Abstract
Anticoagulation-related nephropathy (ARN) is a significant and underdiagnosed complication in patients who receive anticoagulation therapy. It is characterized by acute kidney injury in the setting of excessive anticoagulation defined as an international normalized ratio > 3.0 in patients treated with warfarin. A definitive diagnosis is made by renal biopsy showing acute tubular necrosis with obstruction of the tubuli by red blood cell casts. However, the evidence shows that ARN can occur during treatment with novel oral anticoagulants as well. Although it has been suggested that antiplatelet therapy, such as aspirin, might contribute to coagulopathy (and therefore the hypothetical risk of ARN), there are no reports of ARN induced by antiplatelet therapy according to our knowledge. It is also reported that glomerular lesions (i.e., kidney disease) represent a risk factor for ARN. We present a case of an 82-year-old man who developed biopsy-proven ARN after the administration of dual antiplatelet therapy with no previous anticoagulation treatment and normal coagulation tests.
Keywords: Acute kidney injury, Anticoagulation-related nephropathy, Hematuria, Dual antiplatelet therapy
Introduction
Anticoagulation-related nephropathy (ARN), formerly also referred to as warfarin-related nephropathy, is a form of acute kidney injury (AKI) caused by anticoagulation agents such as warfarin or novel oral anticoagulants [1, 2, 3]. To date, no connection with antiplatelet therapy has been reported.
AKI resulting from glomerular hemorrhage has been described in patients with glomerular lesions in the presence [4] and in the absence likewise [5] of severe coagulopathy which was defined as an International Normalized Ratio (INR) ranging from 6 to 9 in both studies. In more recent studies, AKI associated with glomerular hemorrhage has also been described in patients without underlying kidney disease and with more modest elevations of INR (> 3) [6]. A biopsy study of 9 patients who developed otherwise unexplained AKI in association with a warfarin overdose revealed the predominant lesion of tubular injury caused by obstruction with red blood cells (RBCs) and RBC casts [7]. The recognition of this characteristic histological lesion associated with the clinical presentation of AKI in the setting of over-anticoagulation led to the term “anticoagulant-related nephropathy.”
The incidence of ARN is difficult to determine. In the absence of biopsy data, epidemiologic studies overestimate the incidence of ARN probably because they were based on a presumptive diagnosis of ARN usually defined as an elevation in the serum creatinine within several days of an abnormally elevated INR [8].
Another problem could be that the incidence is frequently overestimated by an ascertainment bias. The usual AKI definition relies on the measurement of increased serum creatinine and increased INR lasting several consecutive days. However, multimorbid patients are more likely to receive anticoagulation therapy; furthermore, they have more frequent creatinine measurements; thus, they are more likely to develop renal insufficiency and ARN.
However, ARN is commonly underdiagnosed in everyday practice. Particularly, two reasons should be considered. Firstly, nephrologists are reluctant to perform kidney biopsy in patients who need therapeutic anticoagulation. In these patients, the biopsy is commonly delayed until the INR returns to the normal range, and in this case, creatinine may also improve; thus, the clinician may conclude that a biopsy is no longer needed. Secondly, the possibility of ARN may be overlooked because it is common mostly among patients with multiple risk factors for AKI of any cause.
In epidemiologic studies, ARN has been generally associated with an increased risk of mortality. AKI in ARN can be severe and can be associated with irreversible kidney injury in some patients, even with the need of maintenance renal replacement therapy.
Case Presentation
An 82-year-old man was admitted to our nephrology ward due to the development of AKI, macroscopic hematuria, fatigue, loss of appetite, and shortness of breath. His medical history was significant for a relapse of unstable angina pectoris treated by a drug-eluting stent implantation 8 months before the admission, also dyslipidemia, arterial hypertension, and benign prostatic hyperplasia. Due to the recent stent implantation, the patient regularly used a standard dual antiplatelet therapy (clopidogrel 75 mg + acetylsalicylic acid 100 mg daily), as well as perindopril (5 mg daily), bisoprolol (2.5 mg daily), and atorvastatin (20 mg daily).
