Abstract
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second leading cause of cancer‐related mortality worldwide. Non‐alcoholic fatty liver disease (NAFLD) encompasses a spectrum of liver pathology that is characterized by the excessive accumulation of fat in the liver attributable to overnutrition and is strongly associated with the metabolic syndrome. Non‐alcoholic steatohepatitis is the more severe form of NAFLD that is defined histologically by the presence of lobular inflammation and hepatocyte ballooning. Non‐alcoholic steatohepatitis patients have a greater tendency to develop advanced liver fibrosis, cirrhosis, and HCC. This review focuses on the epidemiology of NAFLD‐related HCC and its implications. NAFLD has been estimated to contribute to 10–12% of HCC cases in Western populations and 1–6% of HCC cases in Asian populations. NAFLD‐related HCC is expected to increase in Asian populations, in line with the increased prevalence of NALFD similar to that of Western populations in recent years. The increasing burden of NAFLD‐related HCC over time has been demonstrated in studies from both Western and Asian populations. Certain factors such as ethnicity, obesity, and diabetes mellitus appear to have an incremental effect on the risk of developing HCC among NAFLD patients. The difficulty in identifying NAFLD patients with cirrhosis and the possibility of HCC developing in noncirrhotic NAFLD patients are challenges that need to be addressed. Further understanding of these gaps may contribute to better surveillance strategies for the early detection of HCC in NAFLD patients to reduce the mortality and improve the survival of these patients.
Keywords: epidemiology, hepatocellular carcinomanon‐alcoholic fatty liver diseasenon‐alcoholic fatty liver disease‐related hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second leading cause of cancer‐related mortality worldwide. The burden of HCC is highest in East and Southeast Asia and Northern and Western Africa and lowest in South‐Central Asia and Northern, Central, and Eastern Europe.1 HCC mainly develops in patients with chronic liver disease and cirrhosis, which may be due to various etiologies, including chronic infection with the hepatitis B virus or the hepatitis C virus and alcohol. In recent years, non‐alcoholic fatty liver disease (NAFLD) has emerged as one of the leading causes of HCC. NAFLD encompasses a spectrum of liver pathology that is characterized by the excessive accumulation of fat in the liver. NAFLD can be attributed to overnutrition and is strongly associated with obesity, insulin resistance, glucose intolerance, atherogenic dyslipidemia, and arterial hypertension.2 The prevalence of NAFLD has risen along with the increasing prevalence of obesity and is now recognized as the most common cause of chronic liver disease, affecting an estimated 25% of the general population worldwide.3 Non‐alcoholic steatohepatitis (NASH) is the more severe form of NAFLD that is defined histologically by the presence of lobular inflammation and hepatocyte ballooning. While the prevalence of NAFLD is high, only a small proportion of NAFLD patients have NASH. Studies have shown that NASH patients have a greater tendency to develop advanced liver fibrosis and cirrhosis and are at an increased risk of HCC.4, 5, 6 The purpose of this paper is to provide an up‐to‐date review of the current literature on the epidemiology of NAFLD‐related HCC.
Methods
We performed a PubMed search using the MeSH terms “non‐alcoholic fatty liver disease” or “fatty liver” and “hepatocellular carcinoma” in May 2017. The search yielded 674 articles. Of these, 580 were original articles. The abstracts of the articles were examined, and where doubt existed as to the relevance of an article to the review, the full paper was examined. In total, 35 articles were deemed relevant and were included in this review.
Results
Epidemiology of HCC and NAFLD‐related HCC
The global HCC BRIDGE (“Bridge to Better Outcomes in HCC”) study was the first multiregional, large‐scale, longitudinal cohort study looking at HCC patient experience from diagnosis to death.7 The study recruited 18 031 patients newly diagnosed with HCC from 42 sites in 14 countries from 2005 to 2012. While the primary objective of the study was to provide an improved understanding of HCC treatment and outcomes in real‐world clinical practice across the different regions of the world, it provided some important epidemiological observations. First, it confirmed previously reported regional trends in HCC risk factors, whereby the most common risk factor was hepatitis C virus (HCV) in North America, Europe, and Japan and hepatitis B virus (HBV) in China, South Korea, and Taiwan. It also confirmed that HCC occurred up to four times more commonly in males compared with females. In addition, the study noted that the number of HCC cases was relatively lower, and the age at diagnosis was older in Taiwan compared with China and South Korea. These observations were attributed to the success of the universal HBV vaccination program in Taiwan. Last but not least, the study also clearly demonstrated that NAFLD contributed to a larger proportion of HCC cases in North America and Europe (10–12%) when compared with their Asian counterparts (1–6%). This may be explained by the differences in the etiology of chronic liver diseases in the different parts of the world. The prevalence of NAFLD‐related HCC in the general population is not well documented, with most studies reporting only the proportion of NAFLD‐related HCC in single or multiple institutions and a few studies utilizing a large transplant database or cancer registry to look into the impact of NAFLD‐related HCC in the respective countries where the studies were carried out. A summary of these studies can be found in Table 1.7, 8, 9, 10, 11, 12, 13 These studies are further discussed in the sections that follow.
