Abstract
Background and Aim
A proportion of patients having total proctocolectomy and ileal pouch‐anal anastomosis (IPAA) for ulcerative colitis (UC) are later diagnosed with Crohn's disease (CD). The aim of this study was to identify preoperative and perioperative predictors for the subsequent development of CD in patients who had IPAA for presumed UC.
Methods
A retrospective case–control study of patients undergoing IPAA surgery for presumed UC was undertaken. Cases were patients who had a revised diagnosis of CD after surgery. Preoperative and perioperative variables were examined and analyzed.
Results
Fifteen cases were compared with 39 controls. Patients aged ≤25 years at initial UC diagnosis were more likely to develop CD compared to those aged >25 years (odds ratio, OR [95% confidence interval, CI]: 7.1 [1.6–31.3]; P = 0.01). Patients aged ≤30 years at the time of colectomy had an increased risk of subsequent development of CD compared to those aged >30 years (OR [95% CI]: 4.5 [1.3–16.0]; P = 0.02). Cases were more likely to have patchy colitis on their colectomy specimen (OR [95% CI]: 6.7 [1.1–41.8]; P = 0.04). There was no significant difference between groups regarding transmural inflammation, ileitis, or fissuring ulcers on colectomy specimens, or preoperative C‐reactive protein (CRP), albumin, family history, and smoking status.
Conclusion
Predictors of the development of CD in the pouch include young age at diagnosis and at the time of surgery, and patchy colitis in the resected colon.
Keywords: Crohn's disease, ileal pouch‐anal anastomosis, ulcerative colitis
Introduction
Approximately 20–30% of patients with ulcerative colitis (UC) require surgery at some stage of their disease.1 Indications for surgical treatment of UC include chronic refractory disease despite optimal medical therapy, corticosteroid dependence, complications of medical therapy, acute severe UC, and development of colorectal dysplasia or malignancy. There are a number of surgical options, with total proctocolectomy and ileal pouch‐anal anastomosis (IPAA) being the preferred choices of most patients due to maintenance of continence and avoidance of a lifelong stoma.
Treatment of UC and its complications with IPAA is associated with a significant improvement in quality of life and a reduction in malignancy risk.2 However, 5–13% of patients who have IPAA for UC subsequently develop clinical, radiological, or endoscopic changes that resemble Crohn's disease (CD).3 These changes have been referred to as “CD of the pouch” or sometimes “Crohn's‐like” or “CD‐like” complications of the pouch.2, 4, 5, 6 Following IPAA, CD of the pouch can significantly reduce the quality of life. It can cause pouchitis, cuffitis, strictures, deep ulcers, and fistulae, ultimately leading to sepsis or pouch failure,7, 8 and incontinence and anastomotic leaks.9 There may also be an increased risk of malignancy.3 Pouch failure can require surgical diversion and/or excision of the pouch.7 Alternately, in order to avoid surgery, patients with CD of the pouch will likely require lifelong medical treatment.3 It is estimated that patients who develop CD of the pouch are five times more likely to develop pouch failure compared to IPAA patients who do not develop CD.3
Clinical features of CD of the pouch are non‐specific. Patients can present with abdominal/pelvic pain, increased stool frequency, incontinence, and extraintestinal symptoms. Symptoms that can be more suggestive of CD of the pouch are fever, weight loss, vomiting, malnutrition, and fistula drainage.3 The differential diagnosis of CD of the pouch includes pouchitis, infectious gastroenteritis, Clostridium difficile and Cytomegalovirus (CMV), Non‐steroidal anti‐inflammatory drug (NSAID) use, ischemia, radiation enteritis, and surgical complications including anastomotic fistulae.2
Because CD of the pouch has significant morbidity, IPAA surgery is not recommended in patients with known CD. However, CD of the pouch can develop subsequently in patients originally believed to have a diagnosis of UC at the time of their IPAA surgery. It is, therefore, important to establish preoperative predictors for developing CD after IPAA for presumed UC, such that IPAA surgery is only recommended for appropriate patients who in turn are fully informed of all the possible risks and benefits of surgery.
