Abstract
Background
The utility of fecal calprotectin (FC) in small intestinal Crohn's disease (CD) is unclear. We examined how reliably FC reflects clinical and mucosal disease activity in small intestinal CD, colonic CD, and ulcerative colitis (UC).
Methods
A total of 72 Inflammatory Bowel Disease (IBD) patients (23 colonic CD, 14 isolated small intestinal CD, and 35 UC) were included. Clinical activity was assessed using the Harvey–Bradshaw Index (HBI) (CD) and Mayo score (UC). Inflammatory activity was assessed through ileocolonoscopy, cross‐sectional imaging, C‐reactive protein (CRP), and FC. Clinical activity was defined as HBI > 4 or Mayo clinical score ≥ 3. Endoscopy activity was defined as Mayo endoscopic subscore ≥ 1, SES‐CD score ≥ 3, and Rutgeerts > i1.
Results
In UC, FC was correlated with the Mayo clinical score (P < 0.0001) and was highly correlated with the total Mayo score (P < 0.0001). A cut‐off value of FC 100 μg/g provided sensitivity of 88% and specificity 100% for endoscopic activity. FC was lower for patients with endoscopic and clinical remission compared to active endoscopic disease (median 100 vs 1180 μg/g, P < 0.0001). In colonic CD, there was a significant correlation between FC and endoscopic activity (P < 0.001). For an FC cut‐off value of 100 μg/g, sensitivity was 100%, and specificity was 67%. In contrast, for isolated small intestinal CD, there was no significant correlation between FC and objective disease activity measured by either endoscopy or imaging (AUC 0.52, P = 0.58).
Conclusion
FC is reliable for the detection of colonic mucosal inflammation in both UC and CD but is less sensitive and reliable in the detection of small intestinal CD.
Keywords: Crohn's colitis, endoscopy remission, fecal calprotectin, isolated small intestinal Crohn's disease, ulcerative colitis
Introduction
The inflammatory bowel diseases (IBD), ulcerative colitis (UC) and Crohn's disease (CD), are idiopathic, lifelong, chronic intestinal inflammatory conditions characterized by periods of remission and recurrent relapses.1 These periods of relapse are typically unpredictable and without clear inciting factors. A subset of patients has persistent symptomatic disease without true clinical remissions. The main goal of medical therapy in patients with UC and CD is to achieve the effective and sustained suppression of intestinal inflammation and, in addition, to induce and maintain clinical remission.2 Identifying patients at a significant risk of relapse during clinical remission or with active intestinal inflammation that is not reflected by other objective measurements might justify changes in maintenance therapy during symptomatic remission.3
Diagnosis and monitoring of IBD rely on clinical, endoscopic, and radiological parameters.4 One of the biggest challenges in the management of IBD is the prediction of relapse in patients in symptomatic remission prior to the onset of subjective symptoms. Ideally, this would be conducted without the use of invasive tests, such as colonoscopy, which can detect minor degrees of mucosal inflammation that appear to predict relapse.
Treatment strategies based only on presenting clinical manifestations have failed to modify the course of IBD.5, 6, 7, 8 Furthermore, clinical disease activity indices, such as the Harvey–Bradshaw Index (HBI) and the Mayo clinical score, do not correlate well with endoscopic activity.9 A significant proportion of patients in symptomatic remission demonstrate evidence of active mucosal inflammation at colonoscopy. Endoscopic mucosal disease activity has been shown to be one of the best predictors of clinical relapse, disease progression, and occurrence of complications.10, 11
Previous studies suggested that fecal markers might fulfill all the criteria of being noninvasive, simple, inexpensive, markers to detect gastrointestinal inflammation.12 Calprotectin is a calcium‐binding protein that accounts for approximately 60% of the cytosolic protein in neutrophils and has antimicrobial and antiproliferative properties.13, 14 As calprotectin is primarily derived from neutrophils, its concentration is directly proportional to the concentration of neutrophils in the colonic/rectal mucosa.12, 14 It is resistant to bacterial degradation in the gut and is stable in the stool for up to 4 days at room temperature.14 During intestinal inflammation, leukocytes invade the mucosa, resulting in an increase in the excretion of calprotectin into the stool.13, 14 Several studies have compared fecal markers with disease activity indices and endoscopic/histological evaluations.15, 16, 17 Previous studies have shown a good correlation between fecal calprotectin (FC) and endoscopic activity for UC, but studies examining CD have not consistently shown correlations with objective measures of intestinal inflammation. Recently published prospective data suggest that low FC levels predict endoscopic remission in postoperative ileal CD.18 Our hypothesis is that FC is a good measure of colonic inflammation, irrespective of whether it is due to UC or CD, but is not as good a measure of small intestinal inflammation.16, 19
The present study was designed to determine how reliably FC levels reflect clinical and mucosal disease activity in small intestinal CD, as compared to colonic CD and UC.
