Abstract
Background and Aim
Predictive factors for hepatocarcinogenesis following eradication of hepatitis C virus by direct‐acting antivirals (DAAs) are unknown. The aim of the study was to investigate the relationships between liver stiffness (LS) using acoustic radiation force impulse (ARFI) erastograghy and the development of hepatocellular carcinoma (HCC) in patients who achieved sustained virological response (SVR) treated with DAA.
Methods
In this prospective study, we enrolled 263 hepatitis C patients with SVR who underwent ARFI before DAA treatment. Thirty patients had previous HCC.
Results
The median LS value according to ARFI measurements was 1.34 m/s (range: 0.67–4.35). During the follow‐up period (median: 18.1 months), development of HCC occurred in 19 patients (7.2%; HCC occurrence in 7 patients and HCC recurrence in 12 patients). By multivariate Cox regression analysis, HCC history (hazard ratio [HR]: 10.634; 95% confidence interval [CI]: 4.13–27.37; P = 0.001), older age (HR: 4.638; 95% CI: 1.63–13.61; P = 0.004) and higher total bilirubin levels (HR: 4.189; 95% CI: 1.66–10.60; P = 0.002) were independent predictors for the development of HCC, and higher LS value (≥1.73 m/s) at baseline was an independent predictor for HCC occurrence (HR: 8.350; 95% CI: 1.62–43.09; P = 0.011). The cumulative recurrence of HCC was statistically similar according to the degree of LS in patients who were previously treated for HCC.
Conclusion
The LS value at baseline is useful for predicting HCC occurrence. Thus, even if SVR is achieved, patients with higher LS at baseline must be followed carefully for HCC occurrence.
Keywords: acoustic radiation force impulse elastography, chronic hepatitis C, hepatocellular carcinoma, liver stiffness, sustained virological response
Introduction
Hepatitis C virus (HCV) infection is a leading cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC), and is a major global public health issue.1, 2 The emergence of novel direct‐acting antivirals (DAAs) against HCV has dramatically increased the number of patients who achieve a sustained virological response (SVR).3, 4 The elimination of HCV will prevent the progression of chronic hepatitis and associated complications.5 Several studies have reported that achievement of SVR results in the resolution of liver fibrosis6, 7 and a decreased incidence of HCC.8 However, development of HCC is sometimes seen even in patients who achieve SVR after DAA treatment, indicating the need for continuous surveillance for HCC after the eradication of HCV.9, 10, 11, 12 Several previous studies have reported that the degree of liver fibrosis is closely associated with the risk of the development of HCC in patients with chronic hepatitis C.13 Pretreatment staging is important in order to plan initial management of the patient post‐SVR. As liver fibrosis persists even after the elimination of HCV despite its gradual resolution after SVR is achieved, accurate estimation of liver fibrosis is desirable.
Previous studies have reported that advanced fibrosis, advanced age, lower albumin levels, lower platelet counts, and higher α‐fetoprotein (AFP) levels before and after treatment are important predictors of HCC in patients who achieve SVR with interferon‐based treatment.14, 15, 16 However, the risk factors for the development of HCC in patients who achieve SVR after DAA treatment have not been adequately clarified in a prospective study.
Preliminary study results indicate that the acoustic radiation force impulse (ARFI) elastography is a noninvasive method for measuring liver stiffness (LS) and can be used for the diagnosis of liver fibrosis and cirrhosis in chronic liver disease.17, 18, 19, 20, 21, 22, 23 However, the relationship between LS and its impact on the development of HCC remains unclear in chronic hepatitis C patients who achieved SVR following DAA therapy.
To understand this relationship, we enrolled 263 patients who had achieved an SVR following DAA therapy and clinical follow‐up study in which LS measurements were obtained from patients before treatment. The aim of the study was to investigate the relationships between LS using ARFI erastograghy and the development of HCC in this patient population.
