Abstract
Background and Aim
Patients requiring hemodialysis show high morbidity with hepatitis C virus (HCV) infection, but there are difficulties associated with interferon‐based therapies. Asunaprevir and daclatasvir could help patients with HCV genotype 1b because the drugs have a nonrenal metabolism and show good viral eradication. We evaluated the efficacy and safety of combined asunaprevir and daclatasvir therapy.
Methods
This was a multicenter prospective trial of patients with chronic hepatitis or compensated cirrhosis from HCV genotype 1b who had end‐stage renal disease requiring chronic hemodialysis. Asunaprevir and daclatasvir were administered orally (100 mg twice daily and 60 mg once daily, respectively) for 24 weeks. The primary end‐point was the proportion of patients achieving sustained virological response 12, defined as HCV RNA <15 IU/mL undetectable at 12 weeks after completion of asunaprevir and daclatasvir treatment.
Results
Between December 2014 and December 2015, 23 dialysis patients were enrolled, and 22 patients completed the protocol therapy. Sustained virological response 12 rates were 91.3% (95% confidence interval: 72.0–98.9) in the intention‐to‐treat and 95.5% (95% confidence interval: 77.2–99.9) in the per‐protocol populations. Serum aminotransferase significantly decreased after initiation of asunaprevir and daclatasvir (P < 0.01), although the level was low at baseline. Asunaprevir and daclatasvir were well tolerated; however, one patient could not continue because of infective endocarditis and cerebral infarction.
Conclusions
Asunaprevir and daclatasvir could help patients with chronic hepatitis C receiving hemodialysis. Close collaboration with dialysis physicians is important when treating these patients because hemodialysis carries life‐threatening risks.
Keywords: asunaprevir, daclatasvir, direct‐acting antivirals, hepatitis C virus, hemodialysis
Introduction
Hepatitis C virus (HCV) infection in patients undergoing chronic hemodialysis poses some difficulties. The HCV infection rate in dialysis patients is high, with the reported rate ranging between 1.9% and 90%, although it does vary according to geographic location and socioeconomic factors.1 HCV‐positive patients show poorer survival than HCV‐negative patients receiving dialysis because hepatocellular carcinoma (HCC) and cirrhosis are more frequent among HCV‐positive patients.2 The presence of chronic hepatitis and/or the need for antiviral treatment can sometimes be missed in hemodialysis patients with HCV infection because the serum liver transaminase levels in dialysis patients is lower than that in patients with normal renal function.3, 4 Despite low serum liver transaminase levels, severe fibrosis or cirrhosis was observed in 5–32% of hemodialysis patients with HCV infection on histological examination of the liver before renal transplantation.5 Administration of sufficient antiviral treatment is often difficult in dialysis patients because standard and pegylated interferon (IFN) α is recommended at a reduced dose to prevent increasing the blood IFN level. Ribavirin is also contraindicated in dialysis patients.6 For these reasons, a suggestion was made that those hemodialysis patients who require antiviral therapy should be considered for IFN‐free and ribavirin‐free regimens, if possible, to eradicate HCV.6, 7
Treatment for patients infected with HCV has developed greatly in recent years. Although IFN‐based therapies have been used for a long time as the mainstream treatment for HCV, the antiviral effects and safety of treatments have dramatically improved after the emergence of IFN‐free combinations using direct‐acting antivirals (DAA).8
Asunaprevir (ASV) is an NS3 protease inhibitor with antiviral activity against genotypes 1 and 4. Daclatasvir (DCV) is a selective NS5A replication complex inhibitor with broad genotypic antiviral activity.9, 10 Combination therapy with ASV and DCV had a sustained virological response (SVR) of 87.4% at 24 weeks after treatment in IFN‐ineligible/‐intolerant patients and 80.5% in nonresponder patients in a phase 3 trial for chronic HCV genotype 1b infection.11 Because ASV and DCV are metabolized by the liver and excreted in the feces, this combination therapy could be used in hemodialysis patients with HCV genotype 1b. However, when we planned the clinical study, the antiviral activities and safety in hemodialysis patients were unknown. We conducted this prospective study to evaluate the efficacy and safety of ASV and DCV combination therapy in patients with HCV genotype 1b who had a renal failure requiring chronic hemodialysis.
