Abstract
Stress exacerbates disease, and understanding its molecular mechanisms is crucial to the development of novel therapeutic interventions to combat stress-related disorders. The driver of the stress response in the hypothalamic-pituitary-adrenal axis (HPA) is corticotropin-releasing hormone (CRH), a neuropeptide synthesized in the paraventricular nucleus of the hypothalamus. Evidence supports that CRH expression is epigenetically modified at the molecular level by environmental stimuli, causing changes in the stress response. This effect is mediated by a concert of factors that translate environmental change into alterations in gene expression. An important regulator and epigenetic modulator of CRH expression is neuron-restrictive silencing factor (NRSF). Previously, our lab identified numerous splice variants of NRSF that are specific to humans and predictive of differential regulatory effects of NRSF variants on targeted gene expression. The human cell line BeWo has endogenous CRH and NRSF expression providing an in vitro model system. Here, we show that manipulation of NRSF expression through siRNA technology, overexpression by plasmid vectors, and direct cAMP induction that CRH expression is linked to changes in NRSF expression. Accordingly, this epigenetic regulatory pathway in humans might be a critical mechanism involved in the regulation of the stress response.
Keywords: Corticotropin-releasing hormone, Neuron-restrictive silencing factor, Stress regulation
Introduction
Physiological stress is the primary mediator of homeostatic regulation when an organism is presented with environmental adaptation challenges [1]. Effective integration of stressor stimuli in the central nervous system (CNS), neurotransmission to relevant brain centers, and neurohormonal communication to the periphery confer an adaptive response [1]. Ineffective integration and signal propagation to reestablish equilibrium in the CNS and the periphery has been a hallmark of pathophysiology and illness exacerbation. Understanding the underlying molecular mechanisms that integrate stressful signals into the CNS to adaptive or maladaptive responses is essential to understanding an organism's potential towards challenges and the development of therapeutic avenues to reduce the pathophysiological effects of stress. Due to the temporal abundance of stressors in an organism's development, it is evident that mechanisms that are central to the regulation of integration, signaling, and adaptive potential are mediated through epigenetic alterations in genes that contribute to this process [2].
Central to neurotransmission of stressful stimuli within the CNS and the neurohormonal response to the periphery is the corticotropin-releasing hormone (CRH) neuropeptide [3, 4]. The CRH gene resides in the 8th chromosome of humans and is composed of one intron and two exons flanking a region of 2,343 base pairs. The gene codes for a 196-amino acid propeptide that is enzymatically processed to an active neuropeptide of 41 amino acids. CRH mediates its function through two receptors, CRH-R1 and CRH-R2. Depending on the cellular context, CRH activates CRH-R1 and CRH-R2 that subsequently increase cAMP, ERK1/2, NF-kB, and NO/cGMP [5]. CRH receptors are located in the CNS and peripheral organs with highest expression being present in the pituitary in humans [6]. In mice, CRH receptors are present throughout the cerebrum in regions of the cortex, limbic system, hypothalamus, and cerebellum [7]. In humans, CRH is expressed in the cerebral cortex, hippocampus, caudate, cerebellum, and hypothalamus [8]. Therefore, through receptor-ligand presence in the brain, CRH can act as a neurotransmitter contributing to alterations in the limbic system, cortical processing, memory, autonomic function and mood-related systems [9, 10, 11, 12]. The neurohormonal activity of CRH is primarily mediated through its transducing function in the HPA axis that translates integrated neural information into cortisol release, thus acting as a bridge through the CNS to the periphery.
The stress response has been shown to mediate epigenetic alterations in stress gene networks, and the CRH gene is a primary target of that modulation [13]. In a chronic social defeat paradigm in rodents, the degree of methylation in the CRH gene was decreased in the paraventricular nucleus (PVN) of the hypothalamus [14]. In a chronic variable mild stress paradigm, the CRH promoter and intron showed differential hypo- and hypermethylation among male and female rats in the PVN [15]. In a maternal deprivation paradigm, rats showed hypomethylation in the CRH gene in the PVN [16]. In an early prenatal stress paradigm, mice showed hypomethylation in the CRH gene in the PVN [17]. Therefore, it follows that epigenetic alterations of the CRH gene modulate the differential expression of CRH, which in turn gives rise to pathological phenotypes.
An epigenetic initiator relating to modulation of CRH is the protein neuron-restrictive silencing factor (NRSF). NRSF, a Kruppel-type zinc finger transcription factor, is in the 4th chromosome in humans and contains 5 exons that confer functional output towards target genes containing a neuron-restrictive silencing element (NRSE). NRSF binds to NRSE regions through 9 zinc finger domains that are present across exons 2, 3, and 4 in its coding DNA sequence. NRSF is driven by 3 different promoters present in exon 1 and named a, b, and c providing different transcription activity and variant association [18]. Its epigenetic regulation is mediated through two restrictive domains, RD1 and RD2, located in exon 2 and exon 4, respectively. The RD1 domain recruits Sin3/HDAC, hBRM, BRG1, and SWI/SNF to differentially change core histone structure and to remodel chromatin. The RD2 recruits the CoREST complex, which includes the factors MeCP2, G9A, and LSD1 to mediate DNA methylation changes, and core histone modification. The exact recruitment mechanisms and functional output of each domain is still unknown. NRSF has been shown to be extensively modulated in terms of variant expression by alternative splicing in humans resulting in at least 45 possible coding variants and noncoding RNA [18]. Due to its inherent complexity of regulation and functionality, NRSF might be able to differentially modulate CRH expression through epigenetic alterations in its splicing and gene expression.