Table 1.
Studies on the epidemiology of NAFLD‐related HCC
| Author | Study method and population (place) | Study period | Findings |
|---|---|---|---|
| Park et al.7 | Retrospective study; 18 301 HCC patients from 14 countries (67% from Asia, 20% from Europe, 13% from North America) | 2005–2012 | Proportion of NASH‐related HCC: North America 12%, Europe 10%, China 1%, Taiwan 5%, South Korea 6%, Japan 2% |
| Younossi et al.8 | Retrospective study using the SEER registries; 4979 patients with HCC; 701 had NAFLD‐related HCC (USA) | 2004–2009 | 9% yearly increase in the number of NAFLD‐related HCC |
| Wong et al.9 | Retrospective study using the United Network for Organ Sharing registry; 10 061 HCC patients who underwent liver transplantation; 807 had NAFLD‐related HCC (USA) | 2002–2012 | The proportion of NASH‐related HCC increased from 0% in 2002 to 4% in 2007 and 6% in 2012 |
| Dyson et al.10 | Retrospective study 632 patients with HCC referred to the Newcastle‐upon‐Tyne Hospitals NHS Foundation Trust; 136 had NAFLD‐related HCC (UK) | 2000–2010 | The proportion of NAFLD‐related HCC increased >10‐fold to 35% in 2010 |
| Tateishi et al.11 | 33 782 HCC patients from 53 participating centers | 1991–2010 | The proportion of patients with nonviral etiologies increased from 10% in 1991 to 24.1% in 2010. The proportion of patients with NAFLD among those with nonviral etiologies was 11.2%, while 54% was unclassified (but believed to be largely due to NAFLD) |
| Cho et al.12 | 329 patients with non‐B, non‐C, non‐alcohol, or specific cause‐related HCC | 2001–2010 | The proportion of NAFLD‐related HCC increased from 3.8% in 2001–2005 to 12.2% in 2006–2010 |
| Beste et al.13 | Retrospective study, 17 457 incident HCC cases in the Veteran Affairs database; 15% had NAFLD‐related HCC (USA) | 2002–2012 | Incident NAFLD‐related HCC increased from 3.92 per 100 000 in 2002 to 6.16 per 100 000 in 2012 |
HCC, hepatocellular carcinoma; NAFLD, non‐alcoholic fatty liver disease; NASH, non‐alcoholic steatohepatitis; SEER, Surveillance, Epidemiology and End Results.
Increasing prevalence of NAFLD and its impact on HCC
A recent meta‐analysis has shown that the prevalence of NAFLD is now comparable between the West and the East.3 Obesity, previously largely a problem of the Western world, has rapidly emerged in Asia over the past few decades due to globalization and rapid urbanization. The ensuing changes in the dietary and lifestyle habits of the people inadvertently promoted positive energy balance and fueled the rise of the obesity epidemic.14 In China, for example, the number of obese people has increased from below 0.1 million in 1975 to over 43.2 million in 2014, accounting for 16.3% of obese people worldwide. In India, the number of obese people increased from 0.4 million to 9.8 million during the same period.15 The result of this rapid increase in the prevalence of obesity is a dramatic rise in obesity‐related diseases, including NAFLD. Consequently, NAFLD‐related HCC is expected to become increasingly common in the Asia‐Pacific region. The relative contribution of NAFLD toward HCC cases will only escalate with time, not only from the increasing prevalence of NAFLD but also from the anticipated decreasing burden of HBV and HCV infections as a result of more effective preventive strategies, case finding, and treatment of these other etiologies of chronic liver disease. While primary, secondary, and tertiary preventive strategies for HCC due to chronic HBV and HCV infections are somewhat in place, similar strategies for NAFLD‐related HCC are lacking. This calls for more intensified research into NAFLD and NAFLD‐related HCC to help formulate effective preventive strategies.