Methods
Patients
Between November 2014 and December 2016, a total of 268 patients were treated with oral direct‐acting anti‐HCV drugs (daclatasvir/asunaprevir combination therapy, sofosbuvir/ledipasvir combination therapy, ombitasvir/paritaprevir/ritonavir combination therapy, and sofosbuvir/ribavirin combination therapy) at Komaki City Hospital, Japan. A total of 263 patients achieved an SVR after antiviral therapy (98.1%). This prospective study thus included 263 patients who underwent ARFI elastography before antiviral treatment. Thirty patients had history of curative treatment for HCC and 233 had no history of previous HCC. HCV infection was defined by a real‐time polymerase chain reaction (COBAS TaqMan HCV test; Roche Molecular Systems, Pleasanton, CA, USA; lower limit of detection: 1.2 log10 IU/mL). SVR was determined as undetectable serum HCV RNA, 24 weeks after the completion of antiviral therapy by a real‐time polymerase chain reaction. Clinical data were collected at the time of measurement of ARFI elastography and blood samples were collected no more than 7 days before the DAA treatment. Patients who had antibodies against human immunodeficiency virus or hepatitis B virus surface antigen, excessive active alcohol consumption (daily intake >40 g of ethanol) or drug abuse, or other forms of liver disease (e.g. autoimmune hepatitis, alcoholic liver disease, or hemochromatosis) were excluded. The study was approved by the institutional Review Board of Komaki City Hospital and conducted in accordance with the ethical principles of the Declaration of Helsinki. Written informed consent for use of clinical data was obtained from all patients at the time of the measurement of ARFI elastography.
Antiviral therapy
For chronic HCV genotype 1 infection, 67 patients received fixed doses of daclatasvir (60 mg once daily) and asunaprevir (100 mg twice daily) for 24 weeks. One hundred and twenty‐nine patients received fixed doses combination of sofosbuvir/ledipasvir (400/90 mg once daily) for 12 weeks. Two patients received the fixed doses combination of ombitasvir/paritaprevir/ritonavir (25/150/100 mg twice daily) for 12 weeks. For chronic HCV genotype 2 infection, 65 patients received fixed doses of sofosbuvir (400 mg once daily) and ribavirin (600–1000 mg daily based on bodyweight, orally) for 12 weeks. We treated patients based on a standard treatment protocol for Japanese patients.
Diagnosis of HCC and follow‐up
Before starting antiviral therapy, all patients without history of previous HCC underwent abdomen ultrasound to exclude the presence of HCC. On the other hand, all patients with a history of HCC underwent magnetic resonance imaging (MRI) and dynamic computed tomography (CT), besides ultrasound, to exclude recurrent HCC. After the end of antiviral therapy, patients attended medical consultations at the Komaki City Hospital outpatient clinic every 3–6 months. Biochemical measurements, including AFP and tumor marker levels, were assessed from whole blood samples every 3–6 months; ultrasonography, MRI, and dynamic CT were performed every 6 months. Typical imaging findings for HCC included a high‐density mass in the arterial phase and a low‐density mass in the portal phase of dynamic CT or MRI studies. To investigate the incidence of HCC after SVR, the start date of follow‐up was defined as the date of the start of DAA treatment and the endpoint was the development of HCC or the latest medical follow‐up visit prior to May 2017. The factors associated with the development of HCC were prospectively analyzed.
ARFI elastography measurement
In this prospective study, the baseline ARFI elastography measurements of all patients were performed less than 3 months before HCV treatment initiation. The Siemens Acuson S2000TM ultrasound system (Siemens Medical Solutions, Mountain View, CA, USA) was used for the measurement of ARFI elastography using a curved linear 6C1 transducer in all the patients.24 The measurement of ARFI elastography was performed in the right liver lobe through the 7th to 10th intercostal space while the patient was in a supine position with the right arm in abduction. The patients were asked to hold their breath for a moment at the end of expiration to minimize breathing motion during the examination. ARFI measurements were obtained at a depth of 1–2 cm from the liver capsule, avoiding large vessels and fissures in the liver. A total of 10 valid measurements were performed in every patient and a median value was calculated; the result is expressed in m/s.
Statistical analysis
Categorical data are presented as numbers (percentages). Continuous data are presented as means ± SD) and medians (ranges). Normally distributed variables were compared using Student's t‐test and non‐normally distributed variables were compared using the Mann–Whitney U‐test between the two groups of patients who did and did not develop HCC. Frequency data were compared using a chi‐square test or Fisher's exact test, as appropriate. The cumulative incidence of HCC was calculated using the Kaplan–Meier method. Differences among patients with mild LS, moderate LS, and severe LS were assessed using by the log‐rank test. The time frame for HCC incidence was defined as the time from start of DAA treatment to diagnosis of HCC. The Cox proportional hazard model was used for multivariate analyses of factors associated with the incidence of HCC. We determined the cut‐off values of the factors associated with the incidence of HCC using receiver operating characteristic analyses. Statistical analyses were performed using SPSS Statistics 21.0 (IBM SPSS, Chicago, IL, USA); P < 0.05 was considered statistically significant using a two‐tailed test.