Methods
Ethical considerations
This study was approved by the institutional review board or ethics committee of each participating institution. This study is registered with the University Hospital Medical Information Network Clinical Trial Registry, No. UMIN000015882. All patients provided written informed consent.
Patients
Eligible patients were 20 years old or older, with chronic hepatitis or compensated cirrhosis with HCV genotype 1b infection, and who had end‐stage renal disease requiring chronic hemodialysis. Patients were required to have alanine aminotransferase (ALT) and aspartate aminotransferase levels of no more than five times the upper limit of the normal range, a white blood cell count >1500/μL, a neutrophil count >750/μL, a platelet count >50 000/μL, and a total bilirubin level < 2.0 mg/dL at the time of screening. Patients with a FIB‐4 index of >3.25 and/or an aspartate aminotransferase to platelet ratio index (APRI) >1.0 were considered to have advanced liver fibrosis.12, 13 There was no limit on history of HCV treatment. Patients were considered ineligible if they had a malignant disease, including viable HCC or decompensated cirrhosis.
Study design and assessments
This study was conducted by the Saga Study Group of Liver Diseases (SASLD), which comprises specialists in hepatology in Saga Prefecture, Japan. In this multicenter, open‐label, phase 2 study, all the patients received ASV and DCV for 24 weeks. ASV was administered orally at a dose of 100 mg twice daily, and DCV was administered orally at a dose of 60 mg once daily.
The primary efficacy end‐point was the proportion of patients achieving SVR12, defined as HCV RNA undetectable at 12 weeks after completion of ASV and DCV treatment. Patients who discontinued treatment early were also included. The secondary end‐points included the proportion of patients with undetectable HCV RNA at the end of treatment (end‐of‐treatment response). Biochemical and hematological effects and adverse events were also assessed.
The serum HCV RNA level was measured using the COBAS® TaqMan® test (Roche Molecular Diagnostics, Pleasanton, CA), which has a lower limit of quantification of 15 IU/mL. HCV RNA was tested at baseline, week 2 of treatment, every 4 weeks during treatment, end of treatment, and week 12 after completion of treatment. Pre‐existing NS5A resistance‐associated variants were tested using direct sequencing.
Statistical analyses
We assumed an expected SVR12 of 0.85 and a threshold SVR12 of 0.60, which were determined in previous studies.11, 14 The planned sample size was 20 patients in a one‐sided test with 90% power and an α error of 0.1.
All analyses were performed on the intention‐to‐treat population, which was defined as patients who received at least one dose of the protocol treatment (ASV and DCV). The primary end‐point was also tested in the per‐protocol population, which was defined as patients who achieved the protocol treatment for 24 weeks. The 95% confidence intervals (CI) were based on two‐sided binomial tests. Categorical data were summarized using counts and percent. Continuous data were summarized with univariate statistics. Mann–Whitney U tests and Friedman's tests were used to test for changes in biochemical and hematological values. All statistical analyses were performed using R ver. 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients
Between December 2014 and December 2015, 23 dialysis patients were enrolled from seven sites of the SASLD. All enrolled patients started combined ASV and DCV, and 22 patients completed the protocol therapy.
Patient characteristics at baseline are shown in Table 1. Median age was 65 years, and the majority were male (78%). Five patients were considered to have advanced liver fibrosis, and two patients had an NS5A resistance‐associated variant at baseline. Three patients had previously received treatment for HCC; however, they maintained complete remission at baseline in this study. Two patients with a treatment history of IFN had been treated with pegylated IFNα 2a for 48 weeks.
Table 1.