Several reports have already demonstrated the increasing burden of NAFLD‐related HCC (Fig. 1). For example, in the first large, population‐based study on NAFLD‐related HCC, which utilized Surveillance, Epidemiology and End Results (SEER) registries in the United States, a 9% annual increase in the proportion of NAFLD‐related HCC cases was noted between 2004 and 2009.8 Another study using data from the United Network for Organ Sharing (UNOS) registry noted a nearly fourfold increase in NASH‐related HCC from 2002 to 2012.9 In the United Kingdom, Dyson et al. reported a 10‐fold increase in the proportion of NAFLD‐related HCC from 2000 to 2010.10 Meanwhile, epidemiological studies on NAFLD‐related HCC from Asia were scarce and largely originated from Japan and South Korea. A large multicenter retrospective study in Japan reported that the proportion of HCC patients due to nonviral etiologies has increased from 10.0% in 1991 to 24.1% in 2010. They attributed this finding to the increasingly obese population and the rising proportion of patients with diabetes mellitus as a result of dietary changes.11 A study in Korea has also highlighted the increasing proportion of NAFLD‐related HCC over time.12
Figure 1.
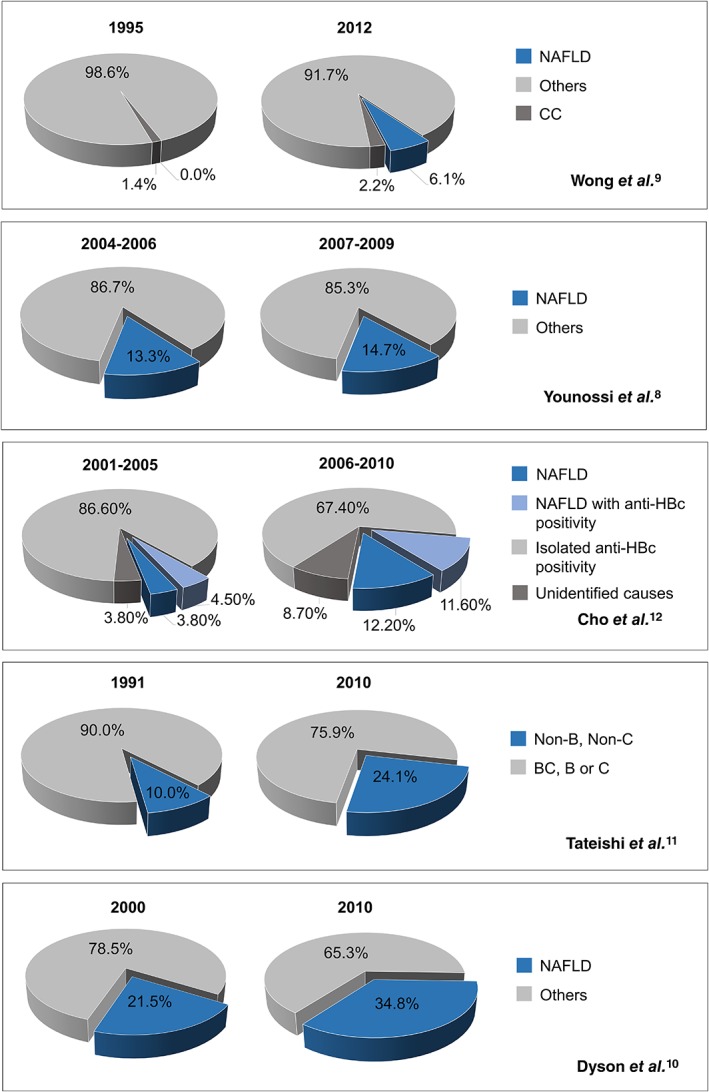
The proportion of non‐alcoholic fatty liver disease‐related hepatocellular carcinoma at different time points based on studies from the United States, United Kingdom, Japan, and Korea.