Results
Baseline characteristics of patients with antiviral treatment
The overall study population of 263 patients consisted of 122 males (46.4%) and 141 females (53.6%). The mean age was 70.7 years (range: 20–88). Detailed demographic data are shown in Table 1. Liver cirrhosis was identified in 49 patients (18.6%), and all patients had well‐preserved liver function of Child–Pugh class A. HCV genotype 1 was present in 198 patients (75.6%). The median V s value according to ARFI measurements was 1.34 m/s (range: 0.67–4.35; Table 1).
Table 1.
Baseline characteristics of patients from the entire study population
| Characteristics | Patients |
|---|---|
| Age (years) | 71.4 (16–88) |
| Sex (female/male) | 141 (53.6%)/122 (46.4%) |
| HCV genotype (1b/2a or 2b) | 198/65 |
| HCC history (absent/present) | 233/30 |
| Body mass index (kg/m2) | 22.6 (15.0–32.9) |
| Serum albumin (g/dL) | 4.0 (2.2–4.9) |
| Total bilirubin (mg/dL) | 0.74 (0.3–2.6) |
| Alanine aminotransferase (IU/L) | 34.0 (10–299) |
| Aspartate aminotransferase (IU/L) | 38.7 (16–224) |
| Gamma‐glutamyl transpeptidase (IU/L) | 31.2 (8–521) |
| Platelet count (×103/μL) | 149 (14–351) |
| α‐Fetoprotein (ng/mL) | 4.5 (0.9–172.3) |
| Liver stiffness (m/s) | 1.41 (0.67–4.35) |
| Antiviral regimens | |
| Daclatasvir + asunaprevir | 67 (25.5%) |
| Ledipasvir/sofosbuvir | 129 (49.1%) |
| Ombitasvir paritaprevir/ritonavir | 2 (0.7%) |
| Sofosbuvir + ribavirin | 65 (24.7%) |
HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
Development of HCC in patients with SVR following DAA therapy
During the follow‐up period (median: 18.1 months; range: 5.6–31.2 months), HCC was identified in 19 patients (7.2%). The median time between the start of DAA treatment and the development of HCC was 8.6 months (range: 6.0–21.3 months). The cumulative incidence of HCC at 1 and 2 years after the start of DAA treatment was 5.4% and 9.7%, respectively. HCC patients included 7 males and 12 females; 12 patients had history of previous HCC and 7 had no history of previous HCC.
Factors associated with the development of HCC in patients with SVR following DAA therapy
The clinical characteristics according to development of HCC are shown in Table 2. LS measurements were significantly higher in patients with the development of HCC than in those without (median: 2.06 m/s vs 1.33 m/s, P < 0.001). Univariate analysis revealed that the presence of HCC history, older age, lower platelet counts, higher total bilirubin levels, and higher AFP levels before treatment were significantly associated with the development of HCC after SVR. The Cox proportional hazards regression analysis confirmed that the presence of HCC history (hazard ratio [HR]: 10.634; 95% confidence interval [CI]: 4.13–27.37; P = 0.001), higher total bilirubin levels (HR: 4.189; 95% CI: 1.66–10.60; P = 0.002), and older age (HR: 4.638; 95% CI: 1.63–13.61; P = 0.004) were significant independent factors associated with the development of HCC in patients who had achieved an SVR following DAA therapy. The cumulative occurrence of HCC in patients with history of HCC was significantly higher than that in patients without history of HCC, based on the Kaplan–Meier analysis and log‐rank test (P < 0.001) (Fig. 1).
Table 2.