Some potential factors that might help predict the subsequent development of CD in IPAA patients have been variably and sometimes inconsistently identified and these include seropositivity for anti‐CBir15, 6, 10 and anti‐Saccharomyces cerevisiae antibody (ASCA),5, 6, 10, 11 a positive family history of CD,6, 12 preoperative presence of extraintestinal manifestations,13, 14 younger age,12, 13, 14, 15 smoking,5 preoperative diagnosis of indeterminate colitis,14, 16 and a longer duration after pouch surgery.12 In contrast, some studies have found no predictors for the postoperative development of CD on preoperative capsule endoscopy17 and on the histopathology of preoperative biopsies or colectomy specimens.4, 18
The aim of the current study was to identify preoperative and perioperative predictors for the subsequent development of CD in Australian patients who had IPAA for presumed UC. If these can be identified, this knowledge could help advise patients of the risk and subsequently prevent its development and/or enable actions to be taken to minimize the impact of its occurrence and associated morbidity.
Methods
A retrospective case–control study was undertaken of patients from two teaching hospitals, The Alfred Hospital and Box Hill Hospital, Melbourne, both of which have specialty inflammatory bowel disease (IBD) units and colorectal surgical services. All patients having IPAA surgery for UC or IBD‐unclassified (IBD‐U) (favor UC) performed between 1993 and 2010 and who were at least 12 months post completion of the final stage of IPAA surgery were searched. Cases were the patients who were subsequently diagnosed with CD after IPAA. Criteria for the diagnosis of CD were the development of one or more changes consistent with CD—complex fistulae of the pouch, other significant perianal disease, significant small bowel disease, linear serpigenous ulcers of the pouch, or granulomas on pouch biopsy.
Patients with a diagnosis of IBD‐U (favor CD) were excluded from the study. Controls were all patients identified via a search of departmental databases that had IPAA for presumed UC and who retained the diagnosis of UC postoperatively until the end of the follow‐up period of the study. From these departmental databases, 39 controls were identified. Controls were not directly matched to cases.
A chart review of cases and controls was performed. Preoperative and perioperative variables that were recorded and analyzed were basic demographics, symptoms in the 2 weeks prior to surgery, most recent blood tests prior to surgery, preoperative endoscopy findings (macroscopic appearances and histology) up to 1 year prior to surgery, colectomy specimen pathology, and the indication for surgery. Where dedicated small bowel imaging was performed, it was normal in all the patients. As this was a retrospective study, there were some missing data for patients, which have been acknowledged as a limitation in the Discussion section.
In cases, the end point of follow up was defined as the time from completion of surgery to the time of a formal re‐diagnosis of CD. In controls, the end point of follow up was defined as the time from completion of surgery to the end of the study, which was 31 January 2013.
The study was approved by the Alfred Health Ethics Committee in February 2013. All study data were de‐identified and written informed consent from patients was not required. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as is recognized by the Alfred Health Ethics Committee.
Statistical analysis
Continuous variables were assessed for normality and expressed as mean (SD) or median (interquartile range) depending on the underlying distribution of the data. Categorical variables were presented as counts and proportions. Comparisons between cases and controls were made using Student's t‐test for normally distributed continuous variables, Wilcoxon rank‐sum test for non‐normally distributed continuous variables, and Chi‐square or Fisher's exact test as appropriate for categorical variables. The risk factors for the development of CD were assessed using univariate logistic regression analysis with results reported as odds ratios (ORs) and 95% confidence intervals (CIs). Statistical significance was set at a two‐sided P‐value of 0.05. All analyses were conducted with SAS software version 9.3 (SAS Institute, Cary, NC, USA).
Results
Cases and controls
Fifteen cases were identified and compared with 39 controls. Most cases had more than one reason to be reclassified as CD, which is shown in Table 1. Ten cases had complex fistulae of the pouch and two cases had other significant perianal disease. Eleven cases had significant small bowel disease, two cases had linear serpigenous ulcers of the pouch, and three cases had a least one granuloma on pouch biopsy. Two cases were found to have an incorrect preoperative diagnosis upon retrospective examination of clinicopathological data.
Table 1.