Incidence of HCC in NAFLD patients
Several studies followed at‐risk patients and reported on the incidence of NAFLD‐related HCC. These studies are summarized in Table 2.16, 17, 18, 19, 20, 21 Studies from both the West and the Asia‐Pacific regions have consistently reported a lower incidence of HCC among NAFLD patients as compared with other liver diseases, such as HCV.16, 17, 18, 20 In the United States, a large, single‐center study of 510 patients with cirrhosis by Ascha et al. reported an annual cumulative incidence of HCC of 2.6% in patients with NASH cirrhosis, compared with 4.0% in patients with HCV cirrhosis.16 This study also clearly demonstrated that any alcohol consumption was associated with an increased risk of HCC for both HCV and NASH patients with cirrhosis. In a separate study from Japan, Yatsuji et al. reported a 5‐year HCC rate of 11.3% for NASH cirrhosis and 30.5% for HCV cirrhosis.20 Nevertheless, the incidence rate shown in these two studies did exceed the 1.5% per year threshold to recommend HCC surveillance, with the aim of reducing mortality and improving survival from incident HCC in patients with NASH cirrhosis.22 On the other hand, HCC may occur in NAFLD patients without cirrhosis (see below). However, the incidence of such cases is low. For example, in a large‐scale retrospective study of Japanese patients with NAFLD, the annual incidence rate of HCC was only 0.043%.21 Therefore, it is neither practical nor cost‐effective to recommend HCC surveillance for noncirrhotic NAFLD patients, and further studies are needed to identify noncirrhotic NAFLD patients who are at increased risk of HCC who will benefit from a HCC surveillance program. In the meantime, all patients with NASH‐related cirrhosis whose general condition and liver function are sufficiently good to allow intervention should receive surveillance for HCC so that curative therapy is more likely to be possible with early diagnosis, while HCC surveillance in patients with advanced fibrosis may be on a case‐by‐case basis. The increasing use of transient elastography, which allows simple and repeated noninvasive assessment of liver fibrosis, will likely change the way patients with chronic liver disease are selected for HCC surveillance, but more data and a consensus on the cut‐off value to be used are needed.
Table 2.
Studies on the incidence of NAFLD‐related HCC in specific populations
| Author | Study method and population (place) | Study period | Findings |
|---|---|---|---|
| Ascha et al.16 | Prospective study; 510 patients (HCV, n = 315; NASH, n = 195) with cirrhosis at the Cleveland Clinic; median follow‐up of 3.2 years (USA) | 2003–2007 | Annual cumulative incidence of HCC was 2.6% in patients with NASH cirrhosis and 4% in patients with HCV cirrhosis |
| Paranagua‐Vezozzo et al.17 | Prospective study; 884 patients with cirrhosis at the Sao Paulo School of Medicine; median follow‐up of 21.4 months; HCV, 57.6%; NASH, 3.1% (Brazil) | 1998–2008 | Incident HCC was 16.9% for HCV cirrhosis and 4% for NASH cirrhosis at 5 years |
| Amarapurkar et al.18 | Prospective study; 585 patients with cirrhosis at the Bombay Hospital and Medical Research Centre; HCV, 14.2%; NASH, 7% (India) | 2010–2011 | Annual incidence of HCC was 3.6% for HCV cirrhosis and 0.46% for NASH cirrhosis |
| Hashimoto et al.19 | Prospective study; 137 NASH patients with advanced fibrosis at the Tokyo Women's Medical University (Japan) | 1990–2007 | The 5‐year cumulative incidence of HCC was 7.6% |
| Yatsuji et al.20 | Prospective study; 68 patients with NASH cirrhosis and 69 patients with HCV cirrhosis at the Tokyo Women's Medical University (Japan) | 1990–2006 | The 5‐year cumulative incidence of HCC was 11.3% for NASH cirrhosis and 30.5% for HCV cirrhosis |
| Kawamura et al.21 | Retrospective cohort study of 6508 patients with ultrasonography‐diagnosed NAFLD | 1997–2010 | Annual incidence of HCC was 0.043%. |
HCC, hepatocellular carcinoma; NAFLD, non‐alcoholic fatty liver disease; NASH, non‐alcoholic steatohepatitis; HCV, hepatitis C virus.