Baseline characteristics of patients according to the development of HCC after DAA therapy
| Characteristics | Patients without HCC after DAA | Patients with HCC after DAA | P‐value |
|---|---|---|---|
| Age (years) | 70.8 (16–88) | 77.0 (65–85) | <0.001 |
| Sex (female/male) | 129 (52.9%)/115 (47.1%) | 12 (63.2%)/7 (36.8%) | 0.477 |
| HCV genotype (1b/2a or 2b) | 181/63 | 17/2 | 0.173 |
| HCC history (absent/present) | 226/18 | 7/12 | <0.001 |
| Body mass index (kg/m2) | 22.5 (15.0–32.9) | 23.3 (16.4–31.5) | 0.563 |
| Serum albumin (g/dL) | 4.0 (2.2–4.9) | 3.8 (2.4–4.5) | 0.097 |
| Total bilirubin (mg/dL) | 0.72 (0.3–2.5) | 0.98 (0.6–2.6) | 0.001 |
| Alanine aminotransferase (IU/L) | 34.5 (10–299) | 24.5 (14–106) | 0.321 |
| Aspartate aminotransferase (IU/L) | 38.4 (16–224) | 43.0 (27–106) | 0.155 |
| Gamma‐glutamyl transpeptidase (IU/L) | 31.7 (8–521) | 21.5 (13–521) | 0.519 |
| Platelet count (×103/μL) | 151 (14–351) | 118 (38–221) | 0.005 |
| α‐Fetoprotein (ng/mL) | 4.4 (0.9–172.3) | 6.3 (2.5–132.5) | 0.011 |
| Liver stiffness (m/s) | 1.39 (0.67–4.35) | 2.04 (1.12–4.00) | <0.001 |
DAA, direct‐acting antiviral; HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
Figure 1.
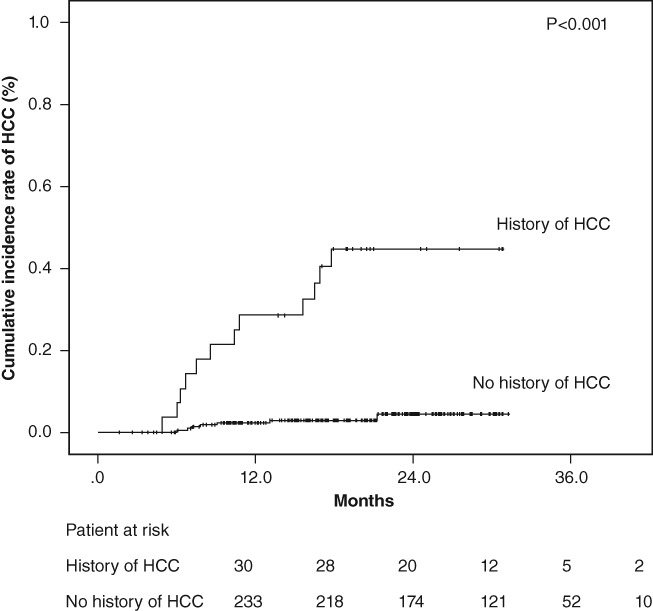
Cumulative incidence rates of hepatocellular carcinoma (HCC) development after sustained virological response following direct‐acting antiviral treatment according to the history of hepatocellular carcinoma. The cumulative incidence rates of HCC development increased significantly in patients with previous HCC treatment (log‐rank test, P < 0.001).
Factors associated with the occurrence of HCC in patients with SVR following DAA therapy
In patients without history of HCC, occurrence of HCC was identified in seven patients (3.0%). The clinical characteristics according to the occurrence of HCC are shown in Table 3. The cumulative incidence of HCC at 1 and 2 years after the start of DAA treatment was 2.3% and 4.3%, respectively. Univariate analysis revealed that higher LS measurement and higher total bilirubin levels before treatment were significantly associated with the occurrence of HCC after SVR. The Cox proportional hazards regression analysis confirmed that higher LS measurement (HR: 8.350; 95% CI: 1.62–43.09; P = 0.011) was significant independent factor associated with the occurrence of HCC in patients who had achieved an SVR following DAA therapy (Table 4).
Table 3.
Baseline characteristics of patients with no history of previous HCC according to HCC occurrence after DAA therapy
| Characteristics | Patients without occurrence of HCC | Patients with occurrence of HCC | P‐value |
|---|---|---|---|
| Age (years) | 70.5 (16–88) | 76.0 (65–84) | 0.051 |
| Sex (female/male) | 119 (51.1%)/107 (48.9%) | 6 (85.7%)/1 (14.3%) | 0.126 |
| HCV genotype (1b/2a or 2b) | 165/61 | 6/1 | 0.678 |
| Body mass index (kg/m2) | 22.4 (15.4–32.9) | 23.5 (18.1–31.5) | 0.171 |
| Serum albumin (g/dL) | 4.1 (2.2–4.9) | 3.8 (2.8–4.4) | 0.307 |
| Total bilirubin (mg/dL) | 0.72 (0.3–2.5) | 0.97 (0.6–1.8) | 0.031 |
| Alanine aminotransferase (IU/L) | 34.1 (10–299) | 25.0 (17–106) | 0.616 |
| Aspartate aminotransferase (IU/L) | 37.7 (16–224) | 43.0 (28–106) | 0.194 |
| Gamma‐glutamyl transpeptidase (IU/L) | 30.7 (8–521) | 22.0 (13–146) | 0.315 |
| Platelet count (×103/μL) | 156 (14–351) | 118 (48–204) | 0.107 |
| α‐Fetoprotein (ng/mL) | 4.2 (0.9–172.3) | 6.4 (2.5–50.5) | 0.099 |
| Liver stiffness (m/s) | 1.33 (0.67–4.35) | 2.06 (1.40–4.00) | 0.005 |
DAA, direct‐acting antiviral; HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
Table 4.