NRSF has been previously shown to modulate CRH expression through a variety of experimental methods. An NRSE domain has been identified in the vertebrate CRH gene in silico, and NRSF has been shown to modulate the CRH gene network [19, 20, 21]. In vitro NRSF has been shown to exert both upregulation and downregulation of the CRH gene, although the mechanisms are still unknown [22]. In a maternal separation stress paradigm, NRSF was shown to be upregulated and more tightly bound to CRH, while CRH expression was lower in the mouse PVN [23]. Moreover, epigenetic factors recruited by NRSF had a temporally mediated activity in the PVN as mice went through development [24, 25]. In a PTSD mouse model, viral delivery of full-length NRSF during chronic and posttrauma stress showed downregulation of CRH in the hypothalamus [26]. In rats, overexpression of the NRSF4 splice variant showed upregulation of CRH along with increased scores in stress-related tasks [27]. In humans with depression, NRSF has been shown to be decreased, while CRH expression was high in plasma [28]. Accordingly, evidence suggests a relationship between NRSF-mediated CRH regulation and stress modulation, although the interaction among NRSF's isoforms and how they might arise through the experience of environmental stressors is unclear in humans.
Here, we investigate for the first time how different elements of the NRSF protein are capable of mediating changes in CRH expression in human cells. We utilize human BeWo cells which express CRH [29]. Knowing the degree of variant complexity of NRSF in humans from previous work in our lab, we utilize siRNAs to downregulate NRSF variants that contain specified domains and investigate downstream effects on CRH expression. We utilize plasmid constructs containing full-length NRSF and the NRSF4 variant to investigate if their overexpression will result in differential regulation of CRH. We then investigate whether activation of cAMP-related pathways will result in changes in expression of NRSF and CRH, using 8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP). Finally, we investigate whether patterns of association between NRSF and CRH indicated by in vitro assays in BeWo also exist in human brain tissue by utilizing expression databases.
Methods
Cell Culture
The BeWo cell line (ATCC®, USA, CCL-98TM) was cultured in Ham's Nutrient Mixture F12 media (HyClone, USA, Cat. SH30026FS) supplemented with 10% fetal bovine serum (Fischer, USA, Cat. S11150) and 1% penicillin-streptomycin (Thermo Fischer, USA, Cat. 15140122). Cells were incubated in a HeracellTM VIOS 160i (Thermo Fischer) at 5% CO2 at 95% humidity and 37°C. Media were replaced every 48 h and when reaching 80%, confluence cells were washed (1×) and then passaged using Trypsin-EDTA (0.05%) containing phenol red (Fischer, Cat. 25300062). Cell were washed with Dulbecco's Phosphate Buffered Saline (Fischer, Cat. D8537).
RNA Isolation
Cells were detached with Trypsin-EDTA and 3 volumes media upon detachment were used for transfer into a 2.0 mL Safe-Lock Tube (Eppendorf, Germany, Cat. 022363352). Cells were pelleted at 500 rcf for 5 min on an Eppendorf 5417R Refrigerated Centrifuge. The liquid phase was collected, and the pellet was washed with PBS. The PBS was aspirated, and the cell pellet was disrupted by flicking the tube. Buffer RLT was used for lysis by vortex using a Vortex-Genie 2 (Sycamore, TX, USA, Cat. SI-0236) at full speed. One volume of 70% ethanol was added into the lysate, prepared fresh with 96% v/v ethanol (Fischer Scientific, Cat. BP8202-1) and Milli-Q® Integral water. After mixing, the solution was placed into an RNeasy Mini Spin Column (Qiagen, Germany, Cat. 74104) and centrifuged at 14,000 rpm for 30 s. The flow through was removed, and a wash was performed with Buffer RW1 (Qiagen, Cat. 1053394) coupled with centrifugation at 14,000 rpm for 30 s. Two subsequent washes were performed with Buffer RPE (Qiagen, Cat. 1018013) coupled with a 14,000 rpm for 30 s spin and a 14,000 rpm for 2 min spin, respectively. The column was then transferred into a new collection tube and centrifuged at 14,000 rpm for 1 min to dry the column. Finally, the column was placed into a new collection tube, RNase-Free Water (Qiagen, Cat. 129112) was added into the column, the column was incubated at room temperature for 5 min, and then centrifuged at 14,000 rpm for 1 min. All centrifugation steps were performed in an Eppendorf® Microcentrifuge 5430R at 25°C. Upon collection, RNA samples were stored in a 20°C freezer.
RNA Quality Control
RNA concentration and purity ratios were measured on a DeNovix DS-11 FX+ Spectrophotometer (Denovix Inc., USA). RNAs were determined viable for downstream applications when their 260/230 ratio was > 1.80 and the 260/280 ratio was > 1.80. In addition, 5 μL of isolated RNA were electrophoresed in a 2% agarose gel that was stained with ethidium bromide and imaged in a Syngene PXi (Syngene Inc., USA) system. The 18S: 16S ratio was assessed to be approximately 2: 1 using OD values calculated with ImageJ software for intact RNA. Any samples deviating from those conditions were cleaned with ethanol precipitation.
cDNA Synthesis
cDNA synthesis was performed with the QuantiTect Reverse Transcription Kit (Qiagen, Cat. 205311). Samples were normalized by always using 1 μg RNA into the synthesis reaction.
