specific treatment is available for most of the viral infections, but we should be aware that some infections, such as Epstein-Barr virus infection, can be treatable with prompt and specific treatment, such as rituximab.
Keywords: Viral infection, Kidney transplantation, Flow cytometry, Polymerase chain reaction, Post-transplantation lymphoproliferative disease, Rituximab
Introduction
The number of solid organ recipients has recently increased. Not only mortality but also graft survival rate of post-transplant patients has remarkably improved with newly developed immunosuppressive agents, such as mycophenolate mofetil (MMF), and molecule-targeting agents, such as basiliximab and rituximab (RTX) [1–4]. However, owing to more sufficient immunosuppression, the bigger risk for infection after organ transplantation still cannot be reduced enough, even until now, which could affect both mortality and graft survival. Appropriate and prompt treatment are needed for infections; however, such treatment is not always easily implemented due to difficulties detecting potential pathogens and focuses, especially when patients are infected with viruses. Here we report three cases with severe viral infection after kidney transplantation. Suitable and rapid treatment could be performed with successful detection of their focuses and pathogens by polymerase chain reaction (PCR) and flow cytometry during the early stages of the infections, which resulted in complete recovery with no subsequent complications.
Case Reports
Case 1
A 5-year-old girl, who was originally diagnosed with autosomal recessive polycystic kidney disease (ARPKD), was admitted to our hospital due to fever and diarrhea for 3 days (Fig. 1a). Up to 17 months had passed since kidney transplantation was performed with her father as the donor. Methylprednisolone (mPSL), MMF, and tacrolimus were needed for immunosuppression after transplantation. Blood examination of case 1 is shown in Table 1. No pathogen could be detected in both blood and stool culture. Anti-viral capsid antigen (VCA)-IgG of Epstein-Barr virus (EBV) was positive, but both EBV nuclear antigen and VCA-IgM of EBV were negative. Cytomegalovirus (CMV) antigen was also negative. Leukopenia and thrombocytopenia were shown on her admission, along with raised serum ferritin and interleukin-2 receptor. We suspected secondary hemophagocytic lymphohistiocytosis (HLH) and in order to exclude leukemia, we decided to conduct bone marrow examination. Hemophagocytosis could be found in her bone marrow aspiration (Fig. 2) and there were no abnormal cells. Finally, she was diagnosed with secondary HLH [5]. MMF was discontinued and dexamethasone was started instead of mPSL. Her fever subsided with these treatments and laboratory data also gradually improved (Fig. 1a). Adenovirus (ADV) was detected by PCR analysis with her stool and blood samples, which finally led us to diagnosing her with ADV-associated HLH. The rapid diagnostic test for ADV had been negative on her admission. ADV antibody titer in her blood serum turned positive 4 months after the aforementioned episode. She did not experienced graft dysfunction.