Reasons for subsequent re‐diagnosis as Crohn's disease
| Case number | Complex fistulae of pouch | Other evidence of perianal disease | Significant small bowel disease | Linear serpigenous ulcers of pouch | Granuloma on biopsy | Incorrect initial diagnosis in retrospect |
|---|---|---|---|---|---|---|
| 1 | X | X | ||||
| 2 | X | X | X | |||
| 3 | X | X | ||||
| 4 | X | |||||
| 5 | X | X | X | X | ||
| 6 | X | X | ||||
| 7 | X | |||||
| 8 | X | X | ||||
| 9 | X | X | ||||
| 10 | X | X | ||||
| 11 | X | X | ||||
| 12 | X | X | ||||
| 13 | X | X | ||||
| 14 | X | X | ||||
| 15 | X | X |
Demographics of cases and controls
Demographic details of the patients studied are outlined in Table 2. The proportion of cases and controls who were females and their median follow‐up times were similar. Among cases, median time to rediagnosis of CD was 6 years. Cases were a mean of 11 years younger than controls (P = 0.01). As shown in Figure 1, patients aged ≤25 years at the time of initial UC diagnosis were more likely to develop CD compared to those aged >25 years (OR [95% CI]: 7.1 [1.6–31.3]; P = 0.01). Cases were also a mean of 12 years younger at the time of colectomy (P = 0.004; Table 1). Patients aged ≤30 years at the time of colectomy had an increased risk of subsequent development of CD compared to those aged >30 years (OR [95% CI]: 4.5 [1.3–16.0]; P = 0.02; Fig. 2).
Table 2.
Demographic information on the cases and controls with IBD
| Cases† | Controls† | P‐value | |
|---|---|---|---|
| Female | 6/15 (40%) | 12/39 (30%) | 0.52 |
| Median follow‐up time, months (IQR) | 72 (23–132) | 59 (34–83) | 0.39 |
| Mean age at IBD diagnosis, years (SD) | 21 (6) | 32 (14) | 0.01 |
| Mean age at colectomy, years (SD) | 27 (7) | 39 (15) | 0.004 |
| Current smoker | 2/14 (14%) | 3/35 (8%) | 0.62 |
| Ex‐smoker | 2/14 (14%) | 11/35 (31%) | 0.22 |
| Never smoked | 10/14 (71%) | 21/35 (60%) | 0.45 |
| Family history of ulcerative colitis | 1/14 (7%) | 5/23 (21%) | 0.24 |
| Family history of Crohn's disease | 1/14 (7%) | 1/23 (4%) | 1.0 |
| No family history of IBD | 12/14 (86%) | 17/23 (74%) | 0.68 |
Variation in the denominator of some variables for cases and controls is due to missing data for that variable.
IBD, inflammatory bowel disease; IQR, interquartile range.
Figure 1.
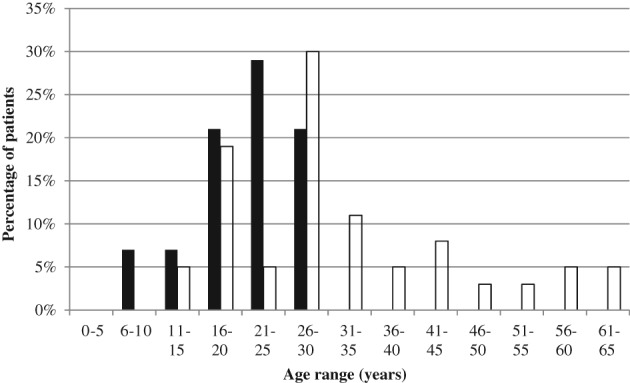
Age of patients at diagnosis of ulcerative colitis. Patients diagnosed ≤25 years of age were more likely to develop Crohn's disease than those aged >25 years (odds ratio [95% confidence interval]: 7.1 [1.6–31.3]; P = 0.01).  , Cases;
, Cases;  , controls.
, controls.
Figure 2.
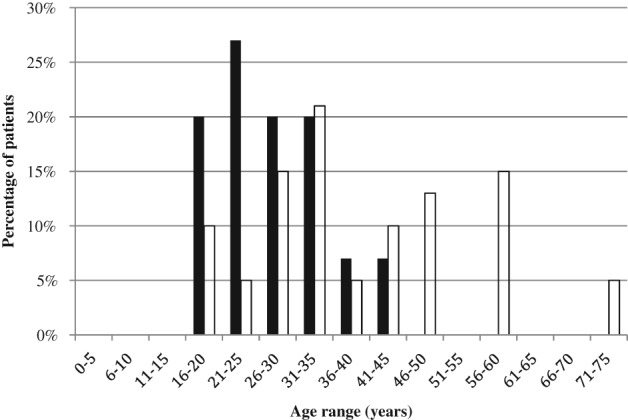
Age of patients at the time of colectomy. Patients aged ≤30 years at the time of colectomy had an increased risk of subsequent development of Crohn's disease compared to those aged >30 years (odds ratio [95% confidence interval]: 4.5 [1.3–16.0]; P = 0.02).  , Cases;
, Cases;  , controls.
