Abstract
Background and Aim
Liver diseases are a major public health problem, accounting for a significant number of hospital visits and admissions and an increasing mortality rate. Melatonin (MLT) is a powerful antioxidant molecule that has been shown to be beneficial under various conditions. The objective was to evaluate the effect of MLT on experimental liver cirrhosis induced by carbon tetrachloride (CCl4) in rats.
Methods
Twenty male Wistar rats (230–250 g) were divided into four groups. I: control group (CO); II: CO + MLT; III: CCl4; and IV: CCl4 + MLT. CCl4 was administered intraperitoneally (i.p.) as follows: 10 doses every 5 days, 10 doses every 4 days, and 7 doses every 3 days. MLT was administered i.p. at a dose of 20 mg/kg from the 10th week to the end of the experiment (16th week).
Results
In the CCl4 + MLT group, we found that MLT caused a decrease in the level of F2‐isoprostanes and NQO1 expression. We also found that MLT reduced the inflammatory process as shown by decreased expressions of NF‐KB/p65 and inducible nitric oxide synthase (iNOS) and a smaller amount of inflammatory infiltrate. MLT reduced the expression of transforming growth factor beta1 (TGF‐β1), alpha‐smooth muscle actin (α‐SMA), and vascular endothelial growth factor (VEGF). Picrosirius staining showed that MLT decreases fibrosis.
Conclusion
MLT has a potent antifibrogenic effect, modulating the parameters of oxidative stress, angiogenesis, and inflammation.
Keywords: antioxidants, melatonin and liver cirrhosis, oxidative damage
Background
Chronic liver diseases cause progressive destruction of the liver parenchyma and accumulation of the extracellular matrix (ECM), increased collagen synthesis, and inability to promote ECM degradation, resulting in fibrosis and subsequent liver cirrhosis, which is the 10th leading cause of death in the Western world.1, 2 Liver fibrogenesis is triggered by increased reactive oxygen species (ROS),3 leading to an inflammatory process by activating profibrogenic mediators, such as transforming growth factor beta (TGF‐β). TGF‐β induces the activation of hepatic stellate cells (HSC) to differentiate into myofibroblasts, thus increasing the expression of cytokines and proteins involved in matrix remodeling.4, 5, 6, 7
In this study, we used the carbon tetrachloride (CCl4) model of cirrhosis in rats because it is similar to human cirrhosis.8, 9 Using CCl4, a widely used solvent in chemical industries, is one of the main pathways for the exposure and absorption of volatile chemicals that may be environmental contaminants, and it is well known for its hepatic and renal toxic actions. After administration, CCl4 is metabolized by the liver via cytochrome P450, leading to the release of free radicals, such as trichloromethyl and its derivatives that have toxic effects on liver cells, causing steatosis, fibrosis, cirrhosis, and eventually cell death.10, 11 These free radicals may act by covalent binding to lipids and initiate lipid peroxidation (LPO).11, 12
Melatonin (MLT) has proved beneficial in many pathological situations. MLT is an indoleamine widely produced and universally distributed, having multiple functions in all organs and organisms. MLT has a potent in vitro and in vivo antioxidant effect.13 It protects various tissues from persistently produced free radicals14, 15 and significant anti‐inflammatory and immunomodulatory activity,13, 16 as well as oncostatic properties.5 Preliminary tests in human subjects have shown the beneficial effect of exogenous MLT in preventing ulcerative colitis, colon cancer, nonalcoholic fatty liver disease, and complications associated with partial liver resection.2, 15
Liver cirrhosis is associated with high risk of mortality, and liver transplantation is not always available in a timely manner. The inhibition of fibrogenic mechanisms represents an important molecular target of therapeutic action, contributing to a temporary support for patients awaiting liver transplantation.17 Therefore, further studies on the effect of MLT on liver diseases may bring basic studies closer to clinical reality.
The objective of the present study was to evaluate the effect of MLT on CCl4‐induced liver cirrhosis in rats in terms of oxidative stress, inflammatory process, and fibrogenic and angiogenic cytokines.
Methods
Animals and procedures
We used 20 male Wistar rats weighing 250 g. The animals were obtained from the Central Laboratory Animal Facility of the Universidade Federal de Pelotas, Rio Grande do Sul (Brazil). They were kept in the Animal Research Unit of the Hospital de Clínicas de Porto Alegre (Brazil) on a 12/12 h light/dark cycle in a temperature‐ and humidity‐controlled environment. The rats had free access to water and received a restricted diet (16 g of chow per day for each animal).12
All experiments were performed in accordance with the guidelines recommended by Research Ethics Committee of the Research and Graduate Studies Group of Hospital de Clínicas de Porto Alegre under protocol number 100316.