Materials and methods
Study population
Adult patients (18–75 years of age) with colonic IBD (colonic CD [Montreal classification L2] or UC) or isolated small intestine CD, ileal, and proximal small bowel disease (Montreal classification L1)20 who were attending the Mount Sinai Hospital IBD Centre, a tertiary referral center, were identified and invited to participate at least 3 days before a scheduled ileocolonoscopy and cross‐sectional imaging study (magnetic resonance enterography). All eligible cases had a definite diagnosis of IBD based on standard clinical, radiological, endoscopic, and histological criteria. Demographics and clinical information were obtained through chart review and patient interview. These patients had a documented ileocolonoscopy and cross‐sectional imaging study (Magnetic Resonance Enterography [MRE]) as a part of routine follow‐up in our institution or for the assessment of symptoms or possible disease relapse. Excluded patients included those with upper gastrointestinal CD involvement (L4), those receiving concomitant nonsteroidal anti‐inflammatory drugs, those with cancer, and those with acute or chronic enteric infections (e.g. Clostridium difficile). The study was approved by the institutional research ethics board at Mount Sinai Hospital.
Data collection
Clinical disease activity was recorded at time of endoscopic assessment by the HBI21 for CD and the Mayo clinical score22 for UC or IBD‐U. Clinical activity was defined as HBI > 4 or Mayo clinical score ≥ 3. The Simple Endoscopic Score‐CD (SES‐CD)23 and the Rutgeerts score24 for postoperative CD subjects were used to assess endoscopic activity in patients with CD. All patients with isolated small intestine CD (L1) were assessed by ileocolonoscopy, C‐reactive protein (CRP), and MRE. Radiographic evidence of activity was defined by the Magnetic Resonance Index of Activity, which incorporates wall thickness, relative contrast enhancement, edema, and ulcers, as previously described by Ordas et al. and Rimola et al.25, 26 The Mayo endoscopic subscore27 was used to assess endoscopic activity in UC (0 = normal 1 = mild, 2 = moderate or 3 = severe). Active endoscopic disease in CD was defined as SES‐CD score ≥ 328 or Rutgeerts > i1.24 CRP (mg/L) was also measured. Patients were instructed to collect the first stool in the morning for FC (μg/g) 2 days before preparation for colonoscopy, in addition to MRE. FC was measured from fresh stool samples using the Buhlmann Quantum Blue Calprotectin High Range immunoassay (100–1800 μg/g).29, 30 FC values < 100 μg/g were considered to be normal.3, 31, 32
Statistical analysis
Spearman's correlations, chi‐square, binary logistic regression, and receiver operator characteristic (ROC) curve analyses were undertaken. For binary data, Fisher's exact test was used to determine significance, and the Kruskall–Wallis test was used to compare continuous and ordinal variables between outcome groups. Preference was given to exact and nonparametric analysis in order to avoid assumptions of distribution in the collected data. Correlations between FC levels and defined clinical, endoscopic, and histological remission were calculated. Sensitivity, specificity, and ROC curve analyses were determined. Statistical analysis was performed using SPSS v 18.0 (IBM, Chicago, IL, USA). A P‐value ≤ 0.05 was deemed to be significant.
Results
A total of 72 IBD patients—23 colonic CD, 14 isolated small intestinal CD (6/14 post ileocolonic resection), and 35 UC—were included. Table 1 displays patient characteristics for the total cohort. Age, gender, CRP, and proportion of patients with clinically active disease or endoscopically/radiographically active disease did not differ significantly among the colonic CD, isolated small intestinal, and UC group. Of the UC patients, 59% were clinically active (Mayo clinical score > 3) as compared to 53.2 and 62% who clinically active (HBI > 4) in the colonic CD and isolated small intestinal CD groups, respectively. Endoscopic disease activity (Mayo endoscopic score ≥ 1) was seen in 69% of the UC patients compared to 43% of patients with colonic CD and 70% of patients with isolated small intestinal CD patients who had endoscopic (SES‐CD score ≥ 3, Rutgeerts > i1) and/or radiological evidence of active disease (P = 0.096). However, there was a trend toward lower‐median FC in the isolated small intestinal CD (135 μg/g, interquartile range (IQR) [100–509.5]) compared to colonic CD (204 μg/g, IQR [100–1091] and UC patients (795 μg/g, IQR [100–1251] (P = 0.06).