, controls.
Preoperative features of cases and controls
Preoperative clinical, laboratory, endoscopic, and histological findings in cases and controls are shown in Table 3. Clinically, only fever occurred more commonly in cases, although this difference did not reach significance. There were no hematological and biochemical differences. Serological markers, ASCA and anti‐neutrophil cytoplasmic antibody (ANCA), were not routinely performed. There were no differences in colonoscopic nor histological findings between cases and controls. However, only 19 of 54 patients had ileoscopy and, therefore, ileal biopsies were performed. One case did have a granuloma consistent with CD on a preoperative biopsy specimen, but the decision was made to proceed with IPAA surgery (at another hospital) regardless.
Table 3.
Preoperative clinical, biochemical, endoscopic, and histopathological data
| Category | Variable | Cases | Controls | P‐value |
|---|---|---|---|---|
| Symptoms and signs | Weight loss | 3/9 (33%) | 10/35 (28%) | 0.79 |
| Fevers | 4/9 (44%) | 5/34 (14%) | 0.05 | |
| Rectal bleeding | 11/11 (100%) | 30/34 (88%) | 0.23 | |
| Extraintestinal symptoms | 1/12 (16%) | 7/34 (20%) | 0.77 | |
| Abdominal pain | 7/9 (77%) | 22/35 (62%) | 0.40 | |
| Laboratory testing | Median C‐reactive protein (IQR) | 28.5 (11–59) | 31 (5–71) | 0.82 |
| Mean ESR (SD) | 42.2 (28.7) | 45.8 (33.9) | 0.81 | |
| Mean hemoglobin (SD) | 121 (20.5) | 123 (23.2) | 0.83 | |
| Mean platelet count (SD) | 353 (79.8) | 385 (143.4) | 0.45 | |
| Mean albumin (SD) | 29.9 (7.5) | 33.8 (7.6) | 0.12 | |
| Positive ASCA IgG | 0/3 | 1/7 | — | |
| Positive ASCA IgA | 2/3 | 1/1 | — | |
| Positive pANCA | 0/4 | 6/14 | — | |
| Endoscopy | Abnormal ileoscopy | 0/4 (0%) | 0/15 (0%) | 1.00 |
| Cobblestoning | 0/10 (0%) | 2/29 (6%) | 1.00 | |
| Skip lesions | 0/10 (0%) | 1/29 (3%) | 1.00 | |
| Patchy macroscopic colitis | 1/10 (10%) | 3/29 (10%) | 1.00 | |
| Relative rectal sparing | 0/10 (0%) | 2/29 (6%) | 1.00 | |
| Histopathology | Small bowel inflammation | 1/4 (25%) | 2/15 (26%) | 1.00 |
| Granuloma | 1/10 (10%) | 0/29 (0%) | 0.26 |
ASCA, anti‐Saccharomyces cerevisiae antibody; ESR, erythrocyte sedimentation rate; pANCA, peri‐nuclear anti‐neutrophil cytoplasmic antibody.
Operative features of cases and controls
Surgical details of the cases and controls, including pathological findings of the colectomy specimen, are shown in Table 4. One case and three controls had a preoperative diagnosis of indeterminate colitis (favor UC). One control had no prior diagnosis and had emergency surgery for uncontrollable rectal bleeding, with pathology of the colectomy specimen confirming a diagnosis of UC. There were no significant differences between cases and controls when analyzed by indication for surgery, nor for surgical technique (two‐stage vs three‐stage operations).
Table 4.