The animals were divided into four groups. CO: control group; CO + MLT: control group receiving MLT; CCl4: receiving carbon tetrachloride; and CCl4 + MLT: receiving CCl4 and MLT. The CCl4 and CCl4 + MLT groups received 27 intraperitoneal doses of CCl4 dissolved in mineral oil (1:6) (volume administered = 0.5 mL). The first 10 doses were administered every 5 days, the next 10 doses were given every 4 days, and the last 7 doses were received every 3 days.18 Phenobarbital was added to the water provided to the animals at a concentration of 0.3 g/L 7 days before the first administration and throughout the experiment to promote cytochrome P450 enzyme induction.12
MLT (Sigma Aldrich, St. Louis, MO, USA) was administered intraperitoneally to the CO + MLT and CCl4 + MLT groups at a dose of 20 mg/kg/day from the 10th week to the end of the experiment (16th week).19
Twenty‐four hours after the last CCl4 administration, the animals were anesthetized with xylazine (5 mg/kg) and ketamine (60 mg/kg), and blood samples were collected from the retro‐orbital plexus. Liver samples were obtained for the other analyses. At the end of the experiment, the animals were killed by exsanguination under deep anesthesia, as described in the American Veterinary Medical Association (AVMA) Guidelines on Euthanasia.20
Histological analysis
Liver tissues were fixed in 4% aqueous formaldehyde solution, embedded in paraffin, and stained with hematoxylin–eosin (HE). Liver fibrosis was assessed with picrosirius and masson trichrome staining.
F2‐isoprostane assay
Livers were excised, weighed, and immediately frozen at −80 °C. The frozen tissues from each rat were homogenized in ice‐cold phosphate buffer (KCl 140 mM, phosphate 20 mM, pH 7.4) and centrifuged at 3000 rpm for 10 min. Protein concentration in the liver homogenates was determined using bovine albumin solution.21 LPO was determined by estimating the level of F2‐isoprostanes, a promising oxidative stress marker, which was detected in the liver using the commercially available Direct 8‐iso‐PGF2α ELISA kit (Enzo Life Sciences, Farmingdale, USA). A true reflection of both free and esterified isoprostane was measured following the manufacturer's instructions.
Western blot
Western blot analysis was performed on cytosolic and nuclear extracts prepared from liver homogenates as previously described.22 The supernatant fraction was collected and stored at −80 °C in aliquots until use. Protein concentration was measured.21 Lysate proteins were fractionated using sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes.23 The membranes were then blocked with 5% nonfat dry milk in tris‐buffered saline containing 0.05% Tween 20 (TTBS) for 1 h at room temperature and probed overnight at 4 °C with polyclonal anti‐NQO1 (SC376023/31 kDa), anti‐TGF‐β (SC31609/25 kDa), anti‐NF‐KB/p65 (SC8008/65 kDa), anti‐iNOSinducible nitric oxide synthase (anti‐iNOS) (SC651/131 kDa), and anti‐vascular endothelial growth factor (anti‐VEGF) (SC7269/42 kDa) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:200–1000 dilution with TTBS in 5% nonfat dry milk. The anti‐alpha‐smooth muscle actin (anti‐α‐SMA) (A2547/42 kDa) antibody (Sigma Aldrich) was at 1:5000 dilution with TTBS in 5% nonfat dry milk. The anti‐β‐actin (A5060/42 kDa) and GAPDH (G9545/37 kDa) antibody (Sigma Aldrich) was at 1:2000 dilution with TTBS in 5% nonfat dry milk. After washing with TTBS, the membranes were incubated for 1 h at room temperature with the secondary horseradish peroxidase (HRP)‐conjugated antibody (Santa Cruz Biotechnology, 1:4000). Protein detection was performed by chemiluminescence using a commercial ECL kit (Amersham Pharmacia Biotech, Little Chalfont, UK).22 The density of the specific bands was quantified using Scion Image software (Scion Corp., Frederick, MD, USA).
Statistical analysis
Means and SDs were calculated for all data. Significant differences between means were evaluated by one‐way anova. Tukey's range test was used when there were significant differences. P values <0.05 were deemed significant. All analyses were carried out using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Effect of MLT on levels of F2‐isoprostanes and expression of NQO1
We found a significant increase in both the F2‐isoprostane level (Fig. 1) and NAD(P)H:quinone oxidoreductase 1 (NQO1) expression (Fig. 2) in the CCI4 group compared with the COs. The use of MLT decreased LPO (F2‐isoprostanes) and NQO1 expression in the CCl4 + MLT group.
Figure 1.
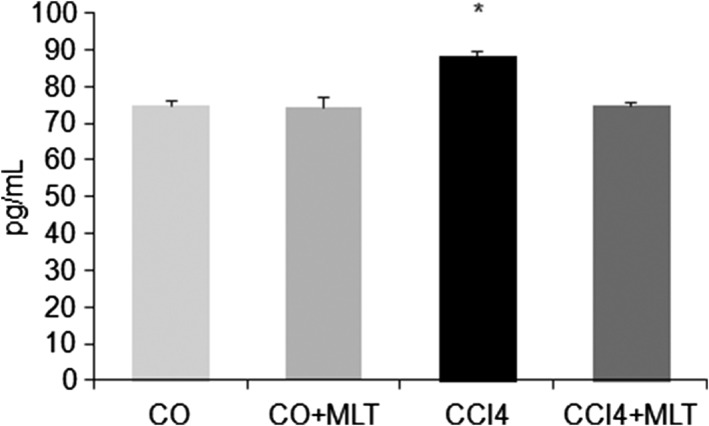
Level of F2‐isoprostanes in rat liver. Results are expressed as mean ± SD. *P < 0.05 carbon tetrachloride (CCl4) versus other groups.