PCR
PCR was performed in an MJ Research PTC-200 Thermal Cycler (MJ Research Inc., USA) with GoTaq Green Master Mix (Promega, USA, Cat. M7122). The reaction mixture was controlled for all samples with composition of 1 μL of 10 μM primer, 1 μL of cDNA, 10 μL of Master Mix, and 12 μL of RNase-free water. For NRSF isoform detection, we utilized our previously reported cycling conditions and primers [18]. For gDNA contamination and loading control we utilized an intron-spanning GAPDH assay, also previously described [18].
Gel Electrophoresis
Electrophoresis of 5 μL of the PCR products or RNA was performed in a 2% agarose gel with ethidium bromide stain for 1 h at 100 V.
Quantitative PCR
Quantitative PCR was performed on a LightCycler® 2.0 Instrument (Roche Holding AG, Switzerland, Cat. 03531414001). PCR reactions were performed with LightCycler® FastStart DNA MasterPLUS SYBR Green I (Roche, Cat. 03515885001) in 20 μL LightCycler® glass capillaries (Roche, Cat. 04929292001). For detection of NRSF variants, we utilized the previously described assays [18]. For CRH, we optimized assays by their respective efficiency calculated through a serial dilution standard curve and established agreement with MIQE guidelines. The assay used the forward primer 5′-TCCCATCTCCCTGGATCTCAC-3′ and the reverse primer, 5′-GTGAGCTTGCTGTGCTAACTGCT-3′. The cycling conditions included an initial 15-min denaturation at 95°C followed by 50 cycles at 95°C for 10 s, 60°C annealing for 10 s, and 72°C extension accompanied with a single fluorescence detection step. After cycling, a melting curve ramp was performed by first raising the temperature to 95°C followed by decrease to 65°C over 1 min, and finally an increase to 95°C with a ramp rate of 0.1°C/s, while continuously measuring fluorescence. Finally, reaction mixtures were cooled to 40°C, and then transferred to 1.5 mL Eppendorf tubes for storage or gel electrophoresis analysis. Experimental analysis of differential expression was assessed with the ΔΔCt method, utilizing GAPDH as the housekeeping gene.
siRNA Transfection
27mer siRNA duplexes were ordered from OriGene Technologies Inc. (USA, Cat. SR304036) targeting differential regions of the NRSF transcript. Target specificity was assessed by NCBI Nucleotide Blast and then integration into the Benchling platform for illustration purposes. Transfection of the siRNAs was performed by harvesting cells at 80% confluency, measuring cells/mL with a Moxi Flow Kit (Orflo, USA, Cat. MXF002) and distributing 150,000 cells in 1 mL media volume in 12-well plates. After adhesion at 24 h, media were changed to media without serum or antibiotics, and transfection was performed with siTran 1.0 siRNA transfection reagent (OriGene Technologies, Cat. TT300001). More specifically, dried 2-nmol siRNAs were first solubilized with 100 μL of supplied duplex buffer, heated at 94°C for 2 min and mixed to create a 20-μM stock. The stock was diluted to 5 μM for each experiment, and a final concentration of 10 nM was used for downregulation of NRSF transcript. Transfected cells were incubated for 48 h before harvesting. For complexing of the siRNAs with the transfection reagent, MilliQ water was utilized.
Plasmid Construction
An alternatively spliced mRNA variant (E1a/E2/E3/N3a/E4i, GenBank accession No. JX896971), expressing the N-terminal NRSF4 isoform had been previously cloned into the pcDNA3.1/V5-His-TOPO® vector (Invitrogen, USA, Cat. K480040) [30]. Due to the failure of obtaining the full-length coding region of NRSF by PCR amplification of cDNA samples derived from human tissues and cell lines [30], two PCR-amplified, overlapping fragments of NRSF – E1a/E2/E3/E4-partial (F1, amplified by primers E1aF2 [5′-ccagcacccaactttaccac-3′] and E4R3 [5′-cacataactgcactgatcacattta-3′]) and E2-partial/E3/E4-full (F2, amplified by primers E2F1 [5′-cagtgagcgagtatcactgga-3′] and E4R2 [5′-caaactaagaactgaaaccttgttca-3′]), were first cloned into pcDNA3.1, respectively. The two constructs (F1 and F2) were then subject to double digestion with SwaI and NotI, which uniquely cuts the sequence at proximal E4 and 3′-end of the TA cloning site, respectively. Briefly, following sequential digestion with SwaI and NotI, the long fragment (∼6.6 kb) of digested pcDNA-F1 and the short fragment (∼2.3 kb) of digested pcDNA-F2 were purified and ligated to make the full-length coding region of NRSF. All the constructs were sequence-verified and were confirmed to be in correct orientation.