Baseline characteristics
| Characteristic | n = 23 |
|---|---|
| Age, years, median (range) | 65 (46–86) |
| Gender, n (%) | |
| Male | 18 (78) |
| Female | 5 (22) |
| Diabetic nephropathy, n (%) | 10 (43) |
| Advanced liver fibrosis, n (%) | 5 (22) |
| Hemoglobin level, g/dL, median (range) | 11.4 (8.2–13.9) |
| Platelet count, ×104/μL, median (range) | 14.4 (5.5–23.0) |
| Serum creatinine level, mg/dL, median (range) | 8.33 (3.61–14.23) |
| Serum albumin level, g/dL, median (range) | 3.6 (2.5–4.6) |
| AST level, U/L, median (range) | 21 (11–36) |
| ALT level, U/L, median (range) | 15 (6–62) |
| γ‐GT level, U/L, median (range) | 28 (11–100) |
| Total bilirubin level, mg/dL, median (range) | 0.4 (0.2–0.8) |
| HCV RNA level, log IU/mL, median (range) | 5.6 (3.7–6.4) |
| Alpha fetoprotein level, ng/mL, median (range) | 3 (1–14) |
| FIB‐4 index, n (%) | |
| >3.25 | 5 (22) |
| APRI, n (%) | |
| >1.0 | 1 (4) |
| Previous interferon treatment, n (%) | 2 (9) |
| Previous HCC treatment, n (%) | 3 (13) |
| NS5A resistance‐associated variant, n (%) | |
| L31M | 1 (4) |
| Y93L | 1 (4) |
APRI, aspartate aminotransferase to platelet ratio index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ‐GT, γ‐glutamyl transpeptidase; HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
Virological response
The lower limit of quantification for serum HCV RNA was 47.8% at week 2 of treatment, and all patients achieved rapid virological response at week 4 (Fig. 1). Rates for the rapid virological response and early virological response were 100%; however, because a patient was withdrawn at week 11 due to infective endocarditis and cerebral infarction, the rate for the end‐of‐treatment response was 95.7% (95% CI: 78.1–99.9). The rate for SVR12 as the primary end‐point was 91.3% (95% CI: 72.0–98.9) as one patient, who did not have NS5A resistance‐associated variant before the protocol treatment, relapsed 4 weeks after completion of treatment. In the per‐protocol population, the rate for SVR12 was 95.5% (95% CI: 77.2–99.9). SVR12 was confirmed in all five patients who were considered to have advanced liver fibrosis, all two patients who had a history of IFN therapy, and all two patients with a pre‐existing NS5A resistance‐associated variant.
Figure 1.
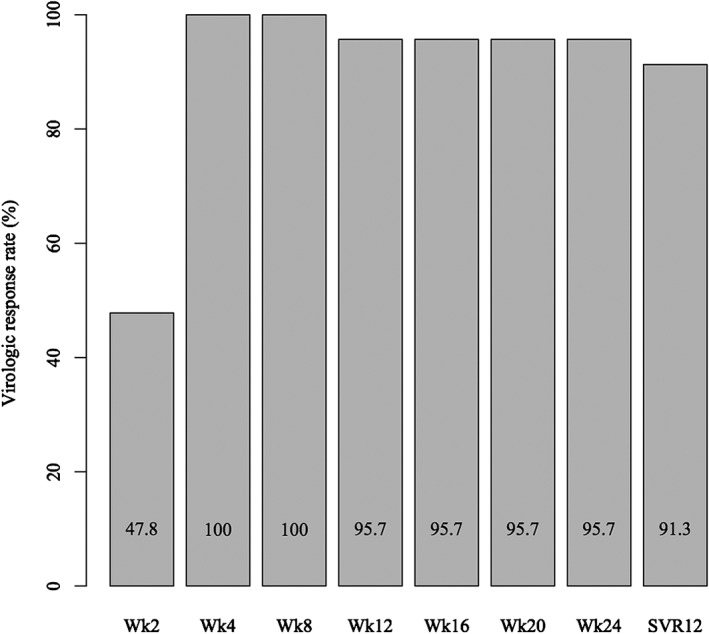
The rates of serum hepatitis C virus (HCV) RNA undetectable from 2 weeks after initiation to 12 weeks after completion with combined asunaprevir and daclatasvir treatment. Rapid virological response and early virological response rates were 100%, and the rate of sustained virological response (SVR) 12 was 91.3% in the intention‐to‐treat population.
Biochemical and hematological response
Serum ALT levels were significantly decreased after initiation of treatment (P < 0.01), although levels in hemodialysis patients were relatively low at baseline (Fig. 2a). The median ALT level changed from 15 U/L at baseline to 8 U/L at the end of treatment. Serum γ‐glutamyl transpeptidase (γ‐GT) levels also decreased gradually (P < 0.01). The median γ‐GT level changed from 28 U/L at baseline to 16 U/L at the end of treatment (Fig. 2b). Bilirubin, hemoglobin, and platelet levels did not show any significant changes. Serum albumin and alpha fetoprotein (AFP) levels were measured at baseline and at the end of treatment, and AFP tended to decrease over time (Fig. 2c). The median AFP level changed from 3.0 ng/mL at baseline to 2.1 ng/mL at the end of treatment; however, there was no statistically significant difference (P = 0.37). Serum albumin levels did not show a significant change. Although the FIB‐4 index and APRI did not significantly improve over the study period, median APRI tended to decrease from baseline (0.39) to week 24 (0.29) (P = 0.08) (Fig. 2d).