Burden and survival outcomes of NAFLD‐related HCC
Despite the relatively lower incidence of HCC among NAFLD patients as compared to other liver diseases, such as HBV and HCV, the high and increasing prevalence of NAFLD has made NAFLD a major contributor to chronic liver disease and HCC cases. For example, NASH has demonstrated the greatest increase and has become the second leading etiology of chronic liver disease among new liver transplant waitlist registrants in the United States.23 NASH is also the most rapidly growing indication and the second leading etiology for liver transplantation among HCC patients in the United States.9 NAFLD‐related HCC patients had significantly shorter survival, and a significantly larger proportion of NAFLD‐related HCC patients died within 1 year of diagnosis compared with HCC related to viral hepatitis. In fact, having NAFLD was an independent factor associated with the risk of death within the first year after diagnosis of HCC. There are several postulations for this seemingly poorer prognosis of NAFLD‐related HCC, including lack of HCC screening for NAFLD patients, limitation of ultrasound to detect small tumors in NAFLD patients (due to the associated obesity), and the development of HCC in noncirrhotic NAFLD patients (see below). Moreover, NAFLD‐related HCC patients had significantly higher cardiovascular disease and were less likely to receive a liver transplant.8
Pitfalls for consideration in epidemiological studies on NAFLD‐related HCC
There are several pitfalls to be considered when conducting epidemiological studies on NAFLD‐related HCC. First, patients with NAFLD are less likely to be transplanted compared to patients with other liver diseases such as viral hepatitis due to their multiple comorbidities and slower progression of the disease.8, 24 Hence, data from the liver transplant registry could be an underestimation of the true magnitude and impact of NAFLD‐related HCC. Second, it is now recognized that the majority of cases of cryptogenic cirrhosis are in fact the advanced stage of NASH, so called “burnt‐out” NASH. Cryptogenic cirrhosis shares many similarities with NASH‐related cirrhosis as both are strongly associated with diabetes mellitus and obesity.25, 26, 27, 28 The increase in visceral fat area and the negative correlation with liver–spleen density ratio measurements further support the theory that most cryptogenic HCC cases are burnt‐out NASH.29 Although most of the histological hallmarks of NASH are not present in cryptogenic cirrhosis, Marrero et al. reported that half of the patients with cryptogenic cirrhosis had prior histological or clinical features associated with NAFLD.30 Hence, the inclusion of only those cases of HCC with a current diagnosis of NAFLD as NAFLD‐related HCC will likely contribute further to the underestimation of NAFLD‐related HCC.25, 31, 32 In order to capture a more accurate estimation of the burden of NASH‐related HCC, Charlton et al. and Wong et al. utilized modified NASH categories (NASH plus 50% of cryptogenic cirrhosis and NASH plus cryptogenic cirrhosis with BMI greater than 30 kg/m2, respectively) in their studies.9, 33 Wong et al. noted that modified NASH category as an etiology of HCC‐related liver transplantation increased significantly over time, and about 25–50% of cryptogenic cirrhosis patients were included into the modified NASH category.9 Last but not least, a large proportion of non‐B, non‐C, non‐alcohol, or specific cause‐related HCC cases in a HBV endemic area actually had serological evidence of prior HBV infection in the form of isolated anti‐HBc IgG positivity. Therefore, in an HBV endemic area, the strategy of including obese HCC patients with cryptogenic cirrhosis without determining anti‐HBc IgG to estimate the burden of NAFLD‐related HCC may not be as appropriate as in other populations where such occurrences would be rare.12
NAFLD‐related HCC and ethnicity
Ethnicity appears to play a role in NAFLD‐related HCC. Couto et al.34 noted that Hispanics were more likely to develop NAFLD‐related HCC compared to non‐Hispanics. Earlier studies have reported that Hispanics were more predisposed to NASH,35, 36, 37 while Altekruse et al. found an increased risk of HCC among Hispanics.38 In addition, US‐born Hispanics had a higher incidence of HCC when compared to foreign‐born Hispanics, while foreign‐born Asians had a higher incidence of HCC than US‐born, suggesting that environmental, socioeconomic, and cultural differences may be contributing factors as well.39 In studies on multiethnic populations in Malaysia,40, 41 the prevalence of NAFLD has been found to be consistently higher among the Malays and the Indians compared with the Chinese, and this ethnic preponderance could be observed as early as young adulthood.42 The etiology of cirrhosis and HCC in the different ethnic groups also mirrored this ethnic preponderance whereby cryptogenic cause, which is now recognized to be associated with NASH in the majority of cases, was more pronounced among the Malays and the Indians compared with the Chinese.43
Obesity, diabetes mellitus, and HCC
Obesity and diabetes mellitus are metabolic comorbidities closely associated with NAFLD that have been found to be independently associated with HCC. In a study utilizing the UNOS database on liver transplantations from 1991 to 2000, which included 19 271 patients, Nair et al. found obesity to be an independent risk factor for HCC.44 However, the risk appeared to be primarily associated with cryptogenic cirrhosis and alcoholic liver disease only. The reason for this disease‐specific association of obesity and HCC is not entirely clear and deserves further exploration. A prospective multicenter study of HCC patients in Italy showed that 73.1% of NAFLD‐related HCC patients have diabetes mellitus compared with only 24.9% of HCV‐related HCC patients.45 In a subsequent larger cohort study from the United States, NAFLD‐related HCC patients were again found to be more likely to have diabetes mellitus compared with HCV‐related HCC patients.46 In another study from the Mayo Clinic, the hazard of HCC among non‐HCV patients was found to be two times higher in patients with diabetes mellitus compared with those without diabetes mellitus.47 In a separate study, diabetes mellitus was also found to be an independent factor associated with HCC among patients with cryptogenic cirrhosis.25 While there is currently much focus on pharmacotherapy for NASH, there is an urgent need for effective public health measures to tackle obesity and the metabolic syndrome as a whole. The latter would have the downstream effect of curbing not only NAFLD‐related HCC but also other associated diseases, especially cardiovascular diseases, which is the leading cause of mortality in these patients.