Table 1.
Patient demographics
| Variable | UC | Colonic CD | Isolated small bowel CD | P value |
|---|---|---|---|---|
| Age (years, median [IQR]) | 34 [24–52] | 39 [25.7–56] | 36 [24–60] | 0.42 |
| Gender (% male) | 44 | 45.9 | 40 | 0.74 |
| Clinically active (%) for CD HBI > 4 and for UC Mayo clinical score ≥ 3 | 59 | 53.2 | 62 | 0.61 |
| Endoscopically/radiographic evidence of activity (%) | 69 | 43 | 70 | 0.096 |
| CRP (median [IQR]; mg/L) | 1.2 [0.6–4.9] | 3.3 [1.3–11.75] | 3.05 [1.1–4.8] | 0.24 |
| FC (median [IQR]; μg/g) | 795 [100–1251] | 204 [100–1091] | 135 [100–509.5] | 0.06 |
CD, Crohn's disease; CRP, C‐reactive protein; FC, fecal calprotectin; HBI, Harvey–Bradshaw Index; IQR, interquartile range; UC, ulcerative colitis.
Clinical activity
For the entire cohort, the AUC for the use of FC in determining the presence of clinical activity (HBI > 4 for CD and Mayo clinical score ≥ 3 for UC) was 0.74 (95% CI [0.62–0.86]; P = 0.001). An FC cut‐off value of 100 μg/g provided a sensitivity of 84% and specificity of 47%, and a cut‐off value of 200 μg/g provided a sensitivity of 90% and specificity of 45%.
When stratifying the cohort by disease location, a distinct difference in the association with active disease became apparent. In UC patients, there was a significant correlation between FC levels and the Mayo clinical score, which excludes the endoscopic subscore (r = 0.63, P < 0.0001). In CD patients, there was a weak correlation between HBI and FC, but this was not statistically significant (r = 0.494, P = 0.11).
The AUC for FC and clinical activity index in the UC population was 0.83 (95% CI [0.70–0.97]; P < 0.001). The AUC for the clinical activity index in isolated small intestinal CD was 0.45 (95% CI [0.13–0.75]; P = 0.6), with sensitivity of 71% and a specificity of 47% for an FC cut‐off value of 100 μg/g and 71 and 56 for an FC cut‐off value 200 μg/g. The AUC for the clinical activity index in colonic CD was 0.72 (95% CI [0.49–0.95]; P = 0.09). A cut‐off value for FC of 100 μg/g had a sensitivity of 80% and a specificity of 50% and 200 μg/g had sensitivity of 83% and specificity of 50% (Table 2).
Table 2.
Summary of predictive values of FC for objective and clinical activity
| AUC for FC | PPV | NPV | Sensitivity | Specificity | |||||
|---|---|---|---|---|---|---|---|---|---|
| >100 | >200 | >100 | >200 | >100 | >200 | >100 | >200 | ||
| UC clinical activity (Mayo clinical score ≥ 3) | 0.83, 95% CI: [0.70–0.97] P < 0.001 | 73 | 73 | 78 | 78 | 91 | 91 | 67 | 67 |
| UC endoscopic activity | 0. 97, 95% CI: [0.91–1] P < 0.001 | 100 | 100 | 100 | 100 | 88 | 88 | 100 | 100 |
| Colonic CD clinical activity (HBI > 4) | 0.72, 95% CI: [0.49–0.95] P = 0.09 | 80 | 84 | 71.4 | 68 | 80 | 83 | 50 | 50 |
| Colonic CD endoscopic activity | 0.95, 95% CI: [0.88–1] P < 0.001 | 67 | 60 | 100 | 100 | 100 | 100 | 67 | 60 |
| Isolated SB CD clinical activity (HBI > 4) | 0.45, 95% CI: [0.13–0.75] P = 0.6 | 50 | 56 | 37.5 | 40 | 71 | 71 | 47 | 56 |
| Isolated SB CD radiographic/endoscopic activity | 0.52, 95% CI [0.22–0.96] P = 0.6 | 75 | 75 | 50 | 53 | 75 | 75 | 50 | 47 |
CD, Crohn's disease; CRP, C‐reactive protein; FC, fecal calprotectin; HBI, Harvey–Bradshaw Index; Isolated SB CD, isolated small bowel CD; PPV, Positive Predictive Value; NPV, Negative Predictive Value; UC, ulcerative colitis.