Plasmid Transfection and Stable Clone Generation
Plasmid NRSF4, NRSF, and pcDNA-empty were diluted into a 0.020 μg/μL concentration in 103 μL MilliQ water. Bewo cells were harvested at 80% confluency and counted with a Moxi Flow System and 320,000 cells were plated into a 12-well plate, in 1 mL media per well without antibiotic. In the plasmid solution, 6.6 μL of FuGENE® HD Transfection Reagent (Promega, Cat. E2311) was added followed by 10–15 pipette mixes and was left to incubate for 10 min at room temperature promoting complexing. Complexed DNA solution was added into the well, mixed and left to incubate for 48 h. Transfection efficiency was assessed with a Zeiss Axio Observer inverted motorized microscope with fluorescence detection (Zeiss, Germany) for turboGFP expression, which served as the plasmid reporter. After 48-h incubation, the cells were harvested and replated into a 6-well plate with 25–30% confluency, with media containing 10% FBS and 200 μg/mL GeneticinTM selective antibiotic (Thermo Fischer, Cat. 10131035). Survival was monitored daily, and selecting media was replaced every 3–4 days for a period of 12 weeks. After clones started appearing as islands, the plate was harvested and plated into a 75 cm2 flask for a final period of 4 days. Then half of the population was isolated and harvested for RNA detection, while the rest was used for imaging and immunohistochemistry. Wells containing cells not utilized for downstream experimentation were cryopreserved in liquid nitrogen.
Compounds and Treatment
Five milligrams of 8-Br-cAMP sodium salt (Sigma, USA, Cat. B7880) was solubilized at a 10 mM concentration in MilliQ water, distributed into 50 μL aliquots, and stored at −20°C. Experiments were performed by harvesting cells at 80% confluency, measuring cells/mL with a Moxi Flow Kit and distributing 150,000 cells in 1-mL media volume in 12-well plates. After 24 h, aliquots of 8-Br-cAMP were used according to the experimental concentration desired ranging from 1 to 500 μM concentrations in 1 mL media. Incubations were performed for 24 and 48 h, and cells were extracted with the method outlined in the RNA isolation section.
GTEx Database Data Mining
GTEx NRSF and CRH RNA-seq data from brain regions of the hippocampus, hypothalamus, cortex, and frontal cortex were imported from the Human Protein Atlas [31]. The RPKM value for each patient within a branch of region and gene were recorded into Microsoft Excel and sorted to have matching RPKMs for CRH and NRSF in that region. Data were transferred into GraphPad Prism of 2017 GraphPad Software, Inc., and plotted either as an X-Y scatter or distribution.
Statistical Analysis
Statistical testing was performed using GraphPad Prism. The data generated from the siRNA experiment were analyzed by one-way ANOVA with the Bonferroni correction for multiple comparisons. The 8-Br-cAMP data were assessed by paired two-tailed t test. The scatters from the GTEx data were tested with linear regression and Pearson correlation, with the null hypothesis being a slope of zero. In all analyses, p values were reported as follows: ns, p > 0.05; * p < 0.05; ** p < 0.01; and *** p < 0.001.
Results
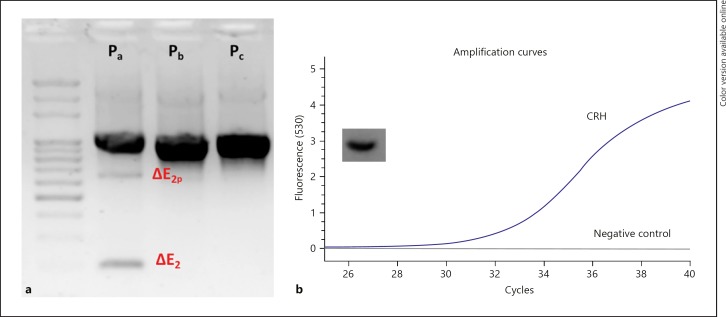
First, we sought to answer the question of whether NRSF and CRH are expressed in BeWo. Previous evidence had suggested that human NRSF can undergo multiple processing to result in a variety of isoforms with predictably different functional characteristics. Moreover, CRH expression has been previously reported to be present in the BeWo cell line [29]. We determined that NRSF is alternatively spliced in BeWo and that splicing is only present in variants driven under promoter, while variants driven by promoter b and c do not exhibit splicing detectable by our PCR assay, although PCR product sequencing would provide higher resolution (Fig. 1a). Using PCR, we observed variants with partial exon 2 exclusion (ΔE2p) and complete exon 2 exclusion (ΔE2) (Fig. 1a). Moreover, expression of CRH was present in BeWo, confirming previous reports [29] (Fig. 1b). Finally, it is important to note that other variants may be expressed in BeWo, and analysis of the complete repertoire of variants expressed in this cell line was not assessed here.
Fig. 1.
a NRSF baseline expression and splicing pattern of variants driven by three alternative promoters (Pa, Pb, Pc) and splicing in NRSF Pa producing variants with partial and complete exon 2 exclusion. b Exponential curve of fluorescence accumulation upon qPCR with CRH-specific primers targeting exon 2, along with no template control, along with single expected amplicon observation by gel electrophoresis of the PCR product, also observed by melting curve analysis.