Development of HCC in noncirrhotic NAFLD
As mentioned earlier, HCC can develop in NAFLD patients in the absence of cirrhosis. Several studies have shown that as much as 40–47% of NAFLD‐related HCC patients had no clinical or histological evidence of cirrhosis.45, 48, 49, 50 A multicenter prospective study in Italy comparing NAFLD‐related HCC and HCV‐related HCC reported that cirrhosis was present in only about 50% of NAFLD‐related HCC, in contrast with the totality of HCV‐related HCC.45 Ertle et al. and Schutte et al. reported that the majority of noncirrhotic HCC patients had NAFLD and the metabolic syndrome, suggesting that NASH per se could promote the development of HCC in the absence of cirrhosis.49, 51 Moreover, Alexander et al. reported a significant association between steatosis and/or steatohepatitis with HCC in 157 HCC patients who had no histological features of cirrhosis.52 This further supports the hypothesis that steatohepatitis alone could have a causative role in hepatocarcinogenesis in noncirrhotic NAFLD. However, in contrast, a study comparing cirrhotic and noncirrhotic NAFLD‐related HCC reported that noncirrhotic patients were less likely to be obese and less likely to have metabolic syndrome and diabetes mellitus.50 However, the relatively small sample size could have impacted the findings from this retrospective study. In terms of clinicopathological findings, most studies agreed that noncirrhotic NAFLD‐related HCC patients were more likely to present with larger tumors.50, 51, 53 This may be due to the lack of HCC screening in noncirrhotic NAFLD patients. Edenvik et al. reported deficiency in HCC surveillance among NAFLD patients, with only 13% of HCC discovered through surveillance, resulting in delayed diagnosis in the majority of patients.54 However, it is not practical to recommend HCC surveillance for all NAFLD patients due to the high prevalence of the disease and the very low incidence of HCC in noncirrhotic NAFLD patients (see above). Another challenge is that cirrhosis may be missed in NAFLD patients as features of cirrhosis may be obscured by fatty liver on ultrasonography. Therefore, NAFLD patients should be carefully assessed for the severity of their liver disease, for example, with the use of noninvasive scores and transient elastography, so that NAFLD patients with cirrhosis who would otherwise have been missed by ultrasonography could be offered HCC surveillance.
Conclusions
While NAFLD‐related HCC is already a major problem in the Western world, it is an emerging threat in the East as the burden of NAFLD increases. Certain factors such as ethnicity, obesity, and diabetes mellitus appear to have an incremental effect on the risk of developing HCC among NAFLD patients, and this deserves further studies. The inherent difficulty in identifying NAFLD patients with cirrhosis and the possibility of HCC developing in noncirrhotic NAFLD patients are other challenges that need to be addressed. Further understanding of these gaps are important and may contribute to better surveillance strategies for early detection of HCC in NAFLD patients to reduce the mortality and improve the survival of these patients.
