Abstract
Background and Aim
Alterations in nutrient metabolism, nutritional requirements, and reduced dietary intakes are common in chronic liver disease (CLD). These result in malnutrition, sarcopenia, and exacerbate progression to decompensation and ascites. We aimed to investigate the effects of continuous tube feeding (TF) on nutritional status and levels of ascites in malnourished individuals with decompensated cirrhosis.
Methods
Fourteen malnourished patients with decompensated cirrhosis and ascites who failed to respond to standard oral nutritional interventions received supplementary continuous nasogastric TF for 7 ± 1 weeks. Liver disease severity was assessed by model for end‐stage liver disease (MELD) and Child–Turcotte–Pugh (CTP) scores.
Results
Continuous TF occurred at home for 7 weeks (1.5–12 weeks). Prior to feeding, 12 patients had severe ascites, 10 required paracentesis, and 13 were severely malnourished. At completion of TF, five patients did not have ascites, four had mild ascites, four had moderate ascites, and only one had severe ascites and 10 no longer required paracentesis (P < 0.001). Median patient survival was 26 ± 7 months. Five survived to transplantation and three remained transplant‐free at 8, 1.9, and 1.7 years. Seven patients were moderately malnourished at completion of TF with an overall improvement in hand grip strength from 51% to 65% of predicted (P = 0.02).
Conclusion
Supplementary continuous TF may help to reduce ascites and paracentesis requirements and improve nutritional status. Supplementary continuous TF should be considered as a treatment for malnourished patients with decompensated cirrhosis and refractory ascites.
Keywords: ascites, cirrhosis, continuous tube feeding, malnutrition, paracentesis
Introduction
Progressive liver disease and disruption of the hepatic architecture are accompanied by a deterioration in the nutritional status of patients with cirrhosis.1 Malnutrition in patients with decompensated cirrhosis is associated with increasing symptoms of decompensation such as increasing ascites, the development of hepatic encephalopathy, and development and progression of esophageal varices.2, 3 Sarcopenia has been identified in 40–70% of patients with cirrhosis4 and is associated with increased mortality independent of disease severity.5
The development of sarcopenia in patients with cirrhosis is multifactorial. Liver function deteriorates with increasing levels of inflammatory mediators, dysbiosis, small bowel bacterial overgrowth, and increased intra‐abdominal pressure from ascites. Inadequate energy and protein intakes have been shown to be associated with the development of sarcopenia in patients with cirrhosis.4
Determination of nutritional status in patients with cirrhosis can be difficult and is often inaccurate.6 Some of the common tools used to assess nutritional status such as whole body weight, unintentional weight loss, body mass index, and serum proteins7 are poor indicators of nutritional status and muscle wasting in patients with decompensated cirrhosis.8 The degree of malnutrition using these parameters is frequently masked by the presence of ascites, peripheral edema, and hepatic hydrothorax. Standard nutritional assessment of the patient with cirrhosis thus includes subjective global assessment (SGA) of nutritional status9 and hand grip strength10 in addition to adequacy of oral intake, frequency of meals or snacks, changes in appetite, taste sensation, early satiety, and nausea.8
Recognizing the contribution of ascites to the patient's overall nutritional requirements remains a topic of debate with some workers maintaining ascites should be included when calculating requirements.11 Additionally, it is particularly difficult to determine the presence of muscle wasting in obese patients with cirrhosis using these methods.12 More reliable indicators of nutritional status and muscle depletion in patients with cirrhosis include SGA,9 hand grip strength,10 mid‐arm circumference (MAC), triceps skinfold (TSF), mid‐arm muscle circumference (MAMC),13 dual‐energy X‐ray absorptiometry (DXA),14 and, more recently, psoas muscle area measured at the L3 vertebral level.5
Alterations in carbohydrate metabolism, fuel utilization, and reduced glycogen storage in particular contribute to the development of malnutrition in patients with chronic liver disease (CLD).15 It has been established that both the macro‐ and micronutrient requirements of patients with decompensated cirrhosis are altered as a result of the disease process.8 In 2006, the European Society for Enteral and Parenteral Nutrition published evidence‐based guidelines for the nutritional management of patients with cirrhosis. These guidelines outline the increased protein and energy requirements for this group and the role of oral sip supplements or tube feeding (TF) to enable patients to meet these increased disease specific requirements.16
Improving the nutritional status of patients with decompensated cirrhosis presents a significant challenge to the practitioner, the patient, their carers, and the healthcare system. The financial burden of liver disease impacts on many aspects of life including adherence to therapy.17 Any intervention which reduces the symptoms and frailty of patients with CLD can improve the quality of life for the patient and reduce the financial burdens of the patient, caregivers, and the healthcare system.