Figure 2.
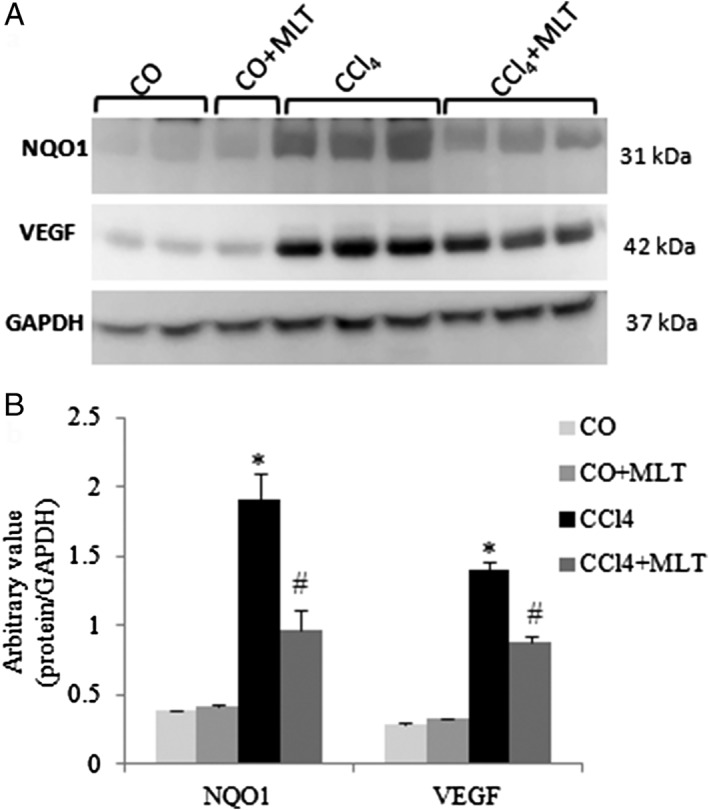
Western blot analysis of NQO1 and vascular endothelial growth factor (VEGF). (a) Cytoplasmic fractions were analyzed by WB with NAD(P)H:quinone oxidoreductase 1 (NQO1) and VEGF and glyceraldehyde phosphate dehydrogenase (GAPDH) antibodies. (b) Arbitrary values expressed as mean and SD. *P < 0.05 carbon tetrachloride (CCl4) versus other groups. #
P < 0.05 CCl4 + melatonin (MLT) versus other groups.  CO,
CO,  CO + MLT,
CO + MLT,  CCl4,
CCl4,  CCl4 + MLT.
CCl4 + MLT.
Effects of MLT on inflammatory markers
Liver histological analysis with HE staining (Fig. 3) showed that the animals of the CCl4 group had histological changes, such as necrosis, hepatocyte degeneration, and the presence of the inflammatory infiltrate. These findings were also found in the CCl4 + MLT groups; however, the incidence and severity of histopathological lesions were lower than those of the CCI4 group.
Figure 3.
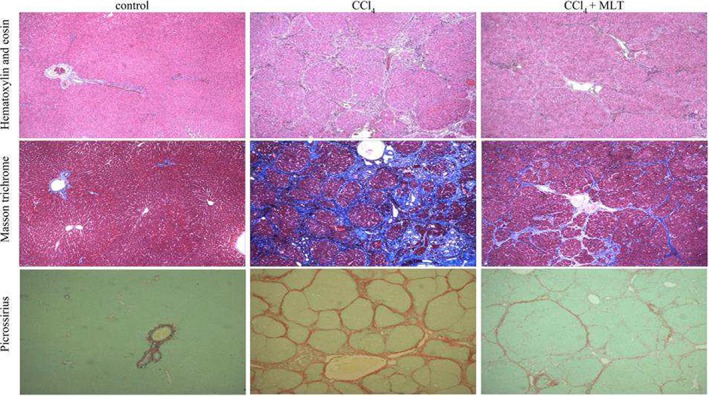
Histological analysis of liver sections by hematoxylin and eosin (10×), masson trichrome (10×), and picrosirius staining (10×).
After confirming the inflammatory environment, possibly caused by the presence of ROS generated by CCl4 metabolism, we assessed NF‐κB p65 expression in the nuclear extract and iNOS expression in the cytoplasmic extract (Fig. 4). The animals of the CCl4 group had significantly increased NF‐κB p65 and iNOS expressions when compared with the COs. The use of MLT caused a significant decrease in the expression of these proteins in the CCl4 + MLT group when compared with the CCl4 group.
Figure 4.