Declaration of conflict of interest: The authors declare that they have no competing interests.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Wong VW, Chan WK, Chitturi S et al The Asia‐Pacific Working Party on Nonalcoholic Fatty Liver Disease Guidelines 2017 Part 1: definition, risk factors and assessment. J. Gastroenterol. Hepatol. 2018; 33: 70–85. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016; 64: 73–84. [DOI] [PubMed] [Google Scholar]
- 4. Adams LA, Lymp JF, St Sauver J et al The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology. 2005; 129: 113–21. [DOI] [PubMed] [Google Scholar]
- 5. Jansen PL. Non‐alcoholic steatohepatitis. Eur. J. Gastroenterol. Hepatol. 2004; 16: 1079–85. [DOI] [PubMed] [Google Scholar]
- 6. Nagaoki Y, Hyogo H, Aikata H et al Recent trend of clinical features in patients with hepatocellular carcinoma. Hepatol. Res. 2012; 42: 368–75. [DOI] [PubMed] [Google Scholar]
- 7. Park JW, Chen M, Colombo M et al Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015; 35: 2155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Younossi ZM, Otgonsuren M, Henry L et al Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015; 62: 1723–30. [DOI] [PubMed] [Google Scholar]
- 9. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014; 59: 2188–95. [DOI] [PubMed] [Google Scholar]
- 10. Dyson J, Jaques B, Chattopadyhay D et al Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 2014; 60: 110–17. [DOI] [PubMed] [Google Scholar]
- 11. Tateishi R, Okanoue T, Fujiwara N et al Clinical characteristics, treatment, and prognosis of non‐B, non‐C hepatocellular carcinoma: a large retrospective multicenter cohort study. J. Gastroenterol. 2015; 50: 350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cho EJ, Kwack MS, Jang ES et al Relative etiological role of prior hepatitis B virus infection and nonalcoholic fatty liver disease in the development of non‐B non‐C hepatocellular carcinoma in a hepatitis B‐endemic area. Digestion. 2011; 84 (Suppl. 1): 17–22. [DOI] [PubMed] [Google Scholar]
- 13. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001‐2013. Gastroenterology. 2015; 149: 1471–82.e5; quiz e17–18. [DOI] [PubMed] [Google Scholar]
- 14. Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013; 9: 13–27. [DOI] [PubMed] [Google Scholar]
- 15. Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J. Hepatol. 2017; 67: 862–73. [DOI] [PubMed] [Google Scholar]
- 16. Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010; 51: 1972–8. [DOI] [PubMed] [Google Scholar]
- 17. Paranagua‐Vezozzo DC, Ono SK, Alvarado‐Mora MV et al Epidemiology of HCC in Brazil: incidence and risk factors in a ten‐year cohort. Ann. Hepatol. 2014; 13: 386–93. [PubMed] [Google Scholar]
- 18. Amarapurkar DN, Dharod M, Gautam S, Patel N. Risk of development of hepatocellular carcinoma in patients with NASH‐related cirrhosis. Trop. Gastroenterol. 2013; 34: 159–63. [DOI] [PubMed] [Google Scholar]
- 19. Hashimoto E, Yatsuji S, Tobari M et al Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J. Gastroenterol. 2009; 44 (Suppl. 19): 89–95. [DOI] [PubMed] [Google Scholar]
- 20. Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non‐alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J. Gastroenterol. Hepatol. 2009; 24: 248–54. [DOI] [PubMed] [Google Scholar]
- 21. Kawamura Y, Arase Y, Ikeda K et al Large‐scale long‐term follow‐up study of Japanese patients with non‐alcoholic fatty liver disease for the onset of hepatocellular carcinoma. Am. J. Gastroenterol. 2012; 107: 253–61. [DOI] [PubMed] [Google Scholar]
- 22. Bruix J, Sherman M, American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53: 1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wong RJ, Aguilar M, Cheung R et al Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015; 148: 547–55. [DOI] [PubMed] [Google Scholar]
- 24. O'Leary JG, Landaverde C, Jennings L, Goldstein RM, Davis GL. Patients with NASH and cryptogenic cirrhosis are less likely than those with hepatitis C to receive liver transplants. Clin. Gastroenterol. Hepatol. 2011; 9: 700–704.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bugianesi E, Leone N, Vanni E et al Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002; 123: 134–40. [DOI] [PubMed] [Google Scholar]
- 26. Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999; 29: 664–9. [DOI] [PubMed] [Google Scholar]
- 27. Kojima H, Sakurai S, Matsumura M et al Cryptogenic cirrhosis in the region where obesity is not prevalent. World J. Gastroenterol. 2006; 12: 2080–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case‐control study. Hepatology. 2000; 32 (4 Pt 1): 689–92. [DOI] [PubMed] [Google Scholar]
- 29. Lee SS, Jeong SH, Byoun YS et al Clinical features and outcome of cryptogenic hepatocellular carcinoma compared to those of viral and alcoholic hepatocellular carcinoma. BMC Cancer. 2013; 13: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002; 36: 1349–54. [DOI] [PubMed] [Google Scholar]