One of the most common symptoms of hepatic decompensation is ascites, often occurring in association with increasing portal hypertension but contributed by the presence of malnutrition. Approximately 50–60% of patients with decompensated cirrhosis will develop ascites18 and up to 22% of patients with ascites develop diuretic‐resistant or refractory ascites with associated reduced 12‐month survival.19 Standard management of ascites centers around sodium restriction, use of diuretics, and in the case of refractory ascites, the use of therapeutic paracentesis, transjugular intrahepatic portosystemic shunts (TIPS), and liver transplantation.20
The aim of this work was to determine the effect of aggressive enteral nutrition intervention on levels of ascites and nutritional status in malnourished patients with decompensated cirrhosis and refractory ascites.
Methods
This is a retrospective case series of patients with refractory ascites who received TF at the A.W. Morrow Gastroenterology and Liver Centre between 2008 and 2016. Patients with underlying liver disease from a variety of etiologies with decompensated cirrhosis complicated by refractory ascites who were malnourished and failed to improve nutritionally with oral nutrition support received continuous supplementary TF with a standard, fiber‐enriched polymeric feed in addition to oral intake. Oral supplementation involved both oral intake as tolerated with the addition of two to three oral sip supplements which provided a total of an additional 38–47 g protein and 500–700 kcal. The composition of the enteral feed was 1.5 kcal/mL and 63 g protein/L. The protein component contained a mixture of whey protein isolate, pea protein isolate, soy protein isolate, and casein. In addition, there were six soluble fibers in the feed. All feeding was via a fine bore feeding tube (#8fr).
Standard practice prior to the insertion of a fine bore feeding tube requires the variceal status of the patient to be identified on endoscopy. If esophageal varices were identified, they were banded and the insertion of the fine bore feeding tube delayed for 10–14 days. These patients underwent repeat endoscopy and, if safe, a fine bore nasogastric (NG) feeding tube was inserted and used only after correct positioning was confirmed by X‐ray.
Feeding was commenced in hospital at a low rate (20 mL/h over 24 h) to avoid refeeding syndrome and reduce nausea with an ultimate goal rate of 50–60 mL high protein (HP)/HE feed/h. Feeding tubes were flushed 4 hourly with 20–30 mL water to prevent blockage. Prior to discharge home, patients were educated in all aspects of managing home enteral feeding including use of the feeding pump, water flushes, and bag changes.
In addition to enteral feeding, patients were advised to eat to tolerance and to continue oral sip supplementation. As the majority of patients experienced nausea, regular anti‐emetics were prescribed in the first 2–3 weeks of supplementary feeding but, in all individuals, long‐term use was not required.
Patient progress was reviewed weekly by the dietitian. Nutritional status and disease severity including ascites, frequency of paracenteses, and presence of hepatic encephalopathy were recorded prior to commencement of feeding, at each review session and at completion of the enteral feeding period. SGA,9 hand grip strength,10 mid‐arm circumference, triceps skinfold, and mid‐arm muscle circumference13, 21 were used to determine nutritional status. Disease severity was assessed using Child–Turcotte–Pugh (CTP) score,22 the model for end‐stage liver disease (MELD),23 and the sodium MELD (MELD‐Na).24 Hepatic encephalopathy was determined by clinical observations and the requirement for use of lactulose and/or rifaximin. Levels of ascites were assessed as mild, moderate, or severe20 and requirement for paracentesis recorded.
The ethics protocol was considered to be a low or negligible risk study and approved by the Sydney Local Health District Human Research Ethics Committee (RPAH Zone) with a waiver of consent.
Results
All patients in this cohort had failed to show signs of improvement in nutritional status or a reduction in their level of ascites following a mean of 5 months of oral intake as tolerated combined with oral nutritional supplementation. Fourteen patients received 7 ± 1 weeks of supplementary TF (1.5–12 weeks). One patient underwent liver transplantation after 2 weeks of feeding.