Fig. 1.
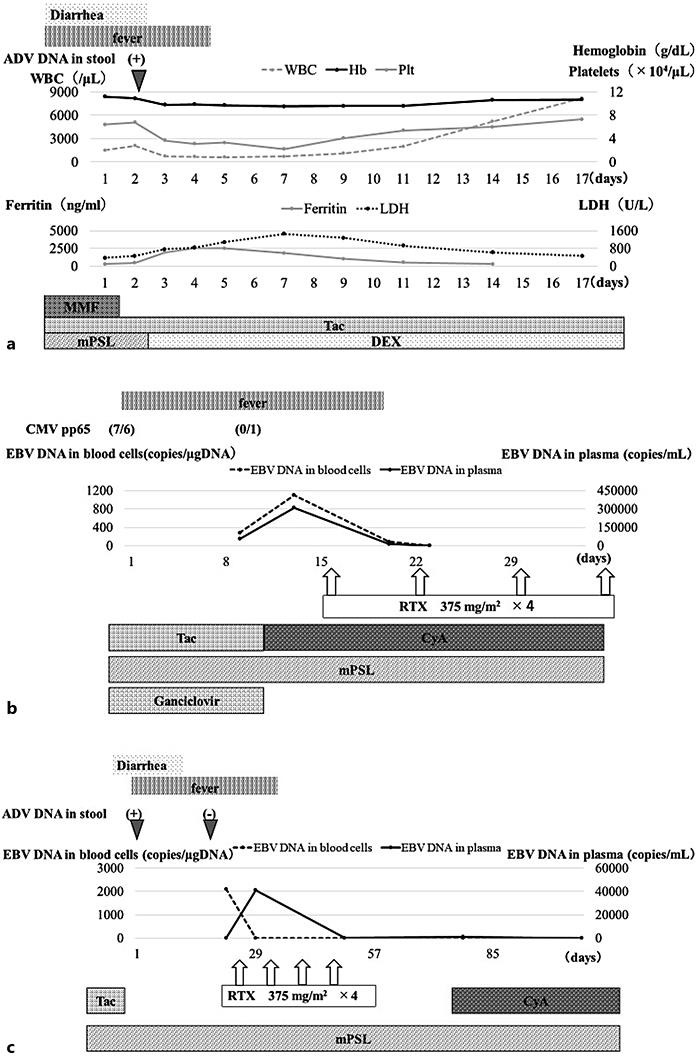
Time courses of three cases. a Case 1. Day 1 is the first day she was admitted. b Case 2. Day 1 is the first day he was admitted. c Case 3. Day 1 is the first day her fever occurred. ADV, adenovirus; CyA, cyclosporine; DEX, dexamethasone; MMF, mycophenolate mofetil; mPSL, methylprednisolone; RTX, rituximab; Tac, tacrolimus.
Table 1.
Blood examinations of the three cases
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Hematology | |||
| WBC, n/µL | 730 | 5,100 | 6,700 |
| Hemoglobin, g/dL | 9.8 | 9.9 | 5.8 |
| Platelets, × 104/µL | 3.7 | 41 | 11.8 |
| Serology | |||
| Adenovirus antibody | – | ||
| EBV-VCA-IgG | + | + | + |
| EBV-VCA-IgM | – | – | – |
| EBNA | – | – | – |
| CMV-pp65 | – | + | – |
| Biochemistry | |||
| Alb, g/dL | 2.9 | 2.5 | 4.5 |
| BUN, mg/dL | 17.1 | 24 | 13 |
| Creatinine, mg/dL | 0.59 | 0.6 | 0.53 |
| Sodium, mmol/L | 136 | 136 | 137 |
| Potassium, mmol/L | 4.6 | 4.7 | 3.8 |
| Chloride, mmol/L | 109 | 107 | 113 |
| AST, IU/L | 230 | 24 | 18 |
| ALT, IU/L | 67 | 8 | 12 |
| LDH, IU/L | 756 | 477 | 232 |
| CRP, mg/dL | 1.13 | 4.44 | <0.1 |
| Ferritin, ng/mL | 1,923 | ||
| TG, mg/dL | 171 | ||
WBC, white blood cells; EBV, Epstein-Barr virus; CMV, cytomegalovirus; TG, triglycerides.
Fig. 2.
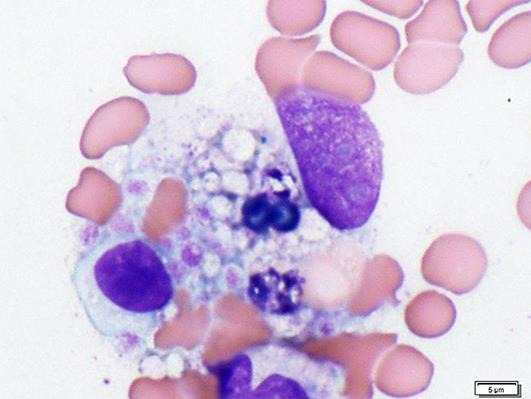
Bone marrow from case 1. Hemophagocytosis by macrophage was indicated.
Case 2
An 8-year-old boy with post-kidney transplantation was admitted to our hospital due to fever for 6 days. He was originally diagnosed with Townes-Brocks syndrome and 4 months had passed since kidney transplantation had been performed from a deceased donor. He had been treated with mPSL, tacrolimus, and MMF for immunosuppression after transplantation. All laboratory data were almost normal except for CMV-pp65 antigen (Table 1). As soon as ganciclovir administration was started and MMF was discontinued, CMV-pp65 antigen turned negative. However, his fever continued and his general condition worsened (Fig. 1b). No pathogen could be detected by blood culturing. PCR analysis with his blood showed that he was positive for EBV but abnormal masses were not evident with chest and adnominal computed tomography. We immediately reduced the dose of tacrolimus and administered intravenous immunoglobulin, but the intervention was not enough for him to show improvement. Additional analyses with flow cytometry and quantitative PCR showed that EBV infected CD19+ cells, but not the other cells, thus indicating CD4+ or CD8+ of T cells, CD56+ of NK cells, and CD14+ of mononuclear cells. He was treated with RTX and was finally confirmed afebrile as the number of EBV copies of peripheral blood cells became negative (Fig. 1b). There were no bacterial infections until his CD20 cells recovered after RTX use, and he did not experienced graft dysfunction.