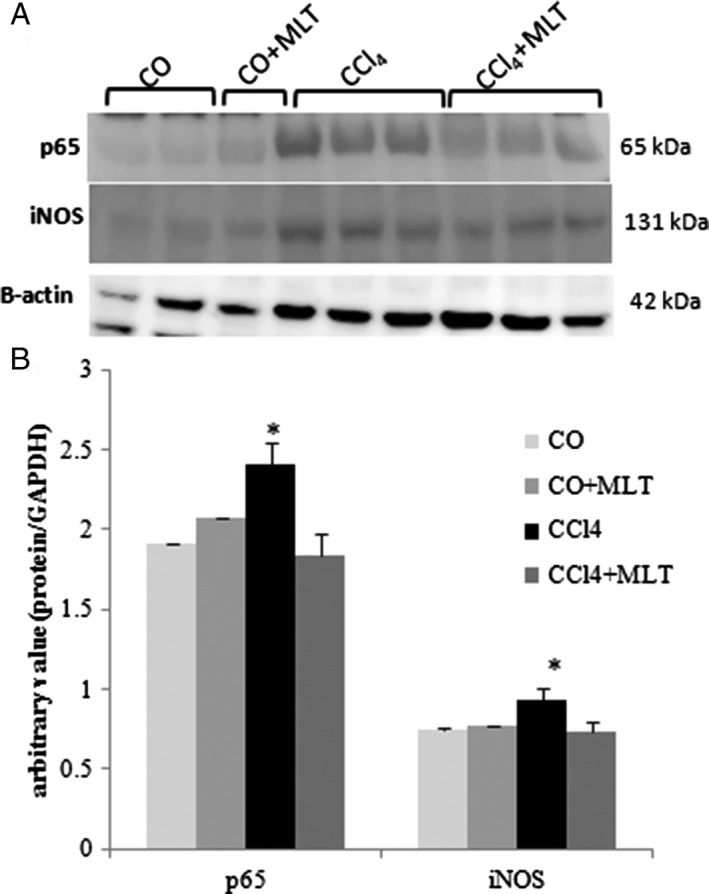
Western blot analysis of p65 and inducible nitric oxide synthase (iNOS). (a) Nuclear fractions were analyzed by WB with NF‐KB/p65 and cytoplasmic iNOS and β‐actin antibodies. (b) Arbitrary values expressed as mean and SD. *P < 0.05 carbon tetrachloride (CCl4) versus other groups.  CO,
CO,  CO + MLT,
CO + MLT,  CCl4,
CCl4,  CCl4 + MLT.
CCl4 + MLT.
Effect of MLT on the expression of TGF‐β1 and α‐SMA in CCl4‐induced liver cirrhosis
We evaluated the expression of TGF‐β1 and α‐SMA (Fig. 5) with the purpose of assessing the effect of MLT on CCl4‐induced liver cirrhosis. CCl4 significantly increased the expression of TGF‐β1 and α‐SMA. In contrast, the group receiving MLT significantly decreased the expression of these proteins when compared with the CCl4 group, thus suggesting the inhibitory effect of MLT on the activation of HSC and ECM deposition.
Figure 5.
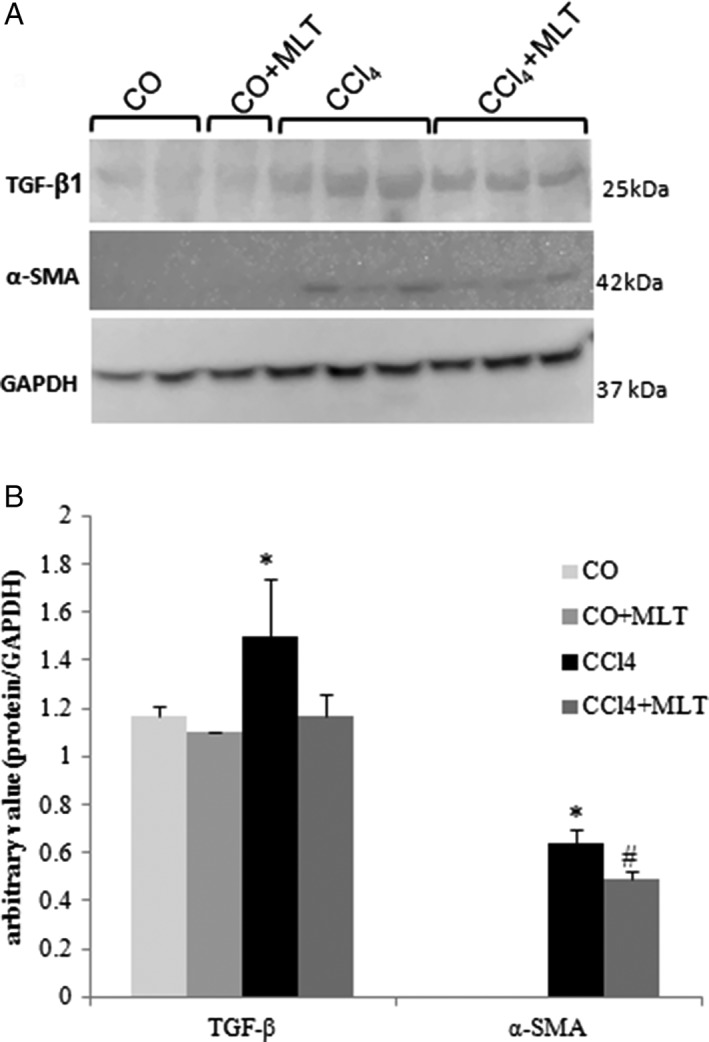
Western blot analysis of transforming growth factor beta (TGF‐β) and alpha‐smooth muscle actin (α‐SMA). (a) Cytoplasmic fractions were analyzed by WB with TGF‐β and α‐SMA and glyceraldehyde phosphate dehydrogenase (GAPDH) antibodies. (b) Arbitrary values expressed as mean and SD. *P < 0.05 carbon tetrachloride (CCl4) versus other groups. #
P < 0.05 CCl4 + melatonin (MLT) versus other groups.  CO,
CO,  CO + MLT,
CO + MLT,  CCl4,
CCl4,  CCl4 + MLT.