Objective activity
For the entire cohort, the AUC for FC for the presence of objective disease activity (Mayo endoscopic sub‐score ≥ 1 for UC and SES‐CD score ≥ 3 and/or radiographic evidence of activity in colonic CD and isolated small intestinal CD, respectively) was 0.91 (95% CI [0.82–1]; P < 0.0001) with a sensitivity of 92% and specificity of 80% for FC > 100 μg/g and 92% sensitivity and 70% specificity for FC > 200 μg/g.
When examining the correlation between FC and objective measurements of disease activity, there was also a distinction in relation to disease phenotype and disease location. There was significant correlation between FC levels and Mayo endoscopy subscore (r = 0.96 P < 0.001). The AUC for FC and endoscopic activity in UC was 0.97 (95% CI [0.91–1.0]; P < 0.001) (Fig. 1). A cut‐off value of FC 100 μg/g had a sensitivity of 88% and a specificity of 100%, and an FC of 200 had a sensitivity of 88% and specificity of 100% (Table 2).
Figure 1.
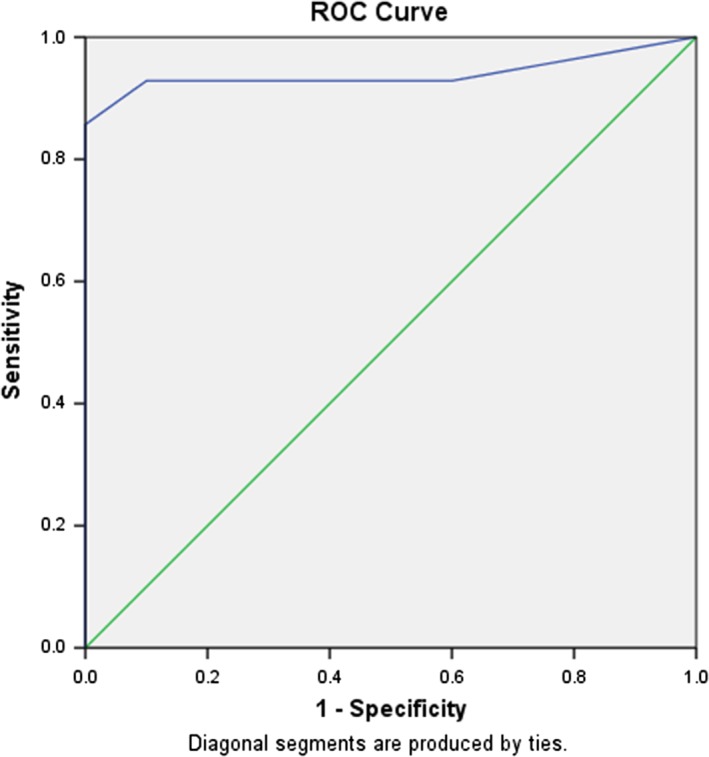
Receiver operator characteristic (ROC) curve for fecal calprotectin (FC) and endoscopic disease activity in ulcerative colitis (UC) patients. The AUC for FC and endoscopic activity in UC was 0.97 (95% CI [0.91–1]; P < 0.001). A cut‐off value of FC 100 μg/g had a sensitivity of 88% and a specificity of 100%.
In colonic CD, there was also a significant correlation between FC level and endoscopic activity (r = 0.74, P < 0.001). The AUC for FC and endoscopic activity in colonic CD was 0.95 (95% CI [0.88–1.0]; P < 0.001). A cut‐off value of FC 100 μg/g had a sensitivity of 100% and a specificity of 67% and 200 μg/g had 100% sensitivity and 60% specificity (Fig. 2). For isolated small bowel CD, there was a poor correlation between FC and objective disease activity measured by endoscopy or imaging (r = 0.24, P = 0.2). The AUC in isolated small bowel CD for FC and endoscopic and/or imaging activity was 0.52 (95% CI [0.22–0.96]; P = 0.6) (Fig. 3). A cut‐off value of FC 100 μg/g had a sensitivity of 75% and a specificity of 50% and 200 μg/g had sensitivity of 75% and specificity of 47%.
Figure 2.