Case 3
A 7-year-old girl, who was originally diagnosed with ARPKD, had fever and diarrhea with massive ascites 9 weeks after kidney transplantation from her mother. She had been treated in a ward with mPSL, MMF, and tacrolimus for immunosuppression after transplantation. Laboratory data showed the same level of anemia and mild liver dysfunction as those before the episode (Table 1). CMV-pp65 antigen was negative. No pathogen could be detected in both blood and stool culture. PCR analysis with blood, urine, and stool showed that she was positive for ADV. Discontinuing MMF and tacrolimus, diarrhea improved and ADV turned negative by PCR analysis with stool (Fig. 1c). However, her fever continued and her general appearance gradually worsened. PCR analysis with her blood cells and ascites showed that she was positive for EBV but abnormal masses were not evident with chest and adnominal computed tomography. Additional analyses with flow cytometry and quantitative PCR showed that EBV infected CD19+ cells, but not other cells, thus indicating CD4+ or CD8+ of T cells, CD56+ of NK cells, and CD14+ of mononuclear cells. RTX was finally administered and her fever and other symptoms were gradually resolved. The copy number of EBV-DNA in her peripheral blood cells turned negative 5 months after RTX. There were no bacterial infections until her CD20 cells recovered after RTX use, and she did not experience graft dysfunction.
Discussion
All cases are summarized in Table 2. Identification of potential pathogens is often difficult in patients with post-kidney transplantation who are possibly infected; as our cases suggest, patients’ signs and symptoms are usually nonspecific and the spectrum of potential pathogens is broad. Rapid and comprehensive microbiologic assays, especially for viruses, are needed for diagnosis, but no tools have been provided so far in clinical settings to satisfy all aspects, including its sensitivity, versatility, and cost [6, 7]. Our case 1 suggests that PCR analyses of a virus to detect potential pathogens would be superior to a rapid antigen test and IgG antibody titer in serum in order to ensure early and effective diagnosis and treatment. We performed PCR analysis of the viruses for all cases to detect pathogens. By PCR analysis, we checked ADV, EBV, CMV, BK virus, JC virus, rhinovirus, enterovirus, human metapneumovirus, norovirus, human herpesvirus (HHV)-6, HHV-7, and parvovirus B19 comprehensively. A previous report has also shown the efficacy of multiplex PCR for patients with post-kidney transplantation who had severe diarrhea to detect pathogenic bacteria [7]. PCR analysis can be a powerful tool to detect causative virus as well as bacteria in the early clinical course of patients with post-kidney transplantation.
Table 2.
Clinical courses of the three cases
| Case No. | Age, years | Disease | Time after KT, months | Antibodies before this episode | Immunosuppression drugs | Chief complaints | Pathogens | Diagnosis | Therapy | Observation period, years | Final eGFR, mL/min/1.73 m2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | ARPKD | 17 | EBV IgG: ND | CMV IgG: ND | mPSL MMF Tac | fever diarrhea | adenovirus | HLH | DEX | 1.5 | 90.4 |
| 2 | 8 | Towns-Brocks syndrome | 4 | EBV IgG: ND | CMV IgG: ND | mPSL MMF Tac | fever diarrhea | EBV | PTLD | RTX | 7.4 | 77.7 |
| 3 | 7 | ARPKD | 03 | EBV IgG: ND | CMV IgG: ND | mPSL MMF Tac | fever diarrhea | EBV | PTLD | RTX | 4.2 | 123 |
ARPKD, autosomal recessive polycystic kidney disease; DEX, dexamethasone; eGFR, estimated glomerular filtration rate; HLH, hemophagocytic lymphohistiocytosis; KT, kidney transplantation; MMF, mycophenolate mofetil; Tac, tacrolimus; mPSL, methylprednisolone; ND, not detected; PTLD, post-transplantation lymphoproliferative disease; RTX, rituximab.