CCl4 + MLT.
Effect of MLT on CCl4‐induced histological changes
Fibrosis was investigated using picrosirius staining (Fig. 3). The animals of the COs showed a normal liver architecture. However, the animals of the CCl4 group showed histological changes, such as disruption of liver parenchyma, intense fibrosis with formation of thick collagen septa and closed nodules. In contrast, the animals of the CCl4 + MLT group showed a significant reduction of fibrosis with incomplete fibrotic septa and nodules.
Effect of MLT on liver angiogenesis
Liver fibrosis changes the liver vascular architecture creating a hypoxic environment, which is an important stimulus for the production of angiogenic factors such as the VEGF (Fig. 2). VEGF expression in the liver of the animals of the CCl4 group was significantly higher when compared with the COs. In the CCl4 + MLT group, this expression was significantly reduced when compared with the CCl4 group.
Discussion
The development and progression of liver cirrhosis is a multistage process involving numerous molecular pathways and genetic changes. The present study investigated the molecular mechanisms of MLT in the oxidative, inflammatory, fibrogenic, and angiogenic damage in the experimental liver cirrhosis process. We found that the use of MLT at a dose of 20 mg/kg was effective to modulate these parameters and reduce liver fibrosis. It is probable that this effect is mainly caused by the attenuation of the oxidative damage and the inflammatory response, thus decreasing the TGF‐β1 expression and the activation of HSC.
An increased deposition of collagen and other ECM proteins is common in many chronic diseases affecting the liver, lungs, arteries, and nervous system.11 In this process, the development and progression of many chronic diseases, including liver diseases, the involvement of oxidative stress, was well described.11 LPO is one of the major consequences of oxidative damage and is suggested as a possible mediator of liver fibrosis, having a strong influence on the synthesis and expression of collagen.3, 11, 12, 24, 25, 26, 27, 28, 29 F2‐isoprostanes are produced from the oxidative degradation of arachidonic acid and released into the circulation. Therefore, they can be easily measured in biological samples as a marker of LPO.24, 30 In agreement with our findings, elevated levels of isoprostanes have been reported in various stages of diseases, such as alcoholic liver disease, hepatorenal syndrome, acute cholestasis, ischemia/reperfusion, diabetes mellitus, chronic obstructive pulmonary disease, etc.11, 24 In our study, the use of MLT significantly reduced the levels of F2‐isoprostanes. According to Zhang et al.,24 MLT reduced the levels of F2‐isoprostanes in the liver of rats with diquat‐induced LPO. In addition, MLT showed a neuroprotective effect in brain lesions, decreasing the availability of free iron and reducing the formation of F2‐isoprostanes.31
As a mechanism for the maintenance of cell redox homeostasis, the body uses significant protection systems, such as NAD(P)H:quinone oxidoreductase 1 (NQO1), which provides cellular protection against free radicals, including superoxide anions.32, 33, 34 Paradoxically, despite its “protective” role, an increased NQO1 expression was correlated with various malignant tumors, including gastric, breast, colon, lung, thyroid, and adrenal gland.27, 32 In our study, an increased NQO1 expression was concomitant with an increased LPO in the CCl4 group, which decreased with the use of MLT. Venugopal and Jaiswal35 suggested that an increased NQO1 expression occurs in response to the generation of ROS caused by inflammation or because of the use of xenobiotic compounds. NQO1 is generally related to cellular redox balance by protecting the cell from free radicals, based on the inhibition of one electron reduction of quinones. In the present study, the authors observe a concomitant increase of NQO1 with Isoprostanes (LPO).