Predictive factors related to the development of HCC after DAA therapy on Cox proportional hazard analysis
| Characteristics | Category | Hazard ratio | 95% Confidence interval | P‐value |
|---|---|---|---|---|
| Development of HCC | ||||
| History of previous HCC | 1: No 2: Yes |
10.634 | 4.132–27.369 | 0.001 |
| Total bilirubin (mg/dL) | 1: <1.0 2: ≥1.0 |
4.189 | 1.656–10.597 | 0.002 |
| Age (years) | 1: <75 2: ≥75 |
4.638 | 1.634–13.161 | 0.004 |
| Occurrence of HCC | ||||
| Liver stiffness (m/s) | 1: <1.73 2: ≥1.73 |
8.350 | 1.618–43.09 | 0.011 |
DAA, direct‐acting antiviral; HCC, hepatocellular carcinoma.
Correlation between LS values and development of HCC
Patients without history of HCC were divided into two groups based on measurement of ARFI elastography levels, with the value as the cutoff (1.73 m/s) using receiver operating characteristic analyses. The cumulative occurrences of HCC in patients with lower LS and higher LS were 1.2% and 6.1%, respectively, at 1 year after the start of DAA treatment and 1.2% and 13.4%, respectively, at 2 years after the start of DAA treatment. The cumulative occurrence of HCC in patients with higher LS was significantly higher than that in patients with lower LS, based on the Kaplan–Meier analysis and log‐rank test (P = 0.003; Fig. 2). The cumulative recurrence of HCC was statistically similar according to the degree of LS in patients who were previously treated for HCC (P = 0.099) (Fig. 3).
Figure 2.
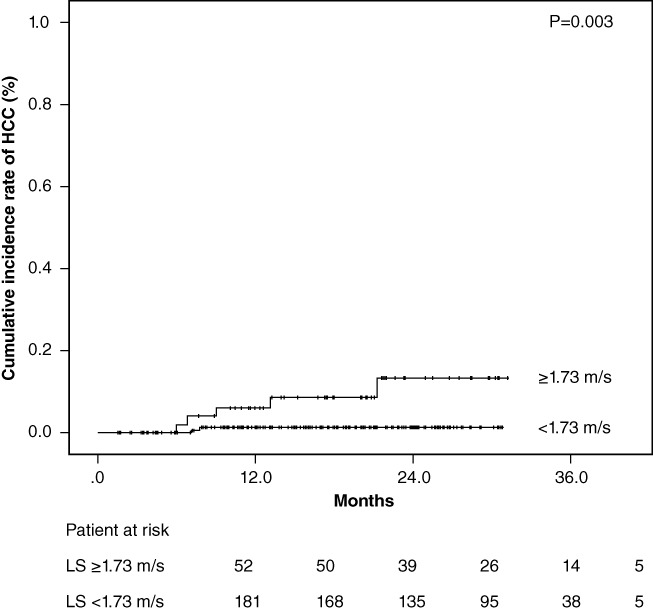
Cumulative incidence rates of hepatocellular carcinoma (HCC) occurrence after sustained virological response following direct‐acting antiviral treatment based on stratified liver stiffness (LS) values. The cumulative incidence rates of HCC occurrence increased significantly in patients with higher LS values at baseline (log‐rank test, P = 0.003).
Figure 3.