It is worthy to note that the original disease in two cases was ARPKD. ARPKD is one of the most common congenital ciliopathies associated with not only kidney but also liver dysfunction. ARPKD patients were reported to show secondary portal hypertension at the median age of 2.8 years [8]. Hypersplenism can be caused by portal hypertension, which has been demonstrated to be capable of bringing about weakened immune systems as well as leukocytopenia itself [9]. Indeed, case 3 had mild leukocytopenia, anemia, and thrombocytopenia before transplantation, which were thought to be related to portal hypertension. Combined with the concept of the net state of immunosuppression [10], severe infections in these two cases might have been due to the condition of their liver. Besides this, severe infections themselves could result in hepatic decompensation with increasing speed in ARPKD patients, who are awaiting liver transplantation [11]. Both of our cases finally needed liver transplantation as early as 18 and 6 months after the aforementioned episodes. Special considerations need to be taken for infections when kidney transplantation is performed in patients with liver dysfunction.
Two out of three cases suffered from post-transplantation lymphoproliferative disease (PTLD) infected by EBV; both cases were categorized with early lesions of PTLD. Our cases with early lesion suggest that treatment only with the reduction of immunosuppression may fail [12, 13]. RTX has been reported to be a powerful option for treatment [13]. However, strict confirmation would be necessary before RTX administration so that cells other than B cells are not infected with EBV by flow cytometry and quantitative PCR.
Quantitative PCR to analyze EBV-DNA is of use for the diagnosis of PTLD [14, 15], but there still remain some points to be fixed in terms of universality; cutoff value and type of specimen (whole blood, plasma, or blood cells), for instance. It is necessary to analyze DNA copies of EBV with both blood cells separated from whole blood and plasma because of the divergence among these two values, as our case 3 suggested. Quantitative PCR and flow cytometry would also be useful for monitoring therapeutic responses and disease activity.
In summary, PCR could become a powerful tool for detecting viral infection in patients with post-kidney transplantation. Flow cytometry as well as quantitative PCR would also be effective for patients with EBV infection, such as PTLD, so that suitable and specific treatment could be performed by the identification of cells infected with EBV. These examinations would also enable us to monitor therapeutic responses and disease activity. We clinicians should be aware of how some viral infections can be treatable with prompt and specific treatment, by applying the above great tools to detect causative pathogens as early as possible.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
Dr. Iijima reports grants from Novartis Pharma K.K., grants from Japan Blood Product Organization, grants from AbbVie LLC, grants from JCR Pharmaceuticals Co., Ltd., grants from Daiichi Sankyo, Co., Ltd., grants from Teijin Pharma Ltd., grants from CSL Behring, grants from Novo Nordisk Pharma Ltd., grants from Air Water Medical Inc., grants from Astellas Pharma Inc., grants from Takeda Pharmaceutical Co., Ltd., grants from Taisho Toyama Pharmaceutical Co., Ltd., grants from Eisai Co. Ltd., grants from Biofermin Pharmaceutical Co., Ltd., from Zenyaku Kogyo Co., personal fees from Zenyaku Kogyo Co., Ltd., personal fees from Novartis Pharma K.K., personal fees from Chugai Pharmaceutical Co., Ltd., personal fees from Astellas Pharma Inc., personal fees from Springer Japan KK, personal fees from Meiji Seika Pharma Co., Ltd., personal fees from Asahi kasei Pharma Corporation, personal fees from Medical Review Co., Ltd, personal fees from Nippon Boehringer Ingelheim Co., Ltd., personal fees from Baxter Limited, personal fees from Ono Pharmaceutical Co., Ltd., personal fees from Sanwa Kagaku Kenkyusho Co., Ltd., personal fees from Sanofi K.K., personal fees from Alexion Pharma LLC., personal fees from Kyowa Hakko Kirin Co., Ltd., outside the submitted work.