Following laboratory results were obtained at admission: serum creatinine 522 μmol/L, urea 22.95 mmol/L, potassium 5.33 mmol/L, uric acid 739 μmol/L, CRP (C-reactive protein) 4.1 mg/L, hemoglobin 107 g/L, leukocytes 35.5 × 109/L, and platelets 117 × 109/L. Proteinuria of 1.0 g/L and erythrocyturia of 460/μL were observed in the urinalysis. Serum electrophoresis proved a deformation of the gamma fraction and IgG kappa monoclonal gammopathy. There were no abnormal findings in the immunological examination except minor elevation of serum IgG (17.6 g/L) and IgM (2.34 g/L).
Abdominal ultrasonography was performed revealing hypertensive nephrosclerosis and signs of acute irritation of the kidneys. The kidneys were symmetric in size (120 mm), had smooth contours and hyperechogenic parenchyma that was in some places extended up to 25 mm. Doppler imaging of the renal arteries found high resistance indexes (> 0.80) in both kidneys. Furthermore, a suspected expansive tumor of the upper pole of the left kidney was also described, as well as splenomegaly and para-aortic lymphadenopathy.
A microscopic differential leukocyte count was performed due to substantial leukocytosis without any signs of infection revealing 68% of atypical neoplastic lymphocytes. Therefore, the blood was examined by flow cytometry which showed B-lymphocyte clonal proliferation with nonspecific phenotype, probably corresponding with a non-Hodgkin's marginal zone lymphoma. Based on these examinations, trepanobiopsy was subsequently performed, revealing lymphoma infiltration which represented 70% of the total bone marrow cell volume. The neoplastic cells morphologically and immunophenotypically corresponded with the diagnosis of non-Hodgkin's mantle cell lymphoma (NH-MCL).
Staging of the neck and chest-abdomen-pelvis CT examination confirmed the findings of splenomegaly and expansive tumor of the left kidney and also revealed diffuse mediastinal, axillar, inguinal, para-aortic, and mesenterial lymphadenopathy.
The patient developed an oligo-anuric renal failure during hospitalization. Original diuresis of approximately 2,000 mL/day decreased to < 500 mL/day, and the serum creatinine increased steadily from 522 to 779 μmol/L within the first week. Regular hemodialysis treatment had to be commenced 8 days after admission due to the progression of renal failure, fluid overload, hyperkalemia, and metabolic acidosis. Our initial workup did not show any explanation for AKI and renal biopsy was delayed due to the discontinuation of dual antiplatelet therapy and clinical bleeding manifestations (hematuria, epistaxis, and spontaneous hematomas). We were not able to distinguish the cause of the persistent bleeding by conventional and routinely available laboratory tests (INR 1.22, aPTT/R 1.13, thrombin time 15.8 s, fibrinogen 2.4 g/L, antithrombin 89%). Renal biopsy was finally performed 17 days after admission. It showed morphology of extensive acute tubular necrosis with numerous RBC casts in the renal tubuli, a histological finding corresponding to ARN, and a concomitant glomerulonephritis (GN) with monoclonal deposits (IgG, IgG3, kappa light chain, and C3 complement component), probably due to NH-MCL. The deposits were localized in the mesangium, and the glomeruli showed the morphological features of mesangioproliferative GN (Fig. 1, 2). Unfortunately, due to the nature of the sample (being subcapsular), no vessels could be discerned, and thus it was impossible to evaluate the arteries and quantify the extent of interstitial fibrosis which may be the limiting factor of further reparation of renal function.
Fig. 1.