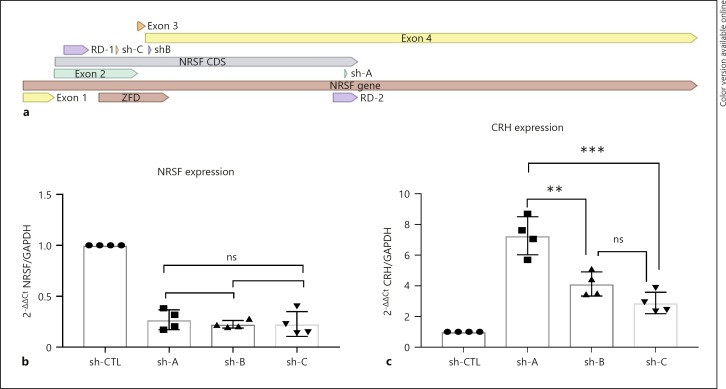
We then sought to investigate what would be the effect of downregulating NRSF on CRH expression by using siRNAs. Three siRNAs were utilized, each with different hybridization sites in the target NRSF mRNA sequence (Fig. 2a). Through delivery of siRNA in the cell we observed downregulation of NRSF driven by promoter a, compared to a scrambled siRNA-treated control (Fig. 2b). The downregulation was not significantly different among siRNAs, suggesting that any observed expression change in CRH would be due to the hybridization target and not the efficacy of the siRNA. It is important to note that unexpectedly downregulation was not observed in NRSF variants driven by promoters b and c, suggesting that those variants might be missing the target site or be present in cellular compartments that are not populated by the siRNA molecule (data not shown). As hypothesized, CRH expression was differentially modulated by targeting different regions of NRSF (Fig. 2c). The highest upregulation in CRH was observed when the siRNA was targeting the region of NRSF responsible for translation of the RD2 domain (2C, shA). When the siRNA was targeting regions within the ZFD of NRSF, there was a modest upregulation, but there was not any significant difference between the two (2C, shB, and shC). Although not able to specifically probe the downregulation of variants containing solely the RD2, it is evident that in this cellular context the RD2 domain primarily acts as a repressive domain. Moreover, targeting of different regions in the ZFD, which has been previously reported to result in upregulation, was also observed in this case due to the reduction of variants containing the region targeting the NRSE domain.
Fig. 2.
a NRSF's coding DNA sequence (CDS) and the respective binding of the siRNA. b Decreased expression of NRSF upon treatment with 10 μM siRNA, no significant difference in total expression decrease by the siRNAs (n = 4). c Differential increase in CRH expression by siRNA downregulation of NRSF, suggesting binding site but not NRSF quantitative decrease is responsible (n = 4). ANOVA with repeated measures was performed to assess significance: ns, p > 0.05; ** p < 0.01; *** p < 0.001.
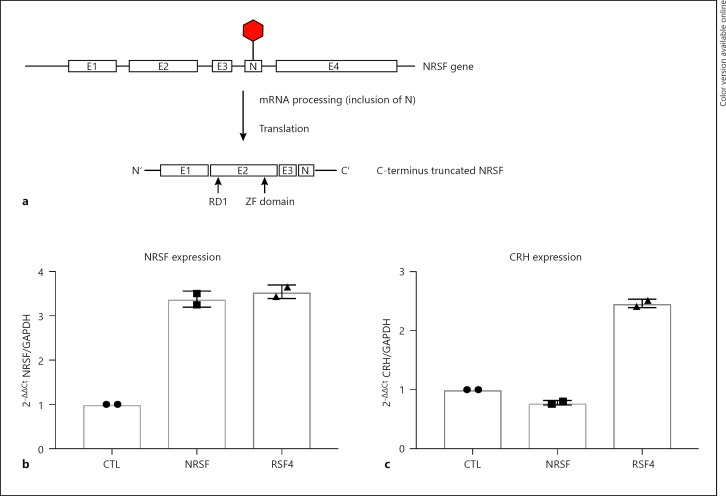
Moreover, we sought to investigate the relationship between different known variants and their effect on CRH expression. Vectors containing the full-NRSF sequence and NRSF4 C-terminus truncated variants missing the RD2 domain were transfected along with a control empty pcDNA3.1 vector. Endogenously, NRSF4 is translated into a C-terminus truncated isoform, through inclusion of exon N, which introduces a premature stop codon upstream of the RD2 in exon 4 (Fig. 3a). Upregulation of the vectors was assessed by mRNA quantification, which showed approximately 3-fold upregulation after clonal selection (Fig. 3b). Interestingly, full-length NRSF vector did not promote any differential expression of CRH in relation to the control, which might be due to the already high occupancy of NRSE sites in the CRH intron (Fig. 3c, NRSF). Moreover, upregulation of NRSF4 showed a remarked 2-fold increase in CRH expression, further supporting the hypothesis that the RD2 domain is a potent repressor of CRH expression (3C, NRSF4).
Fig. 3.
Transfection with 2 μg pcDNA3.0 (CTL), NRSF insert (NRSF), and NRSF4 insert (NRSF4). Cells were transfected in 2 independent experiments and selected over a period of 2 weeks for generation of a clonal population. a The endogenous mechanism of NRSF4 splicing. b NRSF expression induction upon plasmid transfection. c CRH expression changes due to overexpression of NRSF isoform constructs.