Surgical details and the pathological findings of the colectomy specimen
| Category | Variable | Cases (n = 15) |
Controls (n = 39) |
P‐value |
|---|---|---|---|---|
| Suspected diagnosis prior to surgery | Inflammatory bowel disease‐unclassified (favor UC) | 1 (7%) | 3 (8%) | 1.00 |
| UC | 14 (93%) | 35 (90%) | 1.00 | |
| Other | 0 (0%) | 1 (3%) | 1.00 | |
| Indication for surgery | Emergency surgery for fulminant colitis | 2 (13%) | 3 (8%) | 0.86 |
| Planned surgery for severe colitis | 135 (87%) | 32 (82%) | 1.00 | |
| Planned surgery for malignancy | 0 (0%) | 3 (8%) | 0.74 | |
| Number of stages | Two‐stage operation | 6 (40%) | 21 (54%) | 0.36 |
| Three‐stage operation | 9 (60%) | 18 (46%) | 0.36 | |
| Colectomy pathology | Indeterminate colitis | 1/15 (6%) | 3/36 (8%) | 1.00 |
| Dysplasia or malignancy | 0/15 (0%) | 2/35 (6%) | 1.00 | |
| Proctitis | 0/15 (0%) | 2/35 (6%) | — | |
| Left‐sided colitis | 2/15 (13%) | 9/35 (25%) | 0.33 | |
| Extensive colitis | 8/15 (53%) | 13/35 (37%) | 0.29 | |
| Pancolitis | 5/15 (33%) | 11/35 (31%) | 0.89 | |
| Small bowel inflammation | 1/11 (9%) | 2/28 (7%) | 1.00 | |
| Cobblestoning | 2/15 (13%) | 0/39 (0%) | 0.07 | |
| Fissuring ulcers | 2/15 (13%) | 5/39 (12%) | 0.96 | |
| Transmural inflammation | 6/15 (40%) | 11/37 (29%) | 0.47 | |
| Granulomas | 1/15 (6%) | 0/39 (0%) | 0.28 | |
| Patchy colitis | 4/15 (27%) | 2/39 (5%) | 0.04 | |
| Pseudopolyps | 10/15 (66%) | 17/38 (44%) | 0.15 |
UC, ulcerative colitis.
As shown previously, the pathologist reported the presence of patchy colitis, defined as histological areas of normal mucosa within areas of inflammation on colectomy specimen (i.e. histological skip lesions), in 27% of cases and 5% of controls (P = 0.04). On univariate analysis, cases were almost seven times more likely to have patchy colitis on the colectomy specimen than controls (OR [95% CI]: 6.7 [1.1–41.8]; P = 0.04).
Four of the six cases with patchy colitis were under 25 at the time of UC diagnosis and five of the six cases with patchy colitis were under 30 at the time of surgery. One case had a granuloma on the colectomy specimen, but this was only found on retrospective review of the colectomy specimen after a later re‐diagnosis of CD following IPAA surgery. There was no significant difference between cases and controls in other pathological variables on the colectomy specimen, but there was a trend toward increased cobblestoning in cases (P = 0.07). Cases were numerically, but not statistically, more likely to have transmural inflammation (40% vs 29%, P = 0.48), extensive colitis (53% vs 37%, P = 0.29), and pseudopolyps (66% vs 44%, P = 0.15).
Discussion
Attempting to identify patients who may be subsequently diagnosed with CD following IPAA for presumed UC is important due to the significantly worse outcomes in patients subsequently diagnosed with CD. There are two time periods in which risk factors can be identified—pre‐colectomy and pre‐pouch formation. The current multicenter, retrospective case‐control study compared 15 cases of CD of the pouch to 39 controls who retained a diagnosis of UC in order to address these issues. Pre‐colectomy, only a missed diagnosis of CD in the histopathological assessment of colonoscopic biopsies, and a younger age at both diagnosis of UC and at colectomy were identified as predictors of CD in the pouch. Pre‐pouch creation, only patchy colitis was predictive of this developing complication.
Of the 15 cases, it appears that there were two cases where the diagnosis of UC was made incorrectly on colectomy or biopsy specimens prior to the subsequent stages of IPAA surgery. The other 13 cases appeared to evolve to a rediagnosis of CD sometime after surgery. This outlines the importance of careful review of all histopathology results, including the colectomy specimen, by an experienced pathologist prior to proceeding with definitive pouch surgery. This is the standard recommendation for surgery for UC.19 The two cases who were missed diagnoses of CD contributed to our results of the younger age effect as both cases were under 25 at the time of IBD diagnosis, and under 30 at the time of surgery. In addition, one of the two missed diagnoses of CD had patchy colitis on his colectomy specimen.
There were a significant number of patients who developed CD following IPAA for presumed UC. This was possibly because most of the study period (1993–2010) was during the prebiologic era in Australia—biologics were first available on the Pharmaceutical Benefits Scheme in Australia in 2007. During the study period, there were few options for patients with severe colitis such that more surgery was needed, some of which ended up being CD cases.