Patient demographics are outlined in Table 1. The physical and biochemical characteristics of the group and the response to supplementary TF are outlined in Table 2.
Table 1.
Patient demographics prior to continuous supplementary tube feeding
| Total number | 14 |
|---|---|
| Age (mean + SD) | 58 ± 6.4 |
| Gender | |
| Male | 12 |
| Female | 2 |
| Disease etiology | |
| HCV | 5 |
| Hepatitis B | 1 |
| EtOH | 3 |
| HCV + EtOH | 1 |
| Post‐transplant cirrhosis | 2 |
| Non‐alcoholic steatohepatitis | 1 |
| Primary sclerosing cholangitis | 1 |
EtOH, alcohol; HCV, hepatitis C.
Table 2.
Patient characteristics before and after continuous supplementary tube feeding
| Prior to feeding | At completion of feeding | Change | |
|---|---|---|---|
| Bilirubin (μmol/L) | 47 ± 8 | 53 ± 10 | +6 |
| Creatinine (μmol/L) | 87 ± 11 | 81 ± 6 | −6 |
| Albumin (g/L) | 31 ± 2 (supported†) | 32 (unsupported†) ± 2 | 0 |
| INR | 1.8 ± 1 | 1.7 ± 1 | −0.1 |
| MELD | 17 ± 6 | 16 ± 5 | −1 |
| MELD‐Na | 21 ± 1 | 18 ± 2 | −3 |
| CTP | 10 ± 1 | 9 ± 1 | −1 |
| CTP A (n) | 0 | 2 | +2 |
| CTP B (n) | 4 | 5 | +1 |
| CTP C (n) | 9 | 6 | −3 |
| Hepatic encephalopathy | |||
| Yes | 9 | 9 | 0 |
| No | 5 | 5 | 0 |
| Ascites | |||
| None (n) | 0 | 5 | +5** |
| Mild (n) | 2 | 4 | +2** |
| Moderate (n) | 0 | 4 | +4** |
| Severe (n) | 12 | 1 | −11** |
| Body mass index (kg/m2) | 24.7 ± 2 | 23.5 ± 1 | −1.2 |
| SGA | |||
| A (n) | 0 | 0 | 0 |
| B (n) | 1 | 7 | +6* |
| C (n) | 13 | 7 | −6* |
| Hand grip strength (% predicted) | |||
| Left | 51 ± 5 | 65 ± 6 | +15* |
| Right | 50 ± 4 | 63 ± 4 | +13* |
| Mid‐arm circumference (mm) | 237 ± 10 | 239 ± 10 | +2 |
| Triceps skinfold (mm) | 8 ± 1 | 8.0 ± 1 | 0 |
| Mid‐arm muscle circumference (mm) | 212 ± 8 | 214 ± 9 | 0 |
P < 0.05;
P < 0.001.
IV albumin infusion; (mean ± SE).
CTP, Child–Turcotte–Pugh; INR, international normalised ration; MELD, model for end‐stage liver disease; MELD‐Na, sodium MELD; SGA, subjective global assessment.
Nutritional status improved significantly in all patients (P < 0.001). Seven patients improved nutritionally from SGA C (significantly malnourished) to SGA B (moderately malnourished) at the end of the feeding period. Additionally, there was a significant improvement in hand grip strength (P = 0.02).
The mean albumin concentration prior to feeding was similar to that at the conclusion of feeding (Table 2). Serum albumin levels in all patients in the pre‐supplementary TF group were supported by regular intravenous albumin infusion as support for large‐volume paracentesis. Albumin levels in the post‐supplementary TF group were not supported by albumin infusion in 10 patients.
Disease severity measured by MELD, MELD‐Na, and CTP decreased but did not reach statistical significance (Table 2) with MELD reducing from 17±1 to 16 ± 1(ns; P = 0.6), MELD‐Na from 21 ± 1 to 18 ± 2 (ns; P = 0.09), and CTP from 10 ± 1 to 9 ± 1 (ns; P = 0.06).
Nine patients were on lactulose or lactulose and rifaximin during the TF period indicating a level of preexisting hepatic encephalopathy. TF was not associated with any increase in HE (Table 2).