The CCl4 induction of oxidative damage stimulated IkB phosphorylation, which releases NF‐κB p65 to act in the nucleus, with an influence on the transcription of target genes and resulting in the production and secretion of pro‐inflammatory cytokines implicated in promoting fibrosis.36, 37, 38 Once activated, NF‐kB stimulates the expression of iNOS with the consequent increase in the production of nitric oxide (NO), a molecule highly involved in toxin‐induced liver injury.38 Similar findings were found in our study, where CCl4 may have induced IkB phosphorylation based on the increased nuclear expression of NF‐κB p65, as well as the concomitant increase in the expression of iNOS. Several studies have shown that MLT modulates the NF‐kB pathway during inflammation, and the NF‐kB modulation changes the expression of the genes involved in the inflammatory process, including iNOS.38 In the present study, the use of MLT reduced the NF‐kB expression and thus reduced the inflammatory cascade, as evidenced by the decreased iNOS expression. Therefore, MLT acted as an anti‐inflammatory agent in this experimental model of liver cirrhosis. These results are supported by histological findings, as HE staining revealed that MLT was able to reduce necrosis, hepatocyte degeneration, and the extensive presence of the inflammatory infiltrate, as a result of exposure to CCl4, showing further evidence of the hepatoprotective effect of MLT. Our findings are in agreement with previous studies showing that the use of MLT, as well as other substances with antioxidant power, reduces the expression of inflammatory mediators during fibrogenesis and hepatocarcinogenesis induced by hepatotoxins.36, 37 Studies have shown that the treatment with MLT reduces the release of NO in the vasculature and attenuates iNOS expression in the liver, as demonstrated in models of sepsis, ischemia/reperfusion, cholestasis, ionizing radiation, and liver injury caused by toxins such as aflatoxin, CCl4, methanol, and thioacetamide.38, 39
When oxidative stress and inflammation are not interrupted, they may stimulate a fibrotic response characterized by irreversible damage to hepatocytes and decline in liver function.40 As a consequence of liver injury, HSC are activated, thus generating increased synthesis of collagen and increased expression of several cytokines, including TGF‐β, which is a potent stimulus for the synthesis of ECM and expansion of fibrosis.6, 27, 36
The transduction of intracellular signals stimulated by TGF‐β results in the stimulation of α‐SMA, an indicator of HSC activation.41, 42 We evaluated the expression of TGF‐β1 and α‐SMA, which were increased in all rats exposed to CCl4 in accordance with the histological study, where picrosirius staining revealed intense fibrosis with formation of thick collagen septa and closed nodes. The MLT treatment significantly decreased TGF‐β1 and α‐SMA expressions and reduced fibrosis, thus causing the formation of incomplete fibrotic septa and nodules. Similar results were found in rats with liver fibrosis induced by bile duct ligation, using pre‐ and posttreatment with brivanib alaninate, sulforaphane, astaxanthin, quercetin, and MLT.43, 44, 45, 46 In fact, the inhibition of this fibrogenesis pathway is a key strategy for the regulation of the differentiation and proliferation of HSC and the subsequent control of ECM deposition in diseases involving fibrogenesis.
Liver fibrosis is also associated with changes in the liver vascular architecture, creating a hypoxic environment, which is an important stimulus for the angiogenesis. In this environment, HSC, which are closely related to the endothelial cells, produce various angiogenic factors, including the VEGF.44, 47 Liver fibrogenesis, angiogenesis, and hypoxia are associated in the tissue repair process and constitute a pathological vicious circle in the development of cirrhosis. Excess availability of angiogenic factors combined with excess deposition of ECM leads to sinusoidal endothelial cell (SEC) capillarization, producing an intrahepatic shunt, increasing resistance to blood flow and reducing the supply of oxygen, thus resulting in hypoxia, an important contributor to liver cirrhosis.48 Our results suggest that the exposure to CCl4 activates HSC, which induces VEGF expression. Nevertheless, those animals treated with MLT showed significantly reduced VEGF expression, suggesting a possible improvement in the angiogenic process and a decrease in the resistance to blood flow.
Our research group has conducted studies on the effects of MLT on liver injury and diseases and found that MLT can regulate various molecular pathways such as inflammation, proliferation, apoptosis, metastasis, and autophagy in different situations.46, 49, 50
Here, our results suggest that MLT has a powerful antifibrogenic effect, modulating the parameters of oxidative stress, angiogenesis, and inflammation. The action of MLT on the improvement of liver fibrosis seems to make it a promising candidate for clinical trials in chronic liver diseases associated with increased fibrogenesis.
Acknowledgments
This study was supported by grants from the following Brazilian agencies: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundo de Incentivo à Pesquisa e Eventos (FIPE)/Hospital de Clínicas de Porto Alegre (HCPA) and Laboratório Experimental de Hepatologia e Gastroenterologia (HCPA/UFRGS), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), and Laboratório de Estresse Oxidativo e Antioxidantes (ULBRA).