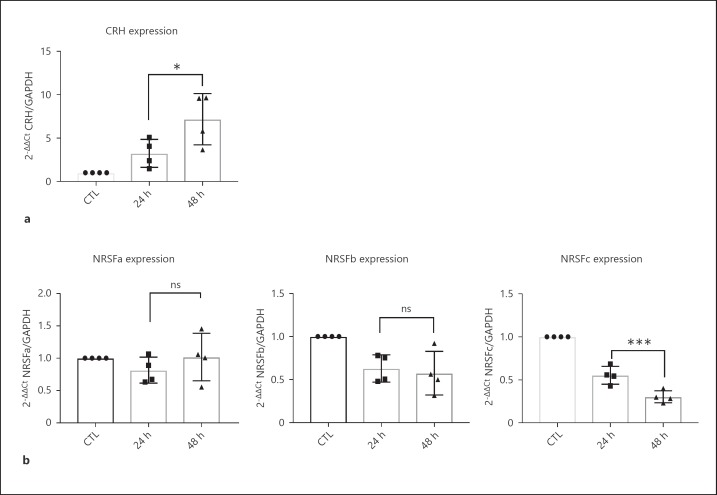
To assess the mechanism under a cell signaling context we utilized 8-Br-cAMP to activate cAMP responsive pathways intracellularly. It has been previously shown that cAMP upregulates CRH through a CRE promoter element [29]. Additionally, NRSF has been shown to be downregulated by cAMP activation, while it also contains CRE elements along its promoter. We performed a time-course experiment by exposing the cells to 250 μM 8-Br-cAMP for 24 and 48 h, while measuring the effects on gene expression. First, we replicated the upregulation effect of cAMP induction in CRH expression (Fig. 4a). We did not observe any effect on NRSF splicing pattern upon treatment with 8-Br-cAMP (data not shown). Interestingly, cAMP pathway induction showed a temporal downregulating effect in NRSF driven by promoter c only, while no significant effect was observed in NRSF promoter a and b variants (Fig. 4b). This effect is puzzling, since research on NRSF has not been focusing on analyzing the location of NRSF variants or their differential activation by signaling pathways. Focusing on the downregulating effect, we might postulate that incremental decreases in NRSF acted to potentiate the upregulation of CRH by cAMP pathway activation.
Fig. 4.
Differential changes in NRSF differential promoter (a, b, c) variant expression (b), and increasing trend in CRH expression (a) upon time course treatment with 250 μM 8-Br-cAMP. Independent experiments (n = 4) where analyzed with paired two-tailed t test. * p < 0.05; ** p < 0.01; *** p < 0.001.
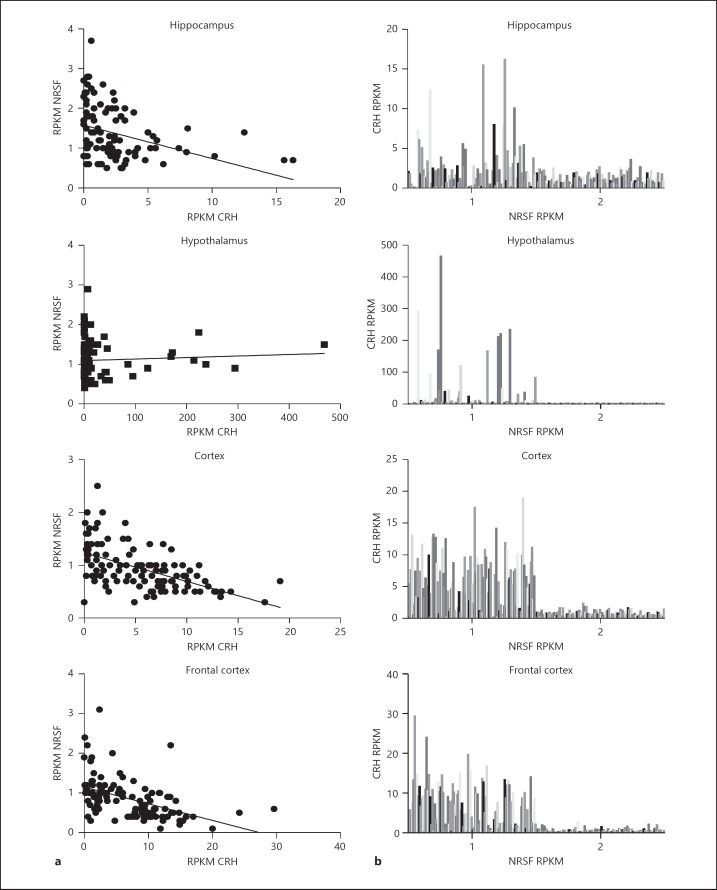
Finally, lacking human neuronal in vitro models for CRH expression, we wanted to investigate if the relationship holds in primary neuronal tissue data. To do so, we utilized the GTEx database to investigate patient RNA sequencing data in the human hippocampus, hypothalamus, cortex, and frontal cortex. We focused on the expression of CRH and NRSF in individual tissue samples collected from patients of age 20–65, while determining that age did not promote any significant alteration in the expression relation of the two genes (data not shown). Pearson correlation analysis showed a statistically significant deviation from a horizontal slope, signifying correlation, in the hippocampus (R = −0.3068, p < 0.0001, n = 181), cortex (R = −0.551, p < 0.0001, n = 114) and frontal cortex (R = −0.4666, p < 0.0001, n = 108), while no significant correlation was observed in the hypothalamus (R = 0.05754, p = 0.5777, n = 96) (Fig. 5a). Then, we decided to plot the data as a histogram to allow for more effective visualization of the underlying relationship of the gene expression of CRH and NRSF (Fig. 5b). Surprisingly, we observed a clear cut-off among gene expression of NRSF and CRH lying at approximately 1.5 RPKM for the NRSF gene, showing that differential quantitative differences in NRSF can influence CRH expression in the human brain. Moreover, we also observed that within the distribution of NRSF, < 1.5 RPKM appears to be a differential expression pattern in CRH RPKM, potentially attributed to differential expression of isoforms of NRSF.
Fig. 5.
a Scatter of NRSF and CRH RPKMs matched to patients across different brain regions; line corresponds to linear regression output. b Distribution of CRH and NRSF RPKMs. A binomial distribution is observed due to the threshold observed in NRSF RPKM (approximately 1.5).