References
- 1.Jensik SC. Tacrolimus (FK 506) in kidney transplantation: three-year survival results of the US multicenter, randomized, comparative trial. FK 506 Kidney Transplant Study Group. Transplant Proc. 1998 Jun;30((4)):1216–8. doi: 10.1016/s0041-1345(98)00216-4. [DOI] [PubMed] [Google Scholar]
- 2.Sollinger HW, Deierhoi MH, Belzer FO, Diethelm AG, Kauffman RS. RS-61443—a phase I clinical trial and pilot rescue study. Transplantation. 1992 Feb;53((2)):428–32. doi: 10.1097/00007890-199202010-00031. [DOI] [PubMed] [Google Scholar]
- 3.Nashan B, Moore R, Amlot P, Schmidt AG, Abeywickrama K, Soulillou JP. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. CHIB 201 International Study Group. Lancet. 1997 Oct;350((9086)):1193–8. doi: 10.1016/s0140-6736(97)09278-7. [DOI] [PubMed] [Google Scholar]
- 4.Tydén G, Kumlien G, Genberg H, Sandberg J, Lundgren T, Fehrman I. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005 Jan;5((1)):145–8. doi: 10.1111/j.1600-6143.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 5.Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007 Feb;48((2)):124–31. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 6.Smith JM, Dharnidharka VR. Viral surveillance and subclinical viral infection in pediatric kidney transplantation. Pediatr Nephrol. 2015 May;30((5)):741–8. doi: 10.1007/s00467-014-2866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coste JF, Vuiblet V, Moustapha B, Bouin A, Lavaud S, Toupance O, et al. Microbiological diagnosis of severe diarrhea in kidney transplant recipients by use of multiplex PCR assays. J Clin Microbiol. 2013 Jun;51((6)):1841–9. doi: 10.1128/JCM.03366-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guay-Woodford LM, Desmond RA. Autosomal recessive polycystic kidney disease: the clinical experience in North America. Pediatrics. 2003 May;111((5 Pt 1)):1072–80. doi: 10.1542/peds.111.5.1072. [DOI] [PubMed] [Google Scholar]
- 9.Nomura Y, Kage M, Ogata T, Kondou R, Kinoshita H, Ohshima K, et al. Influence of splenectomy in patients with liver cirrhosis and hypersplenism. Hepatol Res. 2014 Oct;44((10)):E100–9. doi: 10.1111/hepr.12234. [DOI] [PubMed] [Google Scholar]
- 10.Fishman JA, AST Infectious Diseases Community of Practice Introduction: infection in solid organ transplant recipients. Am J Transplant. 2009 Dec;9(Suppl 4):S3–6. doi: 10.1111/j.1600-6143.2009.02887.x. [DOI] [PubMed] [Google Scholar]
- 11.Duchini A, Viernes ME, Nyberg LM, Hendry RM, Pockros PJ. Hepatic decompensation in patients with cirrhosis during infection with influenza A. Arch Intern Med. 2000 Jan;160((1)):113–5. doi: 10.1001/archinte.160.1.113. [DOI] [PubMed] [Google Scholar]
- 12.González-Barca E, Domingo-Domenech E, Capote FJ, Gómez-Codina J, Salar A, Bailen A, et al. GEL/TAMO (Grupo Español de Linfomas); GELCAB (Grupo para el Estudio de los Linfomas Catalano-Balear); GOTEL (Grupo Oncológico para el Tratamiento y Estudio de los Linfomas) Prospective phase II trial of extended treatment with rituximab in patients with B-cell post-transplant lymphoproliferative disease. Haematologica. 2007 Nov;92((11)):1489–94. doi: 10.3324/haematol.11360. [DOI] [PubMed] [Google Scholar]
- 13.Heslop HE. How I treat EBV lymphoproliferation. Blood. 2009 Nov;114((19)):4002–8. doi: 10.1182/blood-2009-07-143545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiwara S, Kimura H, Imadome K, Arai A, Kodama E, Morio T, et al. Current research on chronic active Epstein-Barr virus infection in Japan. Pediatr Int. 2014 Apr;56((2)):159–66. doi: 10.1111/ped.12314. [DOI] [PubMed] [Google Scholar]
- 15.Imadome K, Fukuda A, Kawano F, Imai Y, Ichikawa S, Mochizuki M, et al. Effective control of Epstein-Barr virus infection following pediatric liver transplantation by monitoring of viral DNA load and lymphocyte surface markers. Pediatr Transplant. 2012 Nov;16((7)):748–57. doi: 10.1111/j.1399-3046.2012.01750.x. [DOI] [PubMed] [Google Scholar]