Declaration of conflict of interests: None.
Funding support: Laboratório de Estresse Oxidativo e Antioxidantes (ULBRA). Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS). Laboratório Experimental de Hepatologia e Gastroenterologia (HCPA/UFRGS). Fundo de Incentivo à Pesquisa e Eventos (FIPE)/Hospital de Clínicas de Porto Alegre (HCPA). Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
REFERENCES
- 1. Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008; 371: 838–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang JJ, Meng X, Li Y et al Effects of melatonin on liver injuries and diseases. Int. J. Mol. Sci. 2017; 18: 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li S, Tan HY, Wang N, Zhang‐Jin Z, Lixing L, Wong C, Feng Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015; 16: 26087–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Novo E, Parola M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair. 2008; 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Girish KS, Paul M, Thushara RM et al Melatonin elevates apoptosis in human platelets via ROS mediated mitochondrial damage. Biochem. Biophys. Res. Commun. 2013; 438: 198–204. [DOI] [PubMed] [Google Scholar]
- 6. Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008; 88: 125–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arauz J, Zarco N, Segovia J, Shibayama M, Tsutsumi V, Muriel P. Caffeine prevents experimental liver fibrosis by blocking the expression of TGF‐β. Eur. J. Gastroenterol. Hepatol. 2014; 26: 164–73. [DOI] [PubMed] [Google Scholar]
- 8. Pérez Tamayo R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology. 1983; 3: 112–20. [DOI] [PubMed] [Google Scholar]
- 9. Jiménez W, Clária J, Arroyo V, Rodés J. Carbon tetrachloride induced cirrhosis in rats: a useful tool for investigating the pathogenesis of ascites in chronic liver disease. J. Gastroenterol. Hepatol. 1992; 7: 90–7. [DOI] [PubMed] [Google Scholar]
- 10. Recknagel RO, Glende EA, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol. Ther. 1989; 43: 139–54. [DOI] [PubMed] [Google Scholar]
- 11. Comporti M, Signorini C, Arezzini B, Vecchio D, Monaco B, Gardi C. F2‐isoprostanes are not just markers of oxidative stress. Free Radic. Biol. Med. 2008; 44: 247–56. [DOI] [PubMed] [Google Scholar]
- 12. Bona S, Filippin LI, Di Naso FC et al Effect of antioxidant treatment on fibrogenesis in rats with carbon tetrachloride‐induced cirrhosis. ISRN Gastroenterol. 2012; 2012: 762920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reiter RJ, Tan DX, Korkmaz A, Rosales‐Corral SA. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update. 2014; 20(2): 293–307. [DOI] [PubMed] [Google Scholar]
- 14. Reiter RJ, Rosales‐Corral SA, Manchester LC, Liu X, Tan DX. Melatonin in the biliary tract and liver: health implications. Curr. Pharm. Des. 2014; 20: 4788–801. [DOI] [PubMed] [Google Scholar]
- 15. Brzozowski T, Jaworek J. Editorial: basic and clinical aspects of melatonin in the gastrointestinal tract. New advancements and future perspectives. Curr. Pharm. Des. 2014; 20: 4785–7. [DOI] [PubMed] [Google Scholar]
- 16. Mauriz JL, Collado PS, Veneroso C, Reiter RJ, González‐Gallego J. A review of the molecular aspects of melatonin's anti‐inflammatory actions: recent insights and new perspectives. J. Pineal Res. 2012; 54: 1–14. [DOI] [PubMed] [Google Scholar]
- 17. Crespo I, Miguel BS, Laliena A et al Melatonin prevents the decreased activity of antioxidant enzymes and activates nuclear erythroid 2‐related factor 2 signaling in an animal model of fulminant hepatic failure of viral origin. J. Pineal Res. 2010; 49: 193–200. [DOI] [PubMed] [Google Scholar]
- 18. Pavanato A, Tuñón MJ, Sánchez‐Campos S et al Effects of quercetin on liver damage in rats with carbon tetrachloride‐induced cirrhosis. Dig. Dis. Sci. 2003; 48: 824–9. [DOI] [PubMed] [Google Scholar]
- 19. Rosa DP, Bona S, Simonetto D, Zettler C, Marroni CA, Marroni NP. Melatonin protects the liver and erythrocytes against oxidative stress in cirrhotic rats. Arq. Gastroenterol. 2010; 47: 72–8. [DOI] [PubMed] [Google Scholar]
- 20. American Veterinary Medical Association 2013 . AVMA guidelines for the euthanasia of animals. [Cited 27 July 2016]. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf [Google Scholar]
- 21. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal. Biochem. 1976; 72: 248–54. [DOI] [PubMed] [Google Scholar]
- 22. Tuñón MJ, San‐Miguel B, Crespo I et al Melatonin treatment reduces endoplasmic reticulum stress and modulates the unfolded protein response in rabbits with lethal fulminant hepatitis of viral origin. J. Pineal Res. 2013; 55: 221–8. [DOI] [PubMed] [Google Scholar]
- 23. Mauriz JL, Molpeceres V, García‐Mediavilla MV, González P, Barrio JP, González‐Gallego J. Melatonin prevents oxidative stress and changes in antioxidant enzyme expression and activity in the liver of aging rats. J. Pineal Res. 2007; 42: 222–30. [DOI] [PubMed] [Google Scholar]
- 24. Zhang L, Wei W, Xu J et al Inhibitory effect of melatonin on diquat‐induced lipid peroxidation in vivo as assessed by the measurement of F2‐isoprostanes. J. Pineal Res. 2006; 40: 326–31. [DOI] [PubMed] [Google Scholar]
- 25. Wasser S, Lim GY, Ong CN, Tan CE. Anti‐oxidant ebselen causes the resolution of experimentally induced hepatic fibrosis in rats. J. Gastroenterol. Hepatol. 2001; 16: 1244–53. [DOI] [PubMed] [Google Scholar]