Discussion
This work supports the hypothesis that NRSF can differentially modulate CRH through its functional domains. It is the first time this pathway has been shown to be endogenously active in a human cell line, more specifically the trophoblast BeWo. We focused on an established in vitro model of CRH expression, which may or may not reflect effects on CRH expression in the brain due to a different cellular context, but nevertheless the pathway is endogenously active in human cells.
Our data suggest that RD2 is a potent repressor of CRH gene expression. This agrees with previous data suggesting that RD2 is recruiting repressive cofactors, such as MeCp2, G9A, and Sin3. Due to absence in resolution at the DNA-histone level, future studies should focus on determining which factors are no longer recruited due to overexpression of truncated isoforms of NRSF, since a delicate balance exists with the absence of certain amino acids, that could result in recruitment in some but not all of the factors recruited by RD2. Another hypothesis is that the RD1 domain acts as an activator due to recruitment of the SWI/SNF complex that can allow for enhancement of binding by certain cofactors [32, 33]. This activating ability of RD1 had been previously reported in a mouse cell line, when the CRH promoter was driving luciferase activity [22].
We also show that NRSF-mediated modulation of CRH is coupled with activation of signaling pathways mediated by cAMP. We observed a novel relationship indicating that cAMP pathway activation can lead to downregulation of the NRSF splice variant driven by promoter c. It has been previously shown in non-human primates and rodents that the transcription factor SP1 has binding sites across the NRSF promoter, although the region of NRSFc is solely regulated by SP1 and has the highest binding sites per promoter [34]. Through sequence investigation of the promoters we observed a similar but less extensive pattern of SP1 regulation in the human NRSF. This leads to the hypothesis that PKA activation by 8-Br-cAMP in this context, phosphorylates SP1 and sends it to degradation machinery in the cell [35]. Since, SP1 is an activator of NRSF transcription, this cAMP-mediated degradation of SP1 may be the cause of the specified decrease. In total, the evidence suggests that temporal decrease in NRSF might allow for increased CREB binding to CRH that leads to increased CRH transcription.
Due to the lack of in vitro human neuronal models of CRH expression, we sought to investigate this relation in patient data. We observed significant correlations among regions of the brain in patients with a surprising finding that a decrease in NRSF expression can hit a threshold level that allows for rapid upregulation of CRH in the brain. Although this was observed on average, certain subpopulations below threshold showed differential CRH expression suggesting that other factors contribute to regulatory function or that NRSF by itself may be regulated to produce these effects, through processes resulting in NRSF isoforms, such as alternative splicing. If the latter is the case, it would support the hypothesis that extensive alternative splicing of NRSF allows for differential molecular phenotypes. Future studies should focus on understanding the phenotypic relation to the different patterns of expression and investigation for certain pathological correlates.
Future work should focus on improving upon the current data, thus providing higher resolution of the discussed mechanism. First, a more suitable model should be used, such as human stem cell-derived hypothalamic neurons expressing CRH, or human organoid models. The investigation at the protein level would also indicate if such a mechanism is protein or RNA mediated. Finally, more high-resolution molecular methods should be employed to pinpoint the exact function of each coding and noncoding RNA of NRSF in relation to the regulation of CRH.
In all, this work provides evidence that the NRSF-mediated modulation of CRH is present in humans. This is exciting since it opens new diagnostic and therapeutic avenues for CRH-related disorders, such as stress disorders. This is primarily the cause, since NRSF is a diverse epigenetic factor that can exist in many different isoforms that confer different functional outputs to CRH expression. This provides the advantage of targeting specificity by small-molecule, receptor-targeted therapeutics, and genetic interventions. Therefore, it is imperative for future studies to focus on the gaps in knowledge surrounding this pathway, now that more evidence supports its existence in humans.
Disclosure Statement
The authors declare no conflict of interest.
Funding Sources
This work was funded by the Nu Rho Psi Research Award and Undergraduate Research and Creative Endeavors Award of Northeastern University.