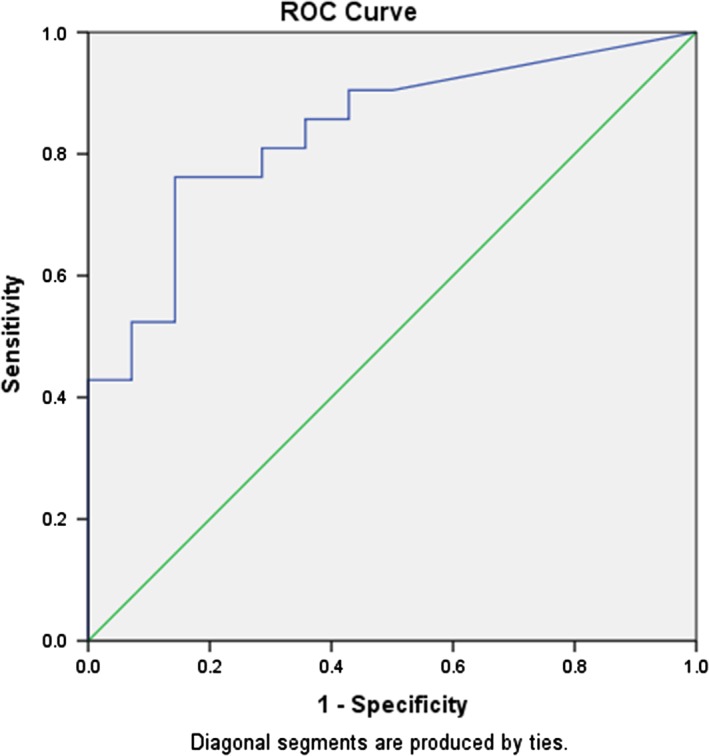
Receiver operator characteristic (ROC) curve for fecal calprotectin (FC) and endoscopic disease activity in colonic Crohn's disease (CD). The AUC for FC and endoscopic activity in colonic CD was 0.95 (95% CI [0.88–1]; P < 0.001). A cut‐off value of FC 100 μg/g had a sensitivity of 100% and a specificity of 67%.
Figure 3.
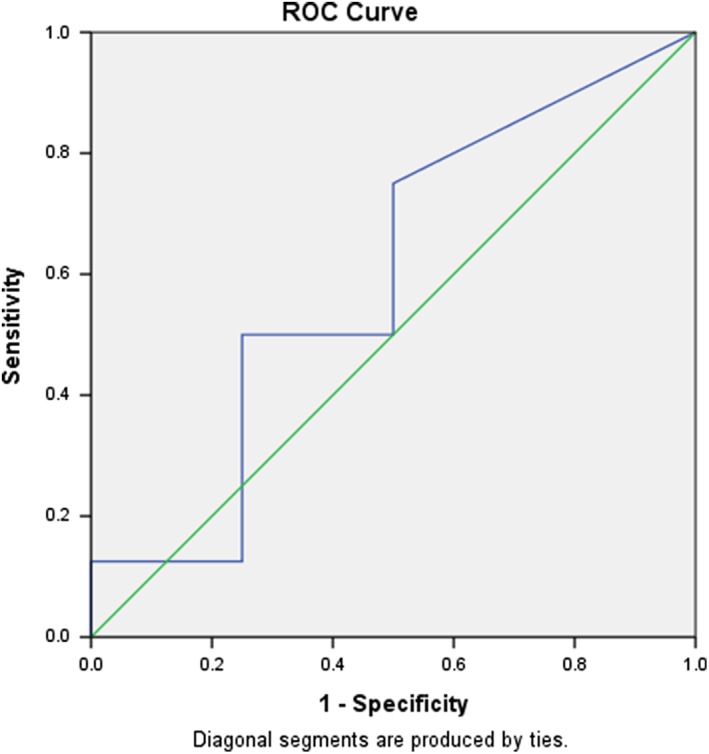
Receiver operator characteristic (ROC) curve for fecal calprotectin (FC) and endoscopic disease activity in isolated small bowel Crohn's disease (CD). The AUC for FC and endoscopic activity was 0.52 (95% CI [0.22–0.96]; P = 0.6). A cut‐off value of FC 100 μg/g had a sensitivity of 75% and a specificity of 50%.