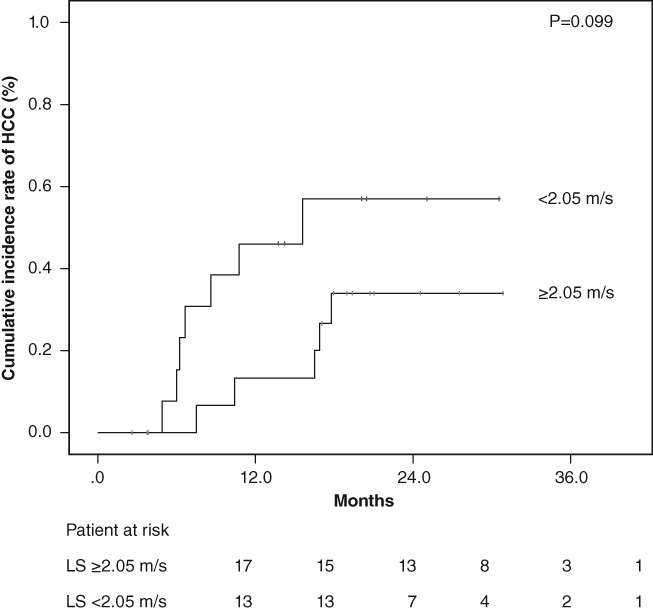
Cumulative incidence rates of hepatocellular carcinoma (HCC) recurrence after sustained virological response following direct‐acting antiviral treatment based on stratified liver stiffness (LS) values. The cumulative incidence rates of HCC recurrence was statistically similar according to the degree of LS in patients who were previously treated for HCC (log‐rank test, P = 0.099).
Discussion
To the best of our knowledge, this is the first study that evaluates the correlation between development of HCC and LS assessed by ARFI elastography in patients who achieved SVR following DAA therapies. The strengths of this study include the information it provides about the impact of LS measurement on development of HCC after SVR following DAA treatment, as assessed by ARFI elastography from 263 patients with HCV. Although patients who achieve SVR have little risk of developing HCC, given the marked increase in the number of patients who achieve SVR, there will also be an increase in the number of patients who develop HCC after SVR in the near future. Indeed, seven of our SVR patients without history of HCC after DAA treatment did develop HCC, demonstrating the need for continued screening of this population. In the present study, we identified the LS measurement before treatment as an independent predictor of HCC occurrence in patients who achieved an SVR following DAA treatment. Because patients with LS values of 1.73m/s have higher risk for HCC development, HCC surveillance strategies might be optimized according to LS values at baseline, even with complete viral eradication.
In the present study, a total of 32 patients with a history of previous HCC were treated with oral direct‐acting anti‐HCV drugs. In these patients, 30 patients achieved an SVR after antiviral therapy (93.8%). Eleven of our SVR patients with history of HCC after DAA treatment did develop HCC. We found 1‐ and 2‐year HCC recurrence rates of 28.6% and 44.7% in a cohort of patients with a history of previous HCC who achieved a complete radiological response after tentatively curative resection or ablation. The risk of recurrence of HCC was statistically similar according to the degree of LS in patients who were previously treated for HCC after SVR following DAA treatment. Several previous studies have reported that HCC recurrence after surgical resection or radiofrequency ablation is not uncommon.25 We found that patients previously treated for HCC have still a high risk of recurrence in the short term, despite DAA treatment, irrespective of the degree of LS.
Liver biopsy had long remained the gold standard for staging fibrosis. However, liver biopsy is no more considered as a perfect methodology because of the invasive nature of the procedure, sampling error, and interobserver variability. In contrast to liver biopsy, ARFI elastography is noninvasive and can be repeated multiple times in the same patient. This is clinically very helpful. Assessment of residual liver fibrosis in patients who achieve SVR is of strategic importance not only for prognostication but also for defining cost‐effective programs of surveillance or liver‐related complications. We previously reported that ARFI elastography is an acceptable method for predicting the severity of fibrosis in HCV patients with SVR.26 Therefore, ARFI elastography will be useful not only during the persistent HCV infection but also after the eradication of HCV.
A recent study has reported that the value of ARFI elastography is closely associated with the risk of the development of HCC in patients with chronic liver diseases.27 In the present study, we also identified that the value of ARFI elastography before treatment correlated with the development of HCC in patients who achieved an SVR following DAA treatment. However, LS measurements may also be affected by factors other than fibrosis stage, for example, inflammatory activity,28, 29 and intrahepatic pressure. There are a few studies demonstrating a decrease in LS in patients on eradication of HCV where both pre‐ and post‐treatment ARFI elastography assessments were available.30 It is probable that such reduction in ARFI elastography values indicates not only the improvement in fibrosis but also inflammation in the liver because of treatment. This is because ARFI elastography reductions are significantly correlated with the grade of activity in the liver.31 Moreover, it is not clear whether the change in LS after treatment, is useful in predicting the development of HCC in patients who achieved an SVR following DAA treatment. Therefore, prospective and more long‐term studies are necessary to confirm whether a change in LS after achieving SVR is correlated with the risk of developing HCC.