There was an overall significant reduction in ascites (Fig. 1) (P < 0.001), which importantly was reflected in reduced requirements for abdominal paracentesis (Fig. 2) (P = 0.003). Ten patients had severe ascites prior to TF and required regular paracentesis at a mean of once every 2 ± 1 weeks. At the completion of the TF period, only one patient had severe ascites, five did not have ascites, four had mild ascites, and four had moderate ascites. Only four patients continued to require paracentesis but with reduced frequency.
Figure 1.
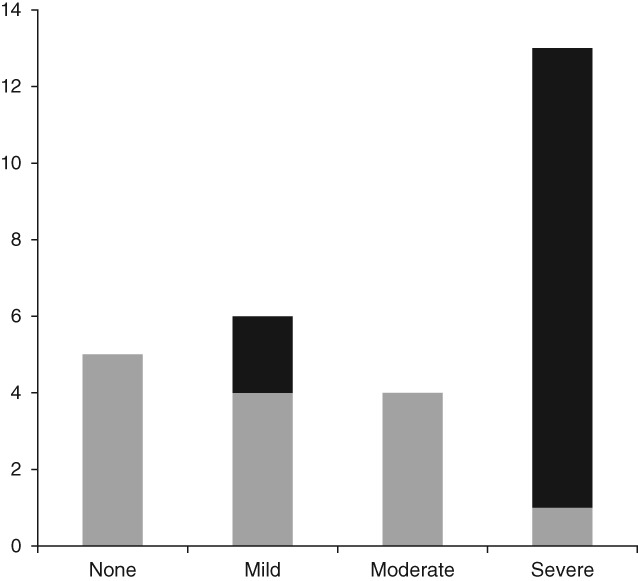
Continuous nasogastric tube feeding reduces levels of ascites in malnourished patients with decompensated cirrhosis. Patients with ascites who failed standard nutrition interventions had significantly reduced levels of ascites following 7 ± 1 weeks of continuous supplementary nasogastric feeding ( , pre‐feeding;
, pre‐feeding;  , post‐feeding).
, post‐feeding).
Figure 2.
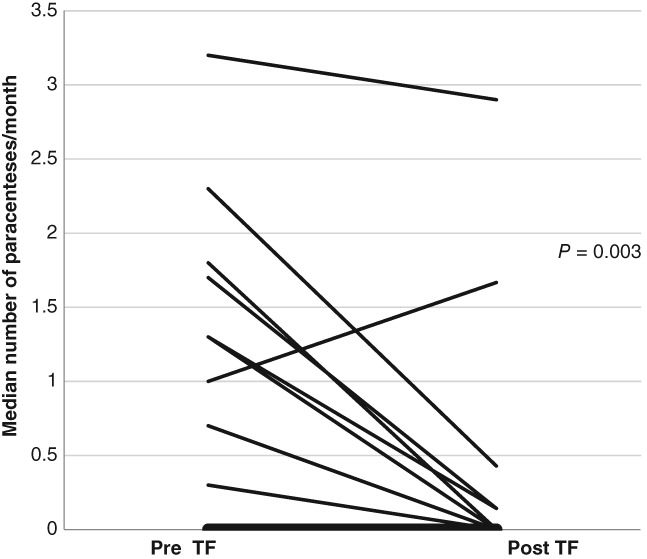
The median paracenteses requirements per month for each individual over 6 months before and 6 months after continuous supplementary feeding. Median monthly requirement for paracentesis over a 6‐month period prior to tube feeding (TF) versus median monthly requirement over a mean follow‐up period of 5.9 ± 0.3 months post continuous TF. Requirements for therapeutic paracentesis were significantly reduced (P = 0.003).
The average monthly paracentesis requirements over a 6‐month period before and 5.9 ± 0.3 months after TF are outlined in Figure 2. Paracentesis requirements were reduced in all but one patient (P < 0.001). This patient initially had a reduction in severity of ascites with reduced paracentesis requirements but ultimately did not improve over the 6 months following TF and did not survive due to poor compliance with feeding regime and ongoing disease progression.