- 26. Bona S, Moreira AJ, Oliveira M, Marroni CA, Marroni NP. Cirrose hepática induzida por tetracloreto de carbono: Modelo inalatório e intraperitoneal In: Marroni NP, Morgan‐Martins MI, Porawski M. (eds). Radicais livres no processo saúde‐doença: da bancada à clínica. Curitiba: CRV, 2012; 182. [Google Scholar]
- 27. Bona S, Moreira AJ, Rodrigues GR et al Diethylnitrosamine induced cirrhosis in wistar rats: an experimental feasibility study. Protoplasma. 2015; 252(3): 825–33. [DOI] [PubMed] [Google Scholar]
- 28. Moreira AJ, Rodrigues GR, Bona S et al Ductular reaction, cytokeratin 7 positivity, and gamma‐glutamyl transferase in multistage hepatocarcinogenesis in rats. Protoplasma. 2017; 254(2): 911–20. [DOI] [PubMed] [Google Scholar]
- 29. Moreira AJ, Rodrigues G, Bona S et al Oxidative stress and cell damage in a model of precancerous lesions and advanced hepatocellular carcinoma in rats. Toxicol. Rep. 2015; 2: 333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Briskey DR, Wilson GR, Fassett RG, Coombes JS. Optimized method for quantification of total F(2)‐isoprostanes using gas chromatography‐tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014; 90: 161–6. [DOI] [PubMed] [Google Scholar]
- 31. Signorini C, Ciccoli L, Leoncini S et al Free iron, total F‐isoprostanes and total F‐neuroprostanes in a model of neonatal hypoxic‐ischemic encephalopathy: neuroprotective effect of melatonin. J. Pineal Res. 2009; 46: 148–54. [DOI] [PubMed] [Google Scholar]
- 32. Lin L, Qin Y, Jin T et al Significance of NQO1 overexpression for prognostic evaluation of gastric adenocarcinoma. Exp. Mol. Pathol. 2014; 96: 200–5. [DOI] [PubMed] [Google Scholar]
- 33. Siegel D, Gustafson DL, Dehn DL et al NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol. Pharmacol. 2004; 65: 1238–47. [DOI] [PubMed] [Google Scholar]
- 34. Yang JJ, Tao H, Huang C, Li J. Nuclear erythroid 2‐related factor 2: a novel potential therapeutic target for liver fibrosis. Food Chem. Toxicol. 2013; 59: 421–7. [DOI] [PubMed] [Google Scholar]
- 35. Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c‐Fos and Fra1 negatively regulate the human antioxidant response element‐mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA. 1996; 93: 14960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heeba GH, Mahmoud ME. Therapeutic potential of morin against liver fibrosis in rats: modulation of oxidative stress, cytokine production and nuclear factor kappa B. Environ. Toxicol. Pharmacol. 2014; 37: 662–71. [DOI] [PubMed] [Google Scholar]
- 37. Hsieh WT, Liu YT, Lin WC. Anti‐inflammatory properties of Ajuga bracteosa in vivo and in vitro study and their effects on mouse model of liver fibrosis. J. Ethnopharmacol. 2011; 135: 116–25. [DOI] [PubMed] [Google Scholar]
- 38. Mauriz JL, Collado PS, Veneroso C, Reiter RJ, González‐Gallego J. A review of the molecular aspects of melatonin's anti‐inflammatory actions: recent insights and new perspectives. J. Pineal Res. 2013; 54: 1–14. [DOI] [PubMed] [Google Scholar]
- 39. Mathes AM. Hepatoprotective actions of melatonin: possible mediation by melatonin receptors. World J. Gastroenterol. 2010; 16: 6087–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J. Hepatol. 2013; 59: 583–94. [DOI] [PubMed] [Google Scholar]
- 41. Gressner OA, Weiskirchen R, Gressner AM. Biomarkers of liver fibrosis: clinical translation of molecular pathogenesis or based on liver‐dependent malfunction tests. Clin. Chim. Acta. 2007; 381: 107–13. [DOI] [PubMed] [Google Scholar]
- 42. Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF‐beta in hepatic fibrosis. Front. Biosci. 2002; 7: d793–807. [DOI] [PubMed] [Google Scholar]
- 43. Oh CJ, Kim JY, Min AK et al Sulforaphane attenuates hepatic fibrosis via NF‐E2‐related factor 2‐mediated inhibition of transforming growth factor‐β/Smad signaling. Free Radic. Biol. Med. 2012; 52: 671–82. [DOI] [PubMed] [Google Scholar]
- 44. Lin HC, Huang YT, Yang YY et al Beneficial effects of dual vascular endothelial growth factor receptor/fibroblast growth factor receptor inhibitor brivanib alaninate in cirrhotic portal hypertensive rats. J. Gastroenterol. Hepatol. 2014; 29: 1073–82. [DOI] [PubMed] [Google Scholar]
- 45. Shen M, Chen K, Lu J et al Protective effect of astaxanthin on liver fibrosis through modulation of TGF‐β1 expression and autophagy. Mediat. Inflamm. 2014; 2014: 954502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Colares JR, Schemitt EG, Hartmann RM et al Antioxidant and anti‐inflammatory action of melatonin in an experimental model of secondary biliary cirrhosis induced by bile duct ligation. World J. Gastroenterol. 2016; 22: 8918–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract. Res. Clin. Gastroenterol. 2011; 25: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mallat A, Lotersztajn S. Cellular mechanisms of tissue fibrosis. 5. Novel insights into liver fibrosis. Am. J. Physiol. Cell Physiol. 2013; 305: C789–99. [DOI] [PubMed] [Google Scholar]
- 49. Salvi JO, Schemitt EG, Colares JR et al Action of melatonin on severe acute liver failure in rats. IOSR J. Pharm. Biol. Sci. 2017; 12: 62–75. [Google Scholar]
- 50. Moreira AJ, Ordoñez R, Cerski CT et al Melatonin Activates Endoplasmic Reticulum Stress and Apoptosis in Rats with Diethylnitrosamine‐Induced Hepatocarcinogenesis. PLoS One. 2015; 10: e0144517. [DOI] [PMC free article] [PubMed] [Google Scholar]