We found that patients who had IPAA for UC (or IBD‐U favor UC) were more likely to be subsequently diagnosed with CD if they were 25 years or younger at the time of initial UC diagnosis or had a colectomy at age ≥30 years. There was no significant difference between groups regarding several variables otherwise associated with CD, namely family history, smoking status, transmural inflammation, ileitis or fissuring ulcers identified on colonoscopy, or preoperative C‐reactive protein (CRP) and albumin levels. A number of other studies have demonstrated that patients who were younger at the time of diagnosis or at the time of surgery were more likely to develop CD after IPAA for UC. Melton et al.,14 using a prospectively maintained database, compared 184 cases and 2630 controls and on multivariate analysis found that cases with a delayed diagnosis of CD after IPAA for UC were significantly younger at the time of surgery compared with controls (mean age: 29.5 years vs 38 years; P < 0.0001). Shen et al.12 in a retrospective case–control study of 116 cases and 442 controls also found younger age as a significant risk factor for developing CD of the pouch, with an OR (95% CI) of 0.84 (0.76–0.93) as compared to patients who were 5 years younger (P = 0.001). Truta et al.13 in a smaller cohort also demonstrated comparable findings to our study. They compared 20 cases and 19 controls, and cases were again diagnosed with UC at a younger mean age than controls (22 years vs 30 years, respectively, P = 0.027). Cases also had IPAA surgery at a younger mean age than controls (31.5 years vs 40 years, respectively, P = 0.035). Similarly, presented to date only in abstract form, of the 149 patients with a preoperative diagnosis of IBD‐U, the only predictor of the subsequent development of CD was a younger age of diagnosis.15
This age difference is consistent with studies demonstrating that patients with CD are usually diagnosed on average 5–10 years earlier than UC patients.20 The peak age of onset of IBD is 20–30 years in CD and 30–40 years in UC.21 Our results were consistent with these observations, with the mean age of onset of IBD being 21 in cases and 32 in controls. In addition, it is well recognized that younger IBD patients tend to have more aggressive and complicated disease (for both CD and UC), which is again consistent with our findings.20, 21 There is, however, conflicting evidence on whether the age of diagnosis independently impacts the risk for surgery.20
Three‐stage operations offer the opportunity for careful pathological examination of the entire colon and of the cuff of terminal ileum before proceeding to pouch formation. Previous studies have suggested that the diagnosis of indeterminate colitis on colectomy specimen14, 16 may indicate a higher risk of subsequent development of CD in the pouch. However, no other features on colectomy have been identified as risk factors. In the current study, there were few clues identified. One in four cases had patchy colitis on their colectomy specimen equating to a near sevenfold increased risk. All these cases and controls with patchy colitis were diagnosed as UC and not indeterminate colitis. However, 5% of the controls also had this feature, which is a well‐documented finding in partially treated disease.9, 22, 23 Because of the small sample size, this finding needs to be replicated in larger studies before further definitive management recommendations can be made, but its presence in 5% of controls would temper any definitive decisions regarding the wisdom of creating a pouch in an individual with such a finding.
It is important to define an exact diagnosis, where possible, before committing a younger patient to IPAA surgery, including a careful review of the histological results of the colectomy specimen with an experienced pathologist. For this reason, two‐ or three‐stage IPAA procedures appear preferable, especially in young patients. The implications of a younger age of diagnosis or colectomy must be discussed with patients such that they can make a fully informed decision about proceeding to IPAA surgery, even though it is likely that most will still choose an IPAA over a permanent end ileostomy.
This study has several limitations, most pertinently its retrospective nature and small sample size. As with all retrospective data collection, there was no standardization of medical and surgical management practices or endoscopic and pathological reporting. In addition, the definition of rediagnosis of CD is not standardized among gastroenterologists, and pathologists also vary in their accuracy in differentiating UC and CD. For example, Farmer et al.24 reviewed 84 IBD colectomy specimens where the diagnosis of UC or CD was made by general pathologists and then compared with the diagnosis made by a pathologist with an interest in IBD. Forty‐five percent of cases were given a different diagnosis and in most cases UC diagnosis was changed to CD. Our study could possibly be improved if we were able to re‐review pathology results of colectomy specimens with a pathologist with a special interest in IBD. Due to the retrospective nature of this study, there were missing data and six controls were lost to follow up. We were unable to find colonoscopy and biopsy reports for all patients; however, all patients would have had initial colonoscopies and biopsies taken with pathology results to confirm their diagnosis of IBD. Presence or absence of small bowel inflammation was not reported on colectomy specimen in 4 cases and 11 controls, and we have made the assumption that this was absent.