TF was well tolerated and no patient ceased TF due to intolerance. Nine patients required anti‐emetics only for the initial 1–2 weeks of TF. All patients required reinsertion of the fine bore feeding tube at least once during the feeding period due to accidental dislodgement or tube blockage. One individual who showed no improvement over the 7‐week period did not have a live‐in carer or relative. He required a longer in‐hospital training period during which there was a reduction in ascites with reduced paracentesis requirements. Following discharge, compliance was variable and the treatment had to be discontinued due to poor adherence with a subsequent deterioration in clinical status.
Participants continued oral sip supplementation following the period of continuous supplementary feeding in order to meet their increased disease‐specific requirements. Median survival during the follow‐up period following continuous supplementary TF was 26 ± 19 months of whom five survived to transplantation and one died from non‐liver‐related causes after 16 months. Three individuals remained transplant free at 8, 1.9, and 1.7 years, respectively, with a return to normal liver function tests. Of those who survived to transplantation, four remained well after a median of 6.8 ± 1.8 years.
Discussion
These results demonstrate that, following continuous supplementary TF in a group of malnourished patients with features of decompensated cirrhosis, there was a significant reduction in levels of ascites and paracentesis requirements. In addition, there was an improvement in nutritional status indicated by a significant improvement in both their SGA score and hand grip strength.
It is important to note that serum albumin concentration at the conclusion of the TF period was the same as that prior to commencement of TF. Prior to commencing TF, 10 patients were receiving albumin infusions to prevent circulatory dysfunction associated with large‐volume paracentesis.20 In patients with cirrhosis, serum albumin concentrations are a marker of hepatic synthetic function.25 At the conclusion of TF, most patients were not receiving regular albumin infusion. However, serum albumin levels before and after TF were not significantly different despite the influence of albumin support prior to TF, which indicates an improvement in hepatic synthetic function and suggesting that this may be a crude marker of clinical improvement.
There was a significant response to supplementary continuous TF that was not apparent in these patients following oral nutrition intervention over a prior mean of 5 months (range: 2–7 months). An evaluation of nutrition intervention in a group of hospitalized, malnourished patients found that patients who did not receive supportive enteral feeding after a month of failed oral intake had poorer outcomes which included increased rates of infection, worsening liver function, and reduced survival.26 The mechanisms of the observed improvements may not be as simple as the provision of additional protein and energy but may be multifactorial. An improvement in nutritional status has been reported in children who had previously failed to thrive on oral nutrition despite meeting their macronutrient requirements only after they received TF for 18–22 h/day.27
A randomized trial investigated the effects of supportive enteral feeding versus oral sip supplementation over 2 weeks in cirrhotic patients.28 This trial found no significant difference in levels of ascites, paracentesis requirements, nutritional status, and disease severity between the two groups.28 Results such as this have led some to believe that enteral nutrition in advanced cirrhosis is a case of “missing the boat”.29 However, patients in our case series received continuous enteral feeding for a longer period (7 ± 1.5 weeks) and there was a trend toward a reduced CTP score (P = 0.06). This was influenced, in part, by the unchanged serum albumin levels before and after continuous feeding, reflecting routine albumin infusions which accompany large‐volume paracentesis.30 Moreover, the post‐feeding serum albumin levels are unsupported by albumin infusion, so are a likely indication of improved hepatic synthetic function. The current scoring systems for disease severity cannot distinguish between improvements secondary to infusion or to improved hepatic synthetic function.22, 23 Our results clearly demonstrate a significant improvement in nutritional status with reduced paracentesis requirements in a selected group of malnourished individuals with decompensated cirrhosis following continuous supplementary TF feeding. Importantly, three individuals who were potential transplant candidates did not require liver transplantation and remain well and transplant‐free at the time of this report.
A number of investigations and clinical guidelines have indicated that there is a reluctance for the introduction of supportive enteral feeding in patients with decompensated cirrhosis who are unable to meet 70–80% of their macronutrient requirements.27, 31, 32 However, although it is widely accepted that this treatment should be adopted,18 the majority of clinicians do not initiate or promote early enteral feeding.32 This is a small uncontrolled series report with a selection bias but adds to previous indications that continuous nasogastric feeding can lead to improved patient outcomes in malnourished patients with decompensated cirrhosis.33, 34
Continuous TF is not appropriate for all patients and some have been shown to decline this intervention.28 Patients need to be well motivated to undertake this treatment. A supportive environment at home is critical to the success of this intervention. This is demonstrated in the case of the individual who deteriorated on cessation of TF.