Figure 2.
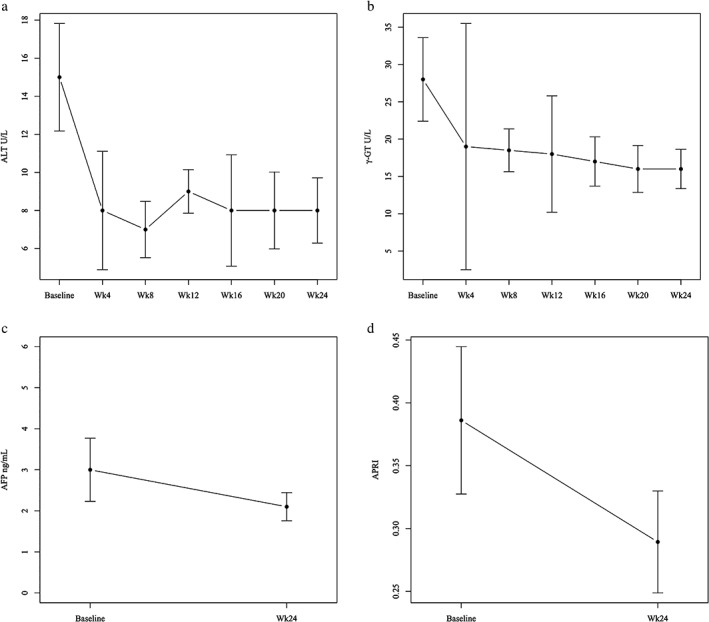
Changes in biochemical data during asunaprevir and daclatasvir treatment. (a) Median serum alanine aminotransferase (ALT), (b) γ‐glutamyl transpeptidase (γ‐GT), (c) alpha fetoprotein (AFP), and (d) aspartate aminotransferase to platelet ratio index (APRI) levels decreased after initiation of combined asunaprevir and daclatasvir treatment. Serum ALT and γ‐GT levels significantly decreased (P < 0.01).
Safety
Serum ALT levels slightly increased in three patients between weeks 6 and 14. Two of these patients, with ALT levels of 35 and 42 U/mL at week 6, respectively, continued the protocol treatment with concurrent ursodeoxycholic acid and showed improvement approximately 1 month later. The other patients, with ALT levels of 100 U/L at week 14, continued the protocol treatment after the rate of a dose reduction of ASV to a dose intensity of ASV was 81%. All the three patients achieved SVR12. Mild fatigue and pruritus were observed in one patient each but did not affect the protocol treatment.
A male patient had repeated hypotension during hemodialysis after initiation of ASV and DCV. This patient did complete the study treatment following careful consideration and cooperation with the dialysis physician. The phenomenon was resolved after the protocol treatment.
A hemorrhagic duodenal ulcer occurred in one patient, who underwent endoscopic hemostasis and blood transfusion. This patient continued the protocol treatment with temporary interruption and achieved SVR12.
A female patient died of infective endocarditis and cerebral infarction during the protocol treatment. She was admitted with a fever at 9 weeks after initiating ASV and DCV and was diagnosed with bacteremia caused by Staphyrococcus epidermidis. Although antibiotics had been administered under continuing ASV and DCV therapy because of fairly general conditions, she had a cerebral infarction 1 week after admission. This was considered to be caused by infective endocarditis. ASV and DCV treatment was stopped. She subsequently developed myocardial ischemia and could not continue hemodialysis. She died 2 weeks after admission. Following careful review, the institutional review board and we did not consider her death related to the study drugs, and the trial was allowed to continue.