The small sample size may have limited the validity of the statistical comparisons between groups and increased the chance of Type II errors. Due to the small number of cases, multivariate analysis could not be performed.
It is unclear why some patients “convert” to CD after IPAA for presumed UC. It has been hypothesized that it may be related to changes in bacterial flora following pouch creation, in association with underlying genetic susceptibility.2, 3, 5, 8 Some authors postulate that the formation of an anastomosis with the potential for fecal stasis above it creates an appropriate environment for the development of CD, analogous to the model of postoperative recurrence of CD at and proximal to an ileocolic anastmosis.2, 3, 12 Others suggest that the non‐physiological environment created in the terminal ileum causes UC to behave differently immunologically in the pouch compared to within the colonic mucosa, thereby becoming phenotypically similar to CD.4 Future studies for identifying potential risk factors for CD of the pouch could also investigate microbiota profiles within the colon.
In conclusion, this study has shown that there are few clinical, endoscopic or pathological clues to those at risk of developing CD of the pouch. However, given the morbidity associated with CD of the pouch, there are some important implications for clinical practice. Clinicians should be alerted to the risk of developing CD of the pouch in patients diagnosed with UC or undergoing colectomy at a young age. This finding has been replicated in multiple other studies and appears robust. Other risk factors identified in this study were missed diagnoses of CD preoperatively and patchy colitis on the colectomy specimen. These results should be confirmed in larger prospective studies. If these findings are reproduced in prospective cohorts, the presence of these factors must alert clinicians to the risk of subsequent Crohn's‐like problems developing if an IPAA is performed.
Declaration of conflict of interest: None.
Author contribution: Dr Miles P Sparrow, Dr Alexandra G Abel, Dr Peter R Gibson, and Dr Alvin Chung were responsible for design and implementation of the study. Mr. Eldho Paul was responsible for the statistical analysis. All authors were involved in the manuscript preparation and approved the final version of the manuscript.
Financial support: None.
References
- 1. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012; 380: 1606–19. [DOI] [PubMed] [Google Scholar]
- 2. Wu H, Shen B. Crohn's disease of the pouch: diagnosis and management. Expert Rev. Gastrenterol. Hepatol. 2009; 3: 155–65. [DOI] [PubMed] [Google Scholar]
- 3. Shen B. Crohn's disease of the ileal pouch: reality, diagnosis, and management. Inflamm. Bowel Dis. 2009; 15: 284–94. [DOI] [PubMed] [Google Scholar]
- 4. Goldstein NS, Sanford WW, Bodzin JH. Crohn's‐like complications in patients with ulcerative colitis after total proctocolectomy and ileal pouch‐anal anastomosis. Am. J. Surg. Pathol. 1997; 21: 1343–53. [DOI] [PubMed] [Google Scholar]
- 5. Tyler AD, Milgrom R, Xu W et al Antimicrobial antibodies are associated with a Crohn's disease‐like phenotype after ileal pouch‐anal anastomosis. Clin. Gastroenterol. Hepatol. 2012; 10: 507–12. [DOI] [PubMed] [Google Scholar]
- 6. Melmed GY, Fleshner PR, Bardakcioglu O et al Family history and serology predict Crohn's disease after ileal pouch‐anal anastomosis for ulcerative colitis. Dis. Colon Rectum. 2008; 51: 100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alexander F. Complications of ileal pouch anal anastomosis. Semin. Pediatr. Surg. 2007; 16: 200–4. [DOI] [PubMed] [Google Scholar]
- 8. Joyce M, Fazio V. Can ileal pouch anal anastomosis be used in Crohn's disease? Adv. Surg. 2009; 43: 111–37. [DOI] [PubMed] [Google Scholar]