Discussion
This study demonstrates that FC for the detection of mucosal inflammation in small bowel CD is not as reliable as it is in colonic CD or in UC patients. These results are in contrast with some previous studies that found FC to be a reliable noninvasive stool biomarker for both UC and CD patients.16, 19, 33 In the last decade, there have been multiple studies supporting the use of FC as a noninvasive stool marker to monitor intestinal inflammation in patients with colonic or ileocolonic disease.11, 19, 34 In 2011, Jensen et al. showed, for the first time, that FC is equally sensitive in CD disease affecting the small bowel and colon.32 However, in that study, 60% of the patients provided a stool sample after ileocolonoscopy, which could have explained elevated levels of FC in CD patients as a result of bleeding or inflammation caused by biopsies taken during the procedure.33 Furthermore, recent study by Cerrillo et al. showed that FC correlates with the degree of MRE inflammatory activity and with surgical pathology damage in ileal CD.35 One caveat of that study was the heterogeneity of the CD population studied, and only 28 patients had confirmed histology results from surgical specimens. Furthermore, none of the 120 patients had an ileocolonoscopy. Unlike Cerrillo et al., in our study, all patients with colonic IBD and isolated small intestine CD (L1) were assessed by ileocolonoscopy and CRP. Moreover, when disease activity was evaluated by objective measures, FC (NPV biomarker) did not correlate well with disease activity in the small bowel CD population. However, when stratified by disease location, endoscopic activity significantly correlated with FC level in those with colonic CD with AUC 0.95 (95% CI [0.88–1]; P < 0.001)) but not with small intestinal CD activity with an AUC of 0.52 (95% CI [0.22–0.96]; P = 0.6). This finding is important because the majority of patients with CD have small bowel involvement and, in one‐third of CD cases, there is isolated small disease.36 Unlike the findings in patients with colonic IBD, either UC, IBD‐U, or colonic CD where FC is a sensitive and specific biomarker of mucosal inflammation, these results suggest that FC is not a reliable biomarker in small intestinal CD.
One explanation for these findings might be the differences in peristalsis and motility between the small and large intestines, with slower transit typically in the colon. This could impact the FC concentration by altering the degradation of calprotectin in the small intestine by proteases that are normally present in the small intestine but not in the large intestine. Another possible explanation may be the differences in the inflammatory disease burden between those with small and large intestinal inflammation. Some of the patients with isolated small intestinal CD had only a short segment of inflamed bowel and, as a result, potentially less loss of neutrophils and their contents into the intestinal lumen.
Our findings in colonic IBD support the current concept that FC correlates closely with endoscopic measures of disease activity in colonic IBD. More specifically, we showed that FC levels were higher with more severe colonic endoscopic disease (Kruskal–Wallis, P < 0.0001). Several studies have previously looked at the value of FC in IBD. In some of those studies, there was a disagreement regarding the FC “cut‐off” value, especially when different CD locations were considered (L1, L2, L3). D'Haens et al. proposed a cut‐off value of 250 μg/g for both CD and UC with specificity of 79% and sensitivity of 60% for predicting the presence of any mucosal inflammation in UC or the presence of more severe lesions, such as large ulcers, in CD. However, in CD, the use of FC was not as reliable as in UC for mucosal inflammation.16 In addition, Sipponen et al.19, 34 and D'Inca et al.37 found that FC was correlated with SES‐CD with a different “cut‐off” value of 100 μg/g. In the present study, the FC test correlated well with clinical symptoms in UC but less well in colonic CD. The same phenomenon has been observed for the correlation between symptoms and endoscopic appearance. In UC, FC correlated with the Mayo clinical score, and this correlation was strengthened by the addition of the endoscopic subscore. Our results are in agreement with previous studies which also showed that, in UC, the subjective symptoms, such as rectal bleeding and stool frequency, reflect that endoscopic status and endoscopy contributed little to the Mayo clinical score.38 In contrast, in CD, our results support the results of previous studies that found that disease activity indices in CD that are primarily clinical, such as HBI or Crohn's Disease Activity Index, are not entirely reflective of objective disease activity and mucosal inflammation.15, 16, 39 Our results also support previous studies which found that FC correlated well with endoscopy and mucosal inflammation but not as well with subjective clinical indices in colonic CD.15, 16, 40 Taken together, these results suggest that FC may be a useful noninvasive biomarker in colonic IBD and that its use might decrease the need for invasive procedures in patients where an objective means of assessing mucosal inflammation is needed.
Our study had several limitations. First, the sample size of this study for both UC and colonic CD was small. A larger sample size would have allowed for a more precise estimation of the reliability of FC in each of the disease subtypes studied. Secondly, the cross‐sectional imaging, MR enterography, was only performed in those patients who had known isolated proximal small intestinal involvement (n = 14) and/or elevated hs‐CRP and/or FC in the absence of disease activity during ileocolonoscopy.
In summary, FC is reliable for the detection of colonic mucosal inflammation in both UC and colonic CD but is less sensitive in small intestinal CD. Further evaluation is required to confirm this finding and determine whether specific features of small intestinal disease may either permit or preclude the use of FC as a useful noninvasive tool that can be used to assess inflammatory disease activity in this context.
Acknowledgments
The authors thank ALPCO for providing the FC test kits.