There are several limitations in this study. The number of patients with the development of HCC was not very large. Because the development of HCC is very rare in patients who achieve SVR after DAA treatment,10–12 this report described a relatively small number of cases. Regardless, this information is useful because risk factors associated with the development of HCC in patients who achieve SVR after DAA treatment were identified. Second, among the enrolled patients, 190 patients underwent liver biopsy (72.2%). We acknowledge that our study could have been strengthened by pretreatment liver biopsies with all of enrolled patients, which, however, was not available. This is due to the difficulty in obtaining informed consent for the invasive liver biopsy before treatment. Finally, prospective data and long‐term clinical follow‐up are needed to assess the clinical course after achieving SVR and the impact of LS measurement after HCV elimination. Future large‐scale studies with sufficient histological data and longer follow‐up periods can resolve these issues.
In conclusion, the degree of LS assessed on ARFI elastography before antiviral treatment was related to the occurrence of HCC in patients who achieved an SVR following DAA treatment. Therefore, even if SVR is achieved, patients with higher LS at baseline must be followed carefully for the occurrence of HCC.
Declaration of conflict of interest: The authors declare that they have nothing to disclose regarding conflicts of interest with respect to this manuscript.
Financial support: The authors declare that they have nothing to disclose regarding funding with respect to this manuscript.
References
- 1. Alter MJ. Epidemiology of hepatitis C in the West. Semin. Liver Dis. 1995; 15: 5–14. [DOI] [PubMed] [Google Scholar]
- 2. Tsukuma H, Hiyama T, Tanaka S et al Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N. Engl. J. Med. 1993; 328: 1797–801. [DOI] [PubMed] [Google Scholar]
- 3. Lok AS, Gardiner DF, Lawitz E et al Preliminary study of two antiviral agents for hepatitis C genotype 1. N. Engl. J. Med. 2012; 366: 216–24. [DOI] [PubMed] [Google Scholar]
- 4. Gane EJ, Stedman CA, Hyland RH et al Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N. Engl. J. Med. 2013; 368: 34–44. [DOI] [PubMed] [Google Scholar]
- 5. Singal AG, Volk ML, Jensen D et al A sustained viral response is associated with reduced liver‐related morbidity and mortality in patients with hepatitis C virus. Clin. Gastroenterol. Hepatol. 2010; 8: 280–8. [DOI] [PubMed] [Google Scholar]
- 6. Shiratori Y, Imazeki F, Moriyama M et al Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann. Intern. Med. 2000; 132: 517–24. [DOI] [PubMed] [Google Scholar]
- 7. Tachi Y, Hirai T, Miyata A et al Progressive fibrosis significantly correlates with hepatocellular carcinoma in patients with a sustained virological response. Hepatol. Res. 2015; 45: 238–46. [DOI] [PubMed] [Google Scholar]
- 8. Ikeda K, Saitoh S, Arase Y et al Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitistype C: a long‐term observation study of 1643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999; 29: 1124–30. [DOI] [PubMed] [Google Scholar]
- 9. Reig M, Marino Z, Perello C et al Unexpected early tumor recurrence in patients with hepatitis C virus–related hepatocellular carcinoma undergoing interferon‐free therapy: a note of caution. J. Hepatol. 2016; 65: 719–26. [DOI] [PubMed] [Google Scholar]
- 10. Conti F, Buonfiglioli F, Scuteri A et al Early occurrence and recurrence of hepatocellular carcinoma in HCV‐related cirrhosis treated with direct‐acting antivirals. J. Hepatol. 2016; 65: 727–33. [DOI] [PubMed] [Google Scholar]
- 11. Nagata H, Nakagawa M, Asahina Y et al Effect of interferon‐based and‐free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J. Hepatol. 2017; doi: 10.1016/j.jhep.2017.05.028. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12. Kanwal F, Kramer J, Asch SM et al Risk of hepatocellular cancer in HCV patients treated with direct acting antiviral agents. Gastroenterology. 2017; doi: 10.1053/j.gastro.2017.06.012. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13. Ogawa E, Furusyo N, Kajiwara E et al Efficacy of pegylated interferon alpha‐2b and ribavirin treatment on the risk of hepatocellular carcinoma of patients with chronic hepatitis C: a prospective multicenter study. J. Hepatol. 2013; 58: 495–501. [DOI] [PubMed] [Google Scholar]