- 9. Odze R. Diagnostic problems and advances in inflammatory bowel disease. Mod. Pathol. 2003; 16: 347–58. [DOI] [PubMed] [Google Scholar]
- 10. Coukos JA, Howard LA, Weinberg JM, Becker JM, Stucchi AF, Farraye FA. ASCA IgG and CBir antibodies are associated with the development of Crohn's disease and fistulae following ileal pouch‐anal anastomosis. Dig. Dis. Sci. 2012; 57: 1544–53. [DOI] [PubMed] [Google Scholar]
- 11. Dendrinos KG, Becker JM, Stucchi AF, Saubermann LJ, LaMorte W, Farraye FA. Anti‐Saccharomyces cerevisiae antibodies are associated with the development of postoperative fistulas following ileal pouch‐anal anastomosis. J. Gastrointest. Surg. 2006; 10: 1060–4. [DOI] [PubMed] [Google Scholar]
- 12. Shen B, Remzi FH, Hammel JP et al Family history of Crohn's disease is associated with an increased risk for Crohn's disease of the pouch. Inflamm. Bowel Dis. 2009; 15: 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Truta B, Li DX, Mahadevan U et al Serologic markers associated with development of Crohn's disease after ileal pouch anal anastomosis for ulcerative colitis. Dig. Dis. Sci. 2014; 59: 135–45. [DOI] [PubMed] [Google Scholar]
- 14. Melton GB, Kiran RP, Fazio VW et al Do preoperative factors predict subsequent diagnosis of Crohn's disease after ileal pouch‐anal anastomosis for ulcerative or indeterminate colitis? Colorectal Dis. 2010; 12: 1026–32. [DOI] [PubMed] [Google Scholar]
- 15. Koh S, Zaghiyan K, Li Q, Rogatko A, Fleshner P. P‐116 clinical factors associated with the development of Crohn's disease in inflammatory bowel disease type‐unclassified patients undergoing IPAA. Inflamm. Bowel Dis. 2014; 20 (Suppl. 1): S75–6. [DOI] [PubMed] [Google Scholar]
- 16. Delaney CP, Remzi FH, Gramlich T, Dadvand B, Fazio VW. Equivalent function, quality of life and pouch survival rates after ileal pouch‐anal anastomosis for indeterminate and ulcerative colitis. Ann. Surg. 2002; 236: 43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murrell Z, Vasiliauskas E, Melmed G, Lo S, Targan S, Fleshner P. Preoperative wireless capsule endoscopy does not predict outcome after ileal pouch‐anal anastomosis. Dis. Colon Rectum. 2010; 53: 293–300. [DOI] [PubMed] [Google Scholar]
- 18. Nasseri Y, Melmed G, Wang HL, Targan S, Fleshner P. Rigorous histopathological assessment of the colectomy specimen in patients with inflammatory bowel disease unclassified does not predict outcome after ileal pouch‐anal anastomosis. Am. J. Gastroenterol. 2010; 105: 155–61. [DOI] [PubMed] [Google Scholar]
- 19. Øresland T, Bemelman WA, Sampietro GM et al European evidence based consensus on surgery for ulcerative colitis. J. Crohns Colitis. 2015; 9: 4–25. 10.1016/j.crohns.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 20. Duricova D, Burisch J, Jess T, Gower‐Rousseau C, Lakatos PL, ECCO‐EpiCom . Age‐related differences in presentation and course of inflammatory bowel disease: an update on the population‐based literature. J. Crohns Colitis. 2014; 8: 1351–61. 10.1016/j.crohns.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 21. Ruel J, Ruane D, Mehandru S, Gower‐Rousseau C, Colombel JF. IBD across the age spectrum—is it the same disease? Nat. Rev. Gastroenterol. Hepatol. 2014; 11: 88–98. [DOI] [PubMed] [Google Scholar]
- 22. De Roche TC, Xiao S‐Y, Liu X. Histological evaluation in ulcerative colitis. Gastroenterol. Rep. (Oxf.). 2014; 2: 178–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joo M, Odze RD. Rectal sparing and skip lesions in ulcerative colitis: a comparative study of endoscopic and histologic findings in patients who underwent proctocolectomy. Am. J. Surg. Pathol. 2010; 34: 689–96. [DOI] [PubMed] [Google Scholar]
- 24. Farmer M, Petras RE, Hunt LE, Janosky JE, Galandiuk S. The importance of diagnostic accuracy in colonic inflammatory bowel disease. Am. J. Gastroenterol. 2000; 95: 3184–8. [DOI] [PubMed] [Google Scholar]