Declaration of conflict of interest: EZ—None. OBK—None. IMG—None. MSS—received research support and consulting fees from Janssen, Abbvie, Takeda, and Prometheus. AHS—received research grants from Abbvie, Amgen, Pfizer, Millennium. Honorarium for educational event presentations—Abbvie, Janssen, Takeda, Shire Advisory Board—Abbvie, Actavis, Janssen, Takeda, Pharmascience, Shire.
Author contributions: EZ, OBK, and AHS—Study concept and design; acquisition of data; analysis; data interpretation; drafting of the manuscript; critical revision of the manuscript for important intellectual content; and statistical analysis. IMG, MSS—Acquisition of data and critical review of manuscript.
Guarantor of the article: EZ, OBK and AHS—accepting full responsibility for the conduct of the study.
References
- 1. Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology. 1994; 107: 3–11. [DOI] [PubMed] [Google Scholar]
- 2. Molander P, Sipponen T, Kemppainen H et al Achievement of deep remission during scheduled maintenance therapy with TNFalpha‐blocking agents in IBD. J. Crohns Colitis. 2013; 7: 730–5. [DOI] [PubMed] [Google Scholar]
- 3. Molander P, af Bjorkesten CG, Mustonen H et al Fecal calprotectin concentration predicts outcome in inflammatory bowel disease after induction therapy with TNFalpha blocking agents. Inflamm. Bowel Dis. 2012; 18: 2011–7. [DOI] [PubMed] [Google Scholar]
- 4. Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007; 133: 1670–89. [DOI] [PubMed] [Google Scholar]
- 5. Bouguen G, Peyrin‐Biroulet L. Surgery for adult Crohn's disease: what is the actual risk? Gut. 2011; 60: 1178–81. [DOI] [PubMed] [Google Scholar]
- 6. Gomes P, du Boulay C, Smith CL, Holdstock G. Relationship between disease activity indices and colonoscopic findings in patients with colonic inflammatory bowel disease. Gut. 1986; 27: 92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baars JE, Nuij VJ, Oldenburg B, Kuipers EJ, van der Woude CJ. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm. Bowel Dis. 2012; 18: 1634–40. [DOI] [PubMed] [Google Scholar]
- 8. Jorgensen LG, Fredholm L, Hyltoft Petersen P, Hey H, Munkholm P, Brandslund I. How accurate are clinical activity indices for scoring of disease activity in inflammatory bowel disease (IBD)? Clin. Chem. Lab. Med. 2005; 43: 403–11. [DOI] [PubMed] [Google Scholar]
- 9. Sandborn WJ, Feagan BG, Hanauer SB et al A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002; 122: 512–30. [DOI] [PubMed] [Google Scholar]
- 10. Rutgeerts P, Sandborn WJ, Feagan BG et al Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005; 353: 2462–76. [DOI] [PubMed] [Google Scholar]
- 11. Rutgeerts P, Van Assche G, Sandborn WJ et al Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology. 2012; 142: 1102–1111.e2. [DOI] [PubMed] [Google Scholar]
- 12. Kane SV, Sandborn WJ, Rufo PA et al Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am. J. Gastroenterol. 2003; 98: 1309–14. [DOI] [PubMed] [Google Scholar]
- 13. Sugi K, Saitoh O, Hirata I, Katsu K. Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil‐derived proteins. Am. J. Gastroenterol. 1996; 91: 927–34. [PubMed] [Google Scholar]
- 14. Voganatsi A, Panyutich A, Miyasaki KT, Murthy RK. Mechanism of extracellular release of human neutrophil calprotectin complex. J. Leukoc. Biol. 2001; 70: 130–4. [PubMed] [Google Scholar]
- 15. Jones J, Loftus EV Jr, Panaccione R et al Relationships between disease activity and serum and fecal biomarkers in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2008; 6: 1218–24. [DOI] [PubMed] [Google Scholar]
- 16. D'Haens G, Ferrante M, Vermeire S et al Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 2012; 18: 2218–24. [DOI] [PubMed] [Google Scholar]
- 17. Zittan E, Kelly OB, Kirsch R et al Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn's disease. Inflamm. Bowel Dis. 2016; 22: 623–30. [DOI] [PubMed] [Google Scholar]
- 18. Wright EK, Kamm MA, De Cruz P et al Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn's disease following surgery. Gastroenterology. 2015; 148: 938–947.e1. [DOI] [PubMed] [Google Scholar]