In addition, multidisciplinary team involvement is critical to support and monitor patient progress. The implications of this intervention are improved quality of life and reduced symptoms of decompensated cirrhosis. In addition, there is a potential for the reduction in medical interventions facilitating reduction in costs and risks associated with paracentesis, which include hematomas, intraperitoneal bleeding, and bacterial peritonitis,35 and the possibility of avoiding or delaying liver transplantation.
In conclusion, continuous supplementary TF may reduce the severity of ascites and the requirement for paracentesis in selected patients with decompensated liver disease who do not respond to standard nutrition intervention. Additionally, continuous supplementary TF appears to be beneficial in improving nutritional status and muscle strength in patients with advanced CLD. Therefore, this treatment option should be considered in the management of malnourished patients with portal hypertension and ascites.
Declaration of conflict of interest: Nothing to disclose.
Financial support: Nothing to disclose.
References
- 1. Campillo B. Nutritional aspects of liver and biliary disease. In: Rodes J, Benhamou JP, Blei AT, Reichen J, Rezzetto M, eds. Textbook of Hepatology From Basic Science to Clinical Practice, 3rd edn. Oxford: Blackwell Publishing Ltd, 2007; 1922–30.
- 2. Moller S, Bendtsen F, Christensen E, Henriksen JH. Prognostic variables in patients with cirrhosis and oesophageal varices without prior bleeding. J. Hepatol. 1994; 21: 940–6. [DOI] [PubMed] [Google Scholar]
- 3. Lautz HU, Selberg O, Körber J, Burger M, Muller MJ. Protein‐calorie malnutrition in liver cirrhosis. Clin. Investig. 1992; 70: 478–86. [DOI] [PubMed] [Google Scholar]
- 4. Sinclair M, Gow PJ, Grossmann M, Angus PW. Review article: sarcopenia in cirrhosis – aetiology, implications and potential therapeutic interventions. Aliment. Pharmacol. Ther. 2016; 43: 765–77. [DOI] [PubMed] [Google Scholar]
- 5. Montano‐Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J. Gastroenterol. 2014; 20: 8061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin. Liver Dis. 2012; 16: 95–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones JM. The methodology of nutritional screening and assessment tools. J. Hum. Nutr. Diet. 2002; 15: 73–5. [DOI] [PubMed] [Google Scholar]
- 8. Bémeur C, Butterworth R. Reprint of: Nutrition in the management of cirrhosis and its neurological complications. J. Clin. Exp. Hepatol. 2014; 4: 141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hasse J, Strong S, Gorman MA, Liepa G. Subjective global assessment: alternative nutrition‐assessment technique for liver‐transplant candidates. Nutrition. 1993; 9: 339–43. [PubMed] [Google Scholar]
- 10. Alvares‐da‐Silva MR, da Silveira RT. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005; 21: 113–18. [DOI] [PubMed] [Google Scholar]
- 11. Dolz C, Raurich JM, Ibanez J, Obrador A, Marse P, Gaya J. Ascites increases the resting energy expenditure in liver cirrhosis. Gastroenterology. 1991; 100: 738–45. [DOI] [PubMed] [Google Scholar]
- 12. Hara N, Iwasa M, Sugimoto R et al Sarcopenia and sarcopenic obesity are prognostic factors for overall survival in patients with cirrhosis. Intern. Med. 2016; 55: 863–70. [DOI] [PubMed] [Google Scholar]
- 13. Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am. J. Clin. Nutr. 1981; 34: 2540–5. [DOI] [PubMed] [Google Scholar]
- 14. Strauss BJ, Gibson PR, Stroud DB, Borovnicar DJ, Xiong DW, Keogh J. Total body dual X‐ray absorptiometry is a good measure of both fat mass and fat‐free mass in liver cirrhosis compared to "gold‐standard" techniques. Melbourne Liver Group. Ann. N. Y. Acad. Sci. 2000; 904: 55–62. [DOI] [PubMed] [Google Scholar]