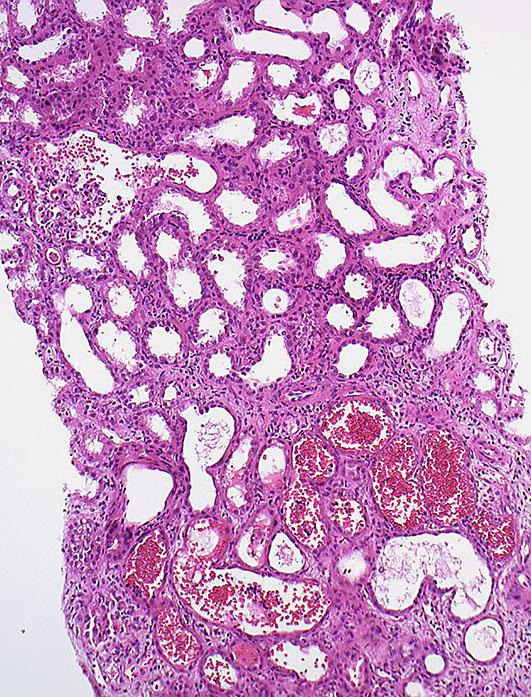
The obvious dilatation of the proximal tubules with flattened epithelia is a morphological sign of AKI. Concurrently, there are RBC casts in the lumen of some of the tubuli. Signs of bleeding in the Bowman's capsule are also visible in the left upper part of the image.
Fig. 2.
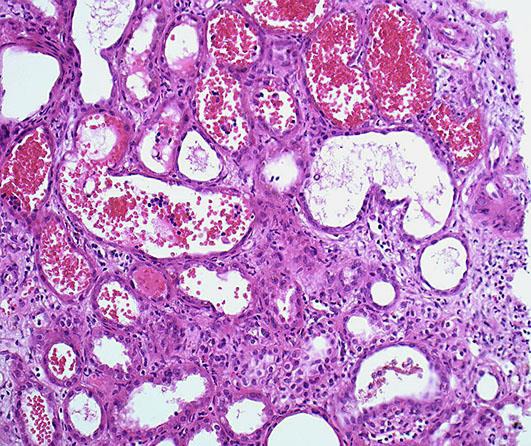
Detail of RBC casts and signs of heavy diffuse damage to the epithelium of the proximal tubuli.
There was no improvement of renal function during the hospitalization nor on any of the other follow-ups, and chronic hemodialysis treatment was required. The patient remained under the observation of the hemato-oncologist. Treatment of the NH-MCL by attenuated immunochemotherapy regimen (miniCHOP) could be started despite the renal failure, and partial remission was achieved. Restaging PET/CT done after 6 cycles of miniCHOP showed regression of the diffuse lymph node infiltration, although the control bone marrow biopsy revealed a slightly hypocellular trilinear bone marrow with nodular infiltration of B-cell non-Hodgkin's lymphoma. A suspected tumor of the left kidney was evaluated as a primary renal tumor. The patient was further investigated, although he was contradicted for surgery treatment later, but no further tumor progression was observed in the follow-up period.
Discussion
The understanding of the pathogenesis of ARN is based upon histological analysis of bioptically obtained kidney tissue and on experiments made in animal models [9]. The likely pathogenesis includes glomerular hemorrhage, obstruction of renal tubuli by RBC casts, and direct tubular epithelial cell injury.
The exact interval between the increased INR and the onset of AKI is not known. However, observational studies suggest that most episodes of AKI occur early (< 8 weeks) after starting warfarin therapy [6, 10].
The clinical symptoms of ARN are nonspecific, and the differential diagnosis of ARN is broad, encompassing the differential diagnosis of AKI. It depends on clinical symptoms and laboratory findings, mainly immunology, urinalysis, and quantitation of protein excretion. GN or vasculitis should be considered in patients presenting with hematuria [11].
There have been no prospective studies on the management of patients with ARN. However, based on clinical experiences, it is believed that restoration of a therapeutic INR may limit the extent of acute and chronic kidney injury that results from the glomerular hemorrhage particularly [11].
In our report, we describe a case with a clinical and histological correlation to ARN in the setting of dual antiplatelet therapy with no anticoagulation. Therefore, we can expect an etiopathogenetic connection. To the best of our knowledge, there has been no similar case reported in the literature to date.
From a differential diagnostic point of view, there are three possible factors, which took place at the same time and each of them could have contributed to the development of ARN: (i) dual antiplatelet therapy, (ii) GN, and (iii) hidden coagulopathy or thrombocytopathy caused by the lymphoma. As mentioned above, the presence of chronic kidney disease almost doubles the risk of developing ARN. We can, therefore, assume that any glomerular damage is a risk factor for ARN.