- 19. Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Farkkila M. Crohn's disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn's disease activity index and endoscopic findings. Inflamm. Bowel Dis. 2008; 14: 40–6. [DOI] [PubMed] [Google Scholar]
- 20. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006; 55: 749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harvey RF, Bradshaw JM. A simple index of Crohn's‐disease activity. Lancet. 1980; 1: 514. [DOI] [PubMed] [Google Scholar]
- 22. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Eng. J. Med. 1987; 317: 1625–9. [DOI] [PubMed] [Google Scholar]
- 23. Daperno M, D'Haens G, Van Assche G et al Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES‐CD. Gastrointest. Endosc. 2004; 60: 505–12. [DOI] [PubMed] [Google Scholar]
- 24. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990; 99: 956–63. [DOI] [PubMed] [Google Scholar]
- 25. Ordas I, Rimola J, Rodriguez S et al Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn's disease. Gastroenterology. 2014; 146: 374–382.e1. [DOI] [PubMed] [Google Scholar]
- 26. Rimola J, Rodriguez S, Garcia‐Bosch O et al Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn's disease. Gut. 2009; 58: 1113–20. [DOI] [PubMed] [Google Scholar]
- 27. Samaan MA, Mosli MH, Sandborn WJ et al A systematic review of the measurement of endoscopic healing in ulcerative colitis clinical trials: recommendations and implications for future research. Inflamm. Bowel Dis. 2014; 20: 1465–71. [DOI] [PubMed] [Google Scholar]
- 28. Sipponen T, Nuutinen H, Turunen U, Farkkila M. Endoscopic evaluation of Crohn's disease activity: comparison of the CDEIS and the SES‐CD. Inflamm. Bowel Dis. 2010; 16: 2131–6. [DOI] [PubMed] [Google Scholar]
- 29. Coorevits L, Baert FJ, Vanpoucke HJ. Faecal calprotectin: comparative study of the Quantum Blue rapid test and an established ELISA method. Clin. Chem. Lab. Med. 2013; 51: 825–31. [DOI] [PubMed] [Google Scholar]
- 30. Oyaert M, Trouve C, Baert F, De Smet D, Langlois M, Vanpoucke H. Comparison of two immunoassays for measurement of faecal calprotectin in detection of inflammatory bowel disease: (pre)‐analytical and diagnostic performance characteristics. Clin. Chem. Lab. Med. 2014; 52: 391–7. [DOI] [PubMed] [Google Scholar]
- 31. Moum B, Jahnsen J, Bernklev T. Fecal calprotectin variability in Crohn's disease. Inflamm. Bowel Dis. 2010; 16: 1091–2. [DOI] [PubMed] [Google Scholar]
- 32. von Roon AC, Karamountzos L, Purkayastha S et al Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am. J. Gastroenterol. 2007; 102: 803–13. [DOI] [PubMed] [Google Scholar]
- 33. Jensen MD, Kjeldsen J, Nathan T. Fecal calprotectin is equally sensitive in Crohn's disease affecting the small bowel and colon. Scand. J. Gastroenterol. 2011; 46: 694–700. [DOI] [PubMed] [Google Scholar]
- 34. Sipponen T, Bjorkesten CG, Farkkila M, Nuutinen H, Savilahti E, Kolho KL. Faecal calprotectin and lactoferrin are reliable surrogate markers of endoscopic response during Crohn's disease treatment. Scand. J. Gastroenterol. 2010; 45: 325–31. [DOI] [PubMed] [Google Scholar]
- 35. Cerrillo E, Beltran B, Pous S et al Fecal calprotectin in Ileal Crohn's disease: relationship with magnetic resonance enterography and a pathology score. Inflamm. Bowel Dis. 2015; 21: 1572–9. [DOI] [PubMed] [Google Scholar]
- 36. Podolsky DK. Inflammatory bowel disease. N. Engl. J. Med. 2002; 347: 417–29. [DOI] [PubMed] [Google Scholar]
- 37. D'Inca R, Dal Pont E, Di Leo V et al Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int. J. Colorectal Dis. 2007; 22: 429–37. [DOI] [PubMed] [Google Scholar]
- 38. Higgins PD, Schwartz M, Mapili J, Zimmermann EM. Is endoscopy necessary for the measurement of disease activity in ulcerative colitis? Am. J. Gastroenterol. 2005; 100: 355–61. [DOI] [PubMed] [Google Scholar]
- 39. Crama‐Bohbouth G, Pena AS, Biemond I et al Are activity indices helpful in assessing active intestinal inflammation in Crohn's disease? Gut. 1989; 30: 1236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kopylov U, Yablecovitch D, Lahat A et al Detection of small bowel mucosal healing and deep remission in patients with known small bowel Crohn's disease using biomarkers, capsule endoscopy, and imaging. Am. J. Gastroenterol. 2015; 110: 1316–23. [DOI] [PubMed] [Google Scholar]