- 15. Owen OE, Trapp VE, Reichard GA et al Nature and quantity of fuels consumed in patients with alcoholic cirrhosis. J. Clin. Investig. 1983; 72: 1821–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Plauth M, Cabre E, Riggio O et al ESPEN guidelines on enteral nutrition: liver disease. Clin. Nutr. 2006; 25: 285–94. [DOI] [PubMed] [Google Scholar]
- 17. Bajaj JS, Wade J, Gibson DP et al The multi‐dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am. J. Gastroenterol. 2011; 106: 1646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gines P, Quintero E, Arroyo V et al Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987; 7: 122–8. [DOI] [PubMed] [Google Scholar]
- 19. Ennaifer R, Elleuch N, Romdhane H et al Refractory ascites in cirrhosis: prevalence and predictive factors. J. Liver. 2014; 3: 162–5. [Google Scholar]
- 20. Aroyo V, Gines P, Gerbes AL et al Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Hepatology. 1996; 23: 164–76. [DOI] [PubMed] [Google Scholar]
- 21. Bishop CW, Pitchey SJ. Estimation of the mid‐upper arm circumference measurement error. J. Am. Diet. Assoc. 1987; 87: 469–74. [PubMed] [Google Scholar]
- 22. Pugh RNH, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973; 60: 646–50. [DOI] [PubMed] [Google Scholar]
- 23. Kamath PS, Wiesner RH, Malinchoc M et al A model to predict survival in patients with end‐stage liver disease. Hepatology. 2001; 33: 464–70. [DOI] [PubMed] [Google Scholar]
- 24. Biggins SW, Kim WR, Terrault NA et al Evidence‐based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006; 130: 1652–61. [DOI] [PubMed] [Google Scholar]
- 25. Hasch E, Jarnum S, Tygstrup N. Albumin synthesis rate as a measure of liver function in patients with cirrhosis. Acta Med. Scand. 1967; 182: 83–92. [DOI] [PubMed] [Google Scholar]
- 26. Campillo B, Richardet JP, Bories PN. Enteral nutrition in severely malnourished and anorectic cirrhotic patients in clinical practice: benefit and prognostic factors. Gastroenterology. 2005; 29: 645–51. [DOI] [PubMed] [Google Scholar]
- 27. Charlton CPJ, Buchanan E, Holden CE et al Intensive enteral feeding in advanced cirrhosis: reversal of malnutrition without precipitation of hepatic encephalopathy. Arch. Dis. Child. 1992; 67: 603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tai MS, Goh K, Taib SHM et al Short‐term nasogastric versus oral feeding in hospitalised patients with advanced cirrhosis. E Spen Eur. E. J. Clin. Nutr. Metab. 2011; 6: e242–7. [Google Scholar]
- 29. Mahadeva S, Goh K‐L. Letter: enteral nutrition in advanced cirrhosis – a case of missing the boat? Aliment. Pharmacol. Ther. 2012; 36: 205–6. [DOI] [PubMed] [Google Scholar]
- 30. Arroyo V, Ginès P, Gerbes AL et al Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Hepatology. 1996; 23: 164–276. [DOI] [PubMed] [Google Scholar]
- 31. Campillo B, Richardet RJ, Scherman E, Bories PN. Evaluation of nutritional practice in hospitalized cirrhotic patients: results of a prospective study. Nutrition. 2003; 19: 515–21. [DOI] [PubMed] [Google Scholar]
- 32. Tsiaousi ET, Hatzitolios AI, Trygonis SK, Savopoulos CG. Malnutrition in end stage liver disease: recommendations and nutritional support. J. Gastroenterol. Hepatol. 2008; 23: 527–33. [DOI] [PubMed] [Google Scholar]
- 33. Campillo B, Richardet JP, Bories PN. Enteral nutrition in severely malnourished and anorectic cirrhotic patients in clinical practice. Gastroenterol. Clin. Biol. 2005; 29: 515–612. [DOI] [PubMed] [Google Scholar]
- 34. Crispin JS. Is tube feeding an option in patients with liver disease? Nutr. Clin. Pract. 2006; 21: 296–8. [DOI] [PubMed] [Google Scholar]
- 35. De Gottardi A, Thévenot T, Spahir L et al risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin. Gastroenterol. Hepatol. 2009; 7: 906–9. [DOI] [PubMed] [Google Scholar]