- 14. Akuta N, Suzuki F, Hirakawa M et al Amino acid substitutions in hepatitis C virus core region predict hepatocarcinogenesis following eradication of HCV RNA by antiviral therapy. J. Med. Virol. 2011; 83: 1016–22. [DOI] [PubMed] [Google Scholar]
- 15. Toyoda H, Tada T, Tsuji K et al Characteristics and prognosis of hepatocellular carcinoma detected in patients with chronic hepatitis C after the eradication of hepatitis C virus: a multicenter study from Japan. Hepatol. Res. 2016; 46: 734–42. [DOI] [PubMed] [Google Scholar]
- 16. Asahina Y, Tsuchiya K, Nishimura T et al α‐Fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 2013; 58: 1253–62. [DOI] [PubMed] [Google Scholar]
- 17. Cassinotto C, Boursier J, Lédinghen V d et al Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016; 63: 1817–27. [DOI] [PubMed] [Google Scholar]
- 18. Attia D, Bantel H, Lenzen H et al Liver stiffness measurement using acoustic radiation force impulse elastography in overweight and obese patients. Aliment. Pharmacol. Ther. 2016; 44: 366–79. [DOI] [PubMed] [Google Scholar]
- 19. Takahashi H, Ono N, Eguchi Y et al Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int. 2010; 30: 538–45. [DOI] [PubMed] [Google Scholar]
- 20. Bota S, Herkner H, Sporea I et al Meta‐analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013; 33: 1138–47. [DOI] [PubMed] [Google Scholar]
- 21. Ogawa E, Furusyo N, Toyoda K et al The longitudinal quantitative assessment by transient elastography of chronic hepatitis C patients treated with pegylated interferon alpha‐2b and ribavirin. Antiviral Res. 2009; 83: 127–34. [DOI] [PubMed] [Google Scholar]
- 22. Arima Y, Kawabe N, Hashimoto S et al Reduction of liver stiffness by interferon treatment in the patients with chronic hepatitis C. Hepatol. Res. 2010; 40: 383–92. [DOI] [PubMed] [Google Scholar]
- 23. Hezode C, Castera L, Roudot‐Thorval F et al Liver stiffness diminishes with antiviral response in chronic hepatitis C. Aliment. Pharmacol. Ther. 2011; 34: 656–63. [DOI] [PubMed] [Google Scholar]
- 24. Nightingale K, Soo MS, Nightingale R et al Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med. Biol. 2012; 28: 227–35. [DOI] [PubMed] [Google Scholar]
- 25. Petta S, Cabibbo G, Barbara M et al Hepatocellular carcinoma recurrence in patients with curative resection or ablation: impact of HCV eradication does not depend on the use of interferon. Aliment. Pharmacol. Ther. 2017; 45: 160–8. [DOI] [PubMed] [Google Scholar]
- 26. Tachi Y, Hirai T, Kojima Y et al Liver stiffness measurement using acoustic radiation force impulse elastography in hepatitis C virus‐infected patients with a sustained virological response. Aliment. Pharmacol. Ther. 2016; 44: 346–55. [DOI] [PubMed] [Google Scholar]
- 27. Aoki T, Iijima H, Tada T et al Prediction of development of hepatocellular carcinoma using a new scoring system involving virtual touch quantification in patients with chronic liver diseases. J. Gastroenterol. 2017; 52: 104–12. [DOI] [PubMed] [Google Scholar]
- 28. Yoon KT, Lim SM, Park JY et al Liver stiffness measurement using acoustic radiation force impulse (ARFI) elastography and effect of necroinflammation. Dig. Dis. Sci. 2012; 57: 1682–91. [DOI] [PubMed] [Google Scholar]
- 29. Bota S, Sporea I, Peck‐Radosavljevic M et al The influence of aminotransferase levels on liver stiffness assessed by acoustic radiation force impulse elastography: a retrospective multicentre study. Dig. Liver Dis. 2013; 45: 762–8. [DOI] [PubMed] [Google Scholar]
- 30. Tada T, Kumada T, Toyoda H et al Improvement of liver stiffness in patients with hepatitis C virus infection who received direct‐acting antiviral therapy and achieved sustained virological response. J. Gastroenterol. Hepatol. 2017; doi: 10.1111/jgh.13788. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31. Tachi Y, Hirai T, Kojima Y et al Liver stiffness reduction correlates with histological characteristics of hepatitis C patients with sustained virological response. Liver Int. 2017; doi: 10.1111/liv.13486. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