Discussion
This study examining combined ASV and DCV therapy in patients with chronic HCV genotype 1b infection and requiring hemodialysis showed promising antiviral effects and met the primary outcome measure. Before the DAA, standard and pegylated IFNα monotherapy were the main treatment strategies for hemodialysis patients with chronic HCV infection. Standard IFNα should be administered at a reduced dose of 3 million units three times a week, and pegylated IFNα 2a or 2b should be administered at a reduced dose of 135 μg and 1 μg/kg once a week, respectively.1 These therapies do not exhibit a satisfactory antiviral effect, with the pooled SVR24 of standard and pegylated IFNα monotherapy reported to be approximately 40% in some meta‐analyses.15, 16, 17 Suda et al. and Toyoda et al. reported that ASV and DCV in patients receiving hemodialysis showed a rate for SVR12 of 95.5% and 100%, respectively.18, 19 Considering these results together with our own findings in the current study, combined ASV and DCV therapy could be a potential standard treatment for patients requiring hemodialysis who have chronic HCV genotype 1b infection. Grazoprevir plus elbasvir for 12 weeks was also effective in patients with HCV genotype 1 and stage 4–5 chronic kidney disease.20 The window of opportunity for hemodialysis patients with HCV has been extended.
Although ASV and DCV is feasible in patients with end‐stage renal disease,18, 19 hemodialysis itself does carry life‐threatening risks. In the current study, one patient had a hemorrhagic duodenal ulcer, and another patient died from infective endocarditis and cerebral infarction during the treatment study period.
In hemodialysis patients with end‐stage renal disease, bacteremia is relatively common (one episode per 100 patient‐care months) because of frequent intravascular access through arteriovenous shunts.21 Additionally, infective endocarditis is significantly more common in these patients and has a higher mortality than in the general population, with previous studies suggesting an incidence of 17–60 times.22 The addition of a protease inhibitor to IFN and ribavirin might increase the risk of infection due to neutrophil dysfunction induced by protease inhibitors.23
It remains unknown whether the SVR with combined ASV and DCV can prevent the development of HCC and improve survival in dialysis patients. A study has reported that the risk of HCC after SVR with DAA was comparable with that of IFN‐based therapies in patients who did not need hemodialysis.24 To determine this question, our group is carrying out a prospective cohort study of hepatocarcinogenesis in patients after SVR with DAAs, including dialysis patients (UMIN000016982).
In conclusion, combined ASV and DCV therapy could be an optimal choice for patients with chronic hepatitis from HCV and who are receiving hemodialysis. Although this treatment shows good safety, hemodialysis itself carries risk factors that can result in lethal events. Particular care should be taken when considering the treatment of these patients, and consultations with dialysis physicians are vital.
Acknowledgments
We thank all the patients and their families for their participation in the study. We also thank all the following hemodialysis physicians for their cooperation with the study: Keisuke Kono (Imamura Hospital), Kazuhide Takahara (Takahara Clinic), Junichi Makino (Makino Clinic), Nobumitsu Okita (Shiroishi Kyouritsu Hospital).
Declaration of conflict of interest: The authors have no competing interests or financial disclosures to declare.
References
- 1. YC Y, Wang Y, He CL, Wang MR, Wang YM. Management of hepatitis C virus infection in hemodialysis patients. World J. Hepatol. 2014; 6: 419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fabrizi F, Takkouche B, Lunghi G, Dixit V, Messa P, Martin P. The impact of hepatitis C virus infection on survival in dialysis patients: meta‐analysis of observational studies. J. Viral Hepat. 2007; 14: 697–703. [DOI] [PubMed] [Google Scholar]
- 3. Espinosa M, Martin‐Malo A, Alvarez de Lara MA, Soriano S, Aljama P, High ALT. levels predict viremia in anti‐HCV‐positive HD patients if a modified normal range of ALT is applied. Clin. Nephrol. 2000; 54: 151–6. [PubMed] [Google Scholar]