- 31. Caldwell SH, Lee VD, Kleiner DE et al NASH and cryptogenic cirrhosis: a histological analysis. Ann. Hepatol. 2009; 8: 346–52. [PMC free article] [PubMed] [Google Scholar]
- 32. Tiniakos DG. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis: histological diagnostic criteria and scoring systems. Eur. J. Gastroenterol. Hepatol. 2010; 22: 643–50. [DOI] [PubMed] [Google Scholar]
- 33. Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011; 141: 1249–53. [DOI] [PubMed] [Google Scholar]
- 34. Couto CA, Gelape CL, Calmet F, Martin P, Levy C. Effect of ethnicity on liver transplant for hepatocellular carcinoma. Exp. Clin. Transplant. 2013; 11: 339–45. [DOI] [PubMed] [Google Scholar]
- 35. Browning JD, Szczepaniak LS, Dobbins R et al Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004; 40: 1387–95. [DOI] [PubMed] [Google Scholar]
- 36. Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009; 49: 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williams CD, Stengel J, Asike MI et al Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle‐aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011; 140: 124–31. [DOI] [PubMed] [Google Scholar]
- 38. Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am. J. Gastroenterol. 2014; 109: 542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang ET, Yang J, Alfaro‐Velcamp T, So SK, Glaser SL, Gomez SL. Disparities in liver cancer incidence by nativity, acculturation, and socioeconomic status in California Hispanics and Asians. Cancer Epidemiol. Biomarkers Prev. 2010; 19: 3106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chan WK, Tan AT, Vethakkan SR, Tah PC, Vijayananthan A, Goh KL. Non‐alcoholic fatty liver disease in diabetics – prevalence and predictive factors in a multiracial hospital clinic population in Malaysia. J. Gastroenterol. Hepatol. 2013; 28: 1375–83. [DOI] [PubMed] [Google Scholar]
- 41. Goh SC, Ho EL, Goh KL. Prevalence and risk factors of non‐alcoholic fatty liver disease in a multiracial suburban Asian population in Malaysia. Hepatol. Int. 2013; 7: 548–54. [DOI] [PubMed] [Google Scholar]
- 42. Chan WK, Bahar N, Razlan H, Vijayananthan A, Sithaneshwar P, Goh KL. Non‐alcoholic fatty liver disease in a young multiracial Asian population: a worrying ethnic predilection in Malay and Indian males. Hepatol. Int. 2014; 8: 121–7. [DOI] [PubMed] [Google Scholar]
- 43. Qua CS, Goh KL. Liver cirrhosis in Malaysia: peculiar epidemiology in a multiracial Asian country. J. Gastroenterol. Hepatol. 2011; 26: 1333–7. [DOI] [PubMed] [Google Scholar]
- 44. Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002; 36: 150–5. [DOI] [PubMed] [Google Scholar]
- 45. Piscaglia F, Svegliati‐Baroni G, Barchetti A et al Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology. 2016; 63: 827–38. [DOI] [PubMed] [Google Scholar]
- 46. Mittal S, Sada YH, El‐Serag HB et al Temporal trends of nonalcoholic fatty liver disease‐related hepatocellular carcinoma in the veteran affairs population. Clin. Gastroenterol. Hepatol. 2015; 13: 594–601.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang JD, Mohamed HA, Cvinar JL, Gores GJ, Roberts LR, Kim WR. Diabetes mellitus heightens the risk of hepatocellular carcinoma except in patients with hepatitis C cirrhosis. Am. J. Gastroenterol. 2016; 111: 1573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Duan XY, Qiao L, Fan JG. Clinical features of nonalcoholic fatty liver disease‐associated hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. 2012; 11: 18–27. [DOI] [PubMed] [Google Scholar]
- 49. Ertle J, Dechene A, Sowa JP et al Non‐alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int. J. Cancer. 2011; 128: 2436–43. [DOI] [PubMed] [Google Scholar]
- 50. Mohamad B, Shah V, Onyshchenko M et al Characterization of hepatocellular carcinoma (HCC) in non‐alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol. Int. 2016; 10: 632–9. [DOI] [PubMed] [Google Scholar]
- 51. Schutte K, Schulz C, Poranzke J et al Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non‐cirrhotic liver. BMC Gastroenterol. 2014; 14: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alexander J, Torbenson M, Wu TT, Yeh MM. Non‐alcoholic fatty liver disease contributes to hepatocarcinogenesis in non‐cirrhotic liver: a clinical and pathological study. J. Gastroenterol. Hepatol. 2013; 28: 848–54. [DOI] [PubMed] [Google Scholar]
- 53. Leung C, Yeoh SW, Patrick D et al Characteristics of hepatocellular carcinoma in cirrhotic and non‐cirrhotic non‐alcoholic fatty liver disease. World J. Gastroenterol. 2015; 21: 1189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Edenvik P, Davidsdottir L, Oksanen A, Isaksson B, Hultcrantz R, Stal P. Application of hepatocellular carcinoma surveillance in a European setting. What can we learn from clinical practice? Liver Int. 2015; 35: 1862–71. [DOI] [PubMed] [Google Scholar]