In addition, it needs to be stated that the bleeding could have been caused by an overdose of antiplatelet drugs. In practice, the effect of antiplatelet therapy is not monitored, even though there are many functional aggregation assays available (light transmission aggregometry, turbidimetric aggregometry, impedance aggregometry, and others). There is a risk of under- or overtreatment even with standard doses of antiplatelet drugs [12]. We did not perform any aggregation testing in our case.
Statement of Ethics
There was no requirement for approval of assessment by the review committee.
Disclosure Statement
The authors declare that they have no conflicts of interest regarding the content of this case report.
References
- 1.Wheeler DS, Giugliano RP, Rangaswami J. Anticoagulation-related nephropathy. J Thromb Haemost. 2016 Mar;14((3)):461–7. doi: 10.1111/jth.13229. [DOI] [PubMed] [Google Scholar]
- 2.Ryan M, Ware K, Qamri Z, Satoskar A, Wu H, Nadasdy G, et al. Warfarin-related nephropathy is the tip of the iceberg: direct thrombin inhibitor dabigatran induces glomerular hemorrhage with acute kidney injury in rats. Nephrol Dial Transplant. 2014 Dec;29((12)):2228–34. doi: 10.1093/ndt/gft380. [DOI] [PubMed] [Google Scholar]
- 3.Narasimha Krishna V, Warnock DG, Saxena N, Rizk DV. Oral anticoagulants and risk of nephropathy. Drug Saf. 2015 Jun;38((6)):527–33. doi: 10.1007/s40264-015-0290-z. [DOI] [PubMed] [Google Scholar]
- 4.Kabir A, Nadasdy T, Nadasdy G, Hebert LA. An unusual cause of gross hematuria and transient ARF in an SLE patient with warfarin coagulopathy. Am J Kidney Dis. 2004 Apr;43((4)):757–60. doi: 10.1053/j.ajkd.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Kincaid-Smith P, Bennett WM, Dowling JP, Ryan GB. Acute renal failure and tubular necrosis associated with hematuria due to glomerulonephritis. Clin Nephrol. 1983 Apr;19((4)):206–10. [PubMed] [Google Scholar]
- 6.Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM, et al. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011 Jul;80((2)):181–9. doi: 10.1038/ki.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodsky SV, Satoskar A, Chen J, Nadasdy G, Eagen JW, Hamirani M, et al. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis. 2009 Dec;54((6)):1121–6. doi: 10.1053/j.ajkd.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 8.An JN, Ahn SY, Yoon CH, Youn TJ, Han MK, Kim S, et al. The occurrence of warfarin-related nephropathy and effects on renal and patient outcomes in korean patients. PLoS One. 2013;8((4)):e57661. doi: 10.1371/journal.pone.0057661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozcan A, Ware K, Calomeni E, Nadasdy T, Forbes R, Satoskar AA, et al. 5/6 nephrectomy as a validated rat model mimicking human warfarin-related nephropathy. Am J Nephrol. 2012;35((4)):356–64. doi: 10.1159/000337918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizk DV, Warnock DG. Warfarin-related nephropathy: another newly recognized complication of an old drug. Kidney Int. 2011 Jul;80((2)):131–3. doi: 10.1038/ki.2011.85. [DOI] [PubMed] [Google Scholar]
- 11.Brodsky SV, Hebert LA. Anticoagulant-Related Nephropathy: Is an AKI Elephant Hiding in Plain View? J Am Coll Cardiol. 2016 Nov;68((21)):2284–6. doi: 10.1016/j.jacc.2016.09.926. [DOI] [PubMed] [Google Scholar]
- 12.Angiolillo DJ. Variability in responsiveness to oral antiplatelet therapy. Am J Cardiol. 2009 Feb;103((3 Suppl)):27A–34A. doi: 10.1016/j.amjcard.2008.11.020. [DOI] [PubMed] [Google Scholar]