- 4. Yuki N, Ishida H, Inoue T et al Reappraisal of biochemical hepatitis C activity in hemodialysis patients. J. Clin. Gastroenterol. 2000; 30: 187–94. [DOI] [PubMed] [Google Scholar]
- 5. Meyers CM, Seeff LB, Stehman‐Breen CO, Hoofnagle JH. Hepatitis C and renal disease: an update. Am. J. Kidney Dis. 2003; 42: 631–57. [DOI] [PubMed] [Google Scholar]
- 6. Akiba T, Hora K, Imawari M et al 2011 Japanese Society for Dialysis Therapy guidelines for the treatment of hepatitis C virus infection in dialysis patients. Ther. Apher. Dial. 2012; 16: 289–310. [DOI] [PubMed] [Google Scholar]
- 7. European Association for Study of Liver . EASL recommendations on treatment of hepatitis C 2015. J. Hepatol. 2015; 63: 199–236. [DOI] [PubMed] [Google Scholar]
- 8. Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet. 2015; 385: 1124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McPhee F, Sheaffer AK, Friborg J et al Preclinical profile and characterization of the Hepatitis C Virus NS3 Protease Inhibitor Asunaprevir (BMS‐650032). Antimicrob. Agents Chemother. 2012; 56: 5387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao M. Antiviral activity and resistance of HCV NS5A replication complex inhibitors. Curr. Opin. Virol. 2013; 3: 514–20. [DOI] [PubMed] [Google Scholar]
- 11. Kumada H, Suzuki Y, Ikeda K et al Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014; 59: 2083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wai C, Greenson JK, Fontana RJ et al A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003; 38: 518–26. [DOI] [PubMed] [Google Scholar]
- 13. Sterling RK, Lissen E, Clumeck N et al Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43: 1317–25. [DOI] [PubMed] [Google Scholar]
- 14. Suzuki Y, Ikeda K, Suzuki F et al Dual oral therapy with daclatasvir and asunaprevir for patients with HCV genotype 1b infection and limited treatment options. J. Hepatol. 2013; 58: 655–62. [DOI] [PubMed] [Google Scholar]
- 15. Gordon CE, Uhlig K, Lau J, Schmid CH, Levey AS, Wong JB. Interferon treatment in hemodialysis patients with chronic hepatitis C virus infection: a systematic review of the literature and meta‐analysis of treatment efficacy and harms. Am. J. Kidney Dis. 2008; 51: 263–77. [DOI] [PubMed] [Google Scholar]
- 16. Fabrizi F, Dixit V, Messa P, Martin P. Pegylated interferon monotherapy of chronic hepatitis C in dialysis patients: Meta‐analysis of clinical trials. J. Med. Virol. 2010; 82: 768–75. [DOI] [PubMed] [Google Scholar]
- 17. Alavian SM, Tabatabaei SV. Meta‐analysis of factors associated with sustained viral response in patients on hemodialysis treated with standard or pegylated interferon for hepatitis C infection. Iran. J. Kidney Dis. 2010; 4: 181–94. [PubMed] [Google Scholar]
- 18. Suda G, Kudo M, Nagasaka A et al Efficacy and safety of daclatasvir and asunaprevir combination therapy in chronic hemodialysis patients with chronic hepatitis C. J. Gastroenterol. 2016; 51: 733–40. [DOI] [PubMed] [Google Scholar]
- 19. Toyoda H, Kumada T, Tada T et al Safety and efficacy of dual direct‐acting antiviral therapy (daclatasvir and asunaprevir) for chronic hepatitis C virus genotype 1 infection in patients on hemodialysis. J. Gastroenterol. 2016; 51: 741–7. [DOI] [PubMed] [Google Scholar]
- 20. Roth D, Nelson DR, Bruchfeld A et al Grazoprevir plus elbasvir in treatment‐naive and treatment‐experienced patients with hepatitis C virus genotype 1 infection and stage 4‐5 chronic kidney disease (the C‐SURFER study): a combination phase 3 study. Lancet. 2015; 386: 1537–45. [DOI] [PubMed] [Google Scholar]
- 21. Powe NR, Jaar B, Furth SL, Hermann J, Briggs W. Septicemia in dialysis patients: incidence, risk factors, and prognosis. Kidney Int. 1999; 55: 1081–90. [DOI] [PubMed] [Google Scholar]
- 22. Nucifora G, Badano LP, Viale P et al Infective endocarditis in chronic haemodialysis patients: an increasing clinical challenge. Eur. Heart J. 2007; 28: 2307–12. [DOI] [PubMed] [Google Scholar]
- 23. Londoño MC, Perelló C, Cabezas J et al The addition of a protease inhibitor increases the risk of infections in patients with hepatitis C‐related cirrhosis. J. Hepatol. 2015; 62: 311–6. [DOI] [PubMed] [Google Scholar]
- 24. Kobayashi M, Suzuki F, Fujiyama S et al Sustained virologic response by direct antiviral agents reduces the incidence of hepatocellular carcinoma in patients with HCV infection. J. Med. Virol. 2017; 89: 476–83. [DOI] [PubMed] [Google Scholar]