References
- 1.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. In pursuit of resilience: stress, epigenetics, and brain plasticity. Ann NY Acad Sci. 2016;1373:56–64. doi: 10.1111/nyas.13020. [DOI] [PubMed] [Google Scholar]
- 3.De Souza EB, Battaglia G. Corticotropin-releasing hormone (CRH) receptors in brain. Adv Exp Med Biol. 1988;245:123–136. doi: 10.1007/978-1-4899-2064-5_9. [DOI] [PubMed] [Google Scholar]
- 4.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grammatopoulos DK. Insights into mechanisms of corticotropin-releasing hormone receptor signal transduction. Br J Pharmacol. 2012;166:85–97. doi: 10.1111/j.1476-5381.2011.01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiroi N, Wong ML, Licinio J, et al. Expression of corticotropin releasing hormone receptors type I and type II mRNA in suicide victims and controls. Mol Psychiatry. 2001;6:540–546. doi: 10.1038/sj.mp.4000908. [DOI] [PubMed] [Google Scholar]
- 7.Dedic N, Deussing AC., and JM The CRF family of neuropeptides and their receptors – mediators of the central stress response. Curr Mol Pharmacol. 2017;10:1–28. doi: 10.2174/1874467210666170302104053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uhlen M, Fagerberg L, Hallstrom BM, et al. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur J Pharmacol. 2008;583:215–225. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci USA. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steckler T, Holsboer F. Corticotropin-releasing hormone receptor subtypes and emotion. Biol Psychiatry. 1999;46:1480–1508. doi: 10.1016/s0006-3223(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 12.Kano M, Muratsubaki T, Van Oudenhove L, et al. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci Rep. 2017;7 doi: 10.1038/s41598-017-09635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stankiewicz AM, Swiergiel AH, Lisowski P. Epigenetics of stress adaptations in the brain. Brain Res Bull. 2013;98:76–92. doi: 10.1016/j.brainresbull.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci. 2010;13:1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- 15.Sterrenburg L, Gaszner B, Boerrigter J, et al. Sex-dependent and differential responses to acute restraint stress of corticotropin-releasing factor-producing neurons in the rat paraventricular nucleus, central amygdala, and bed nucleus of the stria terminalis. J Neurosci Res. 2012;90:179–192. doi: 10.1002/jnr.22737. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Evans AN, Liu Y, Honda M, Saavedra JM, Aguilera G. Maternal deprivation in rats is associated with corticotrophin-releasing hormone (CRH) promoter hypomethylation and enhances CRH transcriptional responses to stress in adulthood. J Neuroendocrinol. 2012;24:1055–1064. doi: 10.1111/j.1365-2826.2012.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen GL, Miller GM. Extensive alternative splicing of the repressor element silencing transcription factor linked to cancer. PLoS One. 2013;8:e62217. doi: 10.1371/journal.pone.0062217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otto SJ, McCorkle SR, Hover J, et al. A new binding motif for the transcriptional repressor REST uncovers large gene networks devoted to neuronal functions. J Neurosci. 2007;27:6729–6739. doi: 10.1523/JNEUROSCI.0091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenherr CJ, Paquette AJ, Anderson DJ. Identification of potential target genes for the neuron-restrictive silencer factor. Proc Natl Acad Sci USA. 1996;93:9881–9886. doi: 10.1073/pnas.93.18.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satoh JI, Kawana N, Yamamoto Y. ChiP-seq data mining: remarkable differences in NRSF/REST target genes between human ESC and ESC-derived neurons. Bioinform Biol Insights. 2013;7:357–368. doi: 10.4137/BBI.S13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seth KA, Majzoub JA. Repressor element silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) can act as an enhancer as well as a repressor of corticotropin-releasing hormone gene transcription. J Biol Chem. 2001;276:13917–13923. doi: 10.1074/jbc.M007745200. [DOI] [PubMed] [Google Scholar]
- 23.Korosi A, Shanabrough M, McClelland S, et al. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J Neurosci. 2010;30:703–713. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh-Taylor A, Molet J, Jiang S, et al. NRSF-dependent epigenetic mechanisms contribute to programming of stress-sensitive neurons by neonatal experience, promoting resilience. Mol Psychiatry. 2018;23:648–657. doi: 10.1038/mp.2016.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh-Taylor A, Molet J, Jiang S, et al. Early-life experience reprograms stress-sensitive neurons and influences adult phenotype via NRSF/REST-dependent epigenetic mechanisms. Neuropsychopharmacology. 2015;40:S477. [Google Scholar]
- 26.Mou H, Zhao X. NRSF and CCR5 established neuron-glia communication during acute and chronic stresses. J Drug Metab Toxicol. 2016;7:1–8. [Google Scholar]
- 27.Uchida S, Hara K, Kobayashi A, et al. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J Neurosci. 2010;30:15007–15018. doi: 10.1523/JNEUROSCI.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otsuki K, Uchida S, Wakabayashi Y, et al. Aberrant REST-mediated transcriptional regulation in major depressive disorder. J Psychiatr Res. 2010;44:378–384. doi: 10.1016/j.jpsychires.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Pan X, Bowman M, Scott RJ, et al. Methylation of the corticotropin releasing hormone gene promoter in BeWo Cells: relationship to gene activity. Int J Endocrinol. 2015;2015:861302. doi: 10.1155/2015/861302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen GL, Miller GM. Tryptophan hydroxylase-2: an emerging therapeutic target for stress disorders. Biochem Pharmacol. 2013;85:1227–1233. doi: 10.1016/j.bcp.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lonsdale J, Thomas J, Salvatore M, et al. The genotype-tissue expression (GTEx) project. Nat Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang L, Nogales E, Ciferri C. Structure and function of SWI/SNF chromatin remodeling complexes and mechanistic implications for transcription. Prog Biophys Mol Biol. 2010;102:122–128. doi: 10.1016/j.pbiomolbio.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe H, Mizutani T, Haraguchi T, et al. SWI/SNF complex is essential for NRSF-mediated suppression of neuronal genes in human nonsmall cell lung carcinoma cell lines. Oncogene. 2006;25:470–479. doi: 10.1038/sj.onc.1209068. [DOI] [PubMed] [Google Scholar]
- 34.Ravache M, Weber C, Mérienne K, Trottier Y. Transcriptional activation of REST by Sp1 in Huntington's disease models. PLoS One. 2010;5 doi: 10.1371/journal.pone.0014311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang F, Hu Y, Huang P, Toleman CA, Paterson AJ, Kudlow JE. Proteasome function is regulated by cyclic AMP-dependent protein kinase through phosphorylation of Rpt6. J Biol Chem. 2007;282:22460–22471. doi: 10.1074/jbc.M702439200. [DOI] [PubMed] [Google Scholar]