Abstract
A 33‐year‐old man was referred with hyperosmotic symptoms of 4 weeks. Clinical examination showed palpable hepatomegaly and no stigmata of liver disease. Findings were random glucose 16.6 mmol/L, HbA1c 12.4%, triglyceride 6.2 mmol/L, normal LFTs and ultrasound liver: increased echogenicity. Management consisted of dietician referral and commencement of metformin 500 mg bd, diamicron MR 60 mg od, and fenofibrate 145 mg od. He was non‐compliant, complaining of “heaviness of head” after consuming oral diabetic agents, without symptoms of hypoglycemia. Treatment was switched to Kombiglyze XR (saxaglipitin 5 mg + metformin 1000 mg) and empagliflozin 25 mg od. He presented 1 week later with generalised pruritus with ALT 307 IU/L and serum GGT 808 IU/L. Following this, a percutaneous liver biopsy was performed, revealing steatohepatitis and marked intra‐hepatic cholestasis. Kombiglyze XR was withheld, with resolution of LFTs to baseline. Phenotypes of liver injury are categorised according to R value, defined as ratio ALT/ULN:ALP/ULN. R value of ≥5:hepatocellular injury, ≤2:cholestatic injury, 2–5:mixed‐type injury. Here, R value points toward mixed type (R = 3.203). Hepatotoxicity in patients with NASH is difficult to diagnose, based on laboratory parameters. Liver histology was useful in indicating additional changes apart from NASH, causing liver derangement. The Rousal Uclaf Causality Assessment Method is a scoring method to determine the probability of drug induced liver injury. RUCAM score for this case was 6 (probable adverse drug reaction). Hepatotoxicity from saxagliptin not been reported prior. Clinicians need to be more vigilant, particularly in patients with NASH.
Keywords: drug‐induced liver injury, fatty liver, hepatology
Introduction
Non‐alcoholic fatty liver disease (NAFLD) is now the most common cause of chronic liver disease in Western and Asian societies. A very close association exists between NAFLD and metabolic syndrome. The prevalence of NAFLD is even higher among patients with diabetes mellitus (T2DM), up to 70%.1 This is because NAFLD and T2DM have similarities in pathogenesis—insulin resistance leading to hyperinsulinemia and gross alterations in carbohydrate and fat metabolism; thus, both often coexist in many individuals with metabolic syndrome. Both the disorders modify the risk of each other in a vicious circle.2
The intestinal hormone glucagon‐like peptide‐1 (GLP‐1), secreted in response to nutrient ingestion, increases insulin secretion from pancreatic β‐cells and reduces glucagon secretion from pancreatic α‐cells. GLP‐1 is inactivated by the dipeptidyl peptidase‐4 (DPP‐4) enzyme. Saxagliptin is a DPP‐4 inhibitor that prevents the degradation of endogenous GLP‐1 and prolongs its effect on insulin and glucagon secretion. Because T2DM is a chronic, progressive disease, limiting hyperglycemic exposure over time requires intensification of treatment. The DPP‐4 inhibitors are largely free of significant interactions, with agents often coadministered to T2DM patients. There are no interactive pharmacokinetic effects between DPP‐4 inhibitors and other oral hypoglycemics, including sulfonylureas, metformin, or thiazolidinediones.3, 4
Kombiglyze MR (metformin with saxagliptin) is a recent combination oral hypoglycemic agent introduced for the management of T2DM. In the phase 3 trial researching the efficacy and safety of initial combination therapy with saxagliptin 5 mg + metformin versus saxagliptin 5 mg or metformin monotherapy in treatment‐naïve patients with T2DM and inadequate glycemic control, it was shown that initial therapy (up to 24 weeks) led to statistically significant improvements in HbA1c (−2.5 vs. −1.7 and −2.0%, all P < 0.0001 vs. monotherapy) compared with either treatment alone across key glycemic parameters, with a tolerability profile similar to the monotherapy components.5
Hepatotoxicity is not a recognized adverse event of this drug. We present a case of Kombiglyze‐induced cholestasis in a patient with non‐alcoholic steatohepatitis.
Case report
A 33‐year‐old man with no known past medical history was referred to the clinic with a 4‐week history of constitutional symptoms, polyuria, and polydipsia. He was teetotal and denied using recreational or over‐the‐counter prescription drugs and consuming herbal and dietary supplements (HDS).
There was a positive family history of diabetes mellitus. Clinical examination showed a nonobese (BMI = 23.2) gentleman with dermatopathic skin changes over his shins and palpable hepatomegaly but no stigmata of chronic liver disease. Initial investigations demonstrated the following: random blood glucose of 16.6 mmol/L and HbA1c of 12.4% (IFCC was 111 mmol/mol), fasting total cholesterol of 7.9 mmol/L with triglyceride of 6.2 mmol/L, normal LFTs, and an ultrasound scan with features of hepatic steatosis. Other viral and autoimmune etiologies were excluded.
The patient was diagnosed with new‐onset T2DM and NAFLD. The initial management consisted of a dietitian referral and commencement of pharmacological therapy for his metabolic syndrome: metformin 500 mg bd, gliclazide MR 60 mg od, and fenofibrate 145 mg od. Unfortunately, he could not comply with the treatment, complaining of “heaviness of the head” after consuming morning doses of metformin and gliclazide MR, without typical symptoms of hypoglycemia. In view of his young age and aggressive disease, his treatment was switched to Kombiglyze XR (saxagliptin 5 mg + metformin 1000 mg) and empagliflozin 25 mg od.
He presented 1 week later with complaints of generalized pruritus. Physical examination was unremarkable, but his LFTs had deteriorated significantly, with a rapid rise of serum Alanine aminotransferase to 307 IU/L and serum gamma glutamyl transferase 808 IU/L (Fig. 1a). A repeat ultrasound of the abdomen showed no changes from the previous scan, and a decision was taken to perform a percutaneous liver biopsy. Liver histology demonstrated the following changes: hepatocytes showed fatty change in 30–40% of liver cells, along with focal ballooning degeneration, bile stasis, focal binucleation, large cell change, and scattered lobular hepatitis. The portal tracts were inflamed with a mixture of inflammatory cells (lymphocyte predominant) with focal interphase hepatitis (Fig. 1b). These changes included a histological diagnosis of non‐alcoholic steatohepatitis (NASH) and marked intrahepatic cholestasis.
Figure 1.
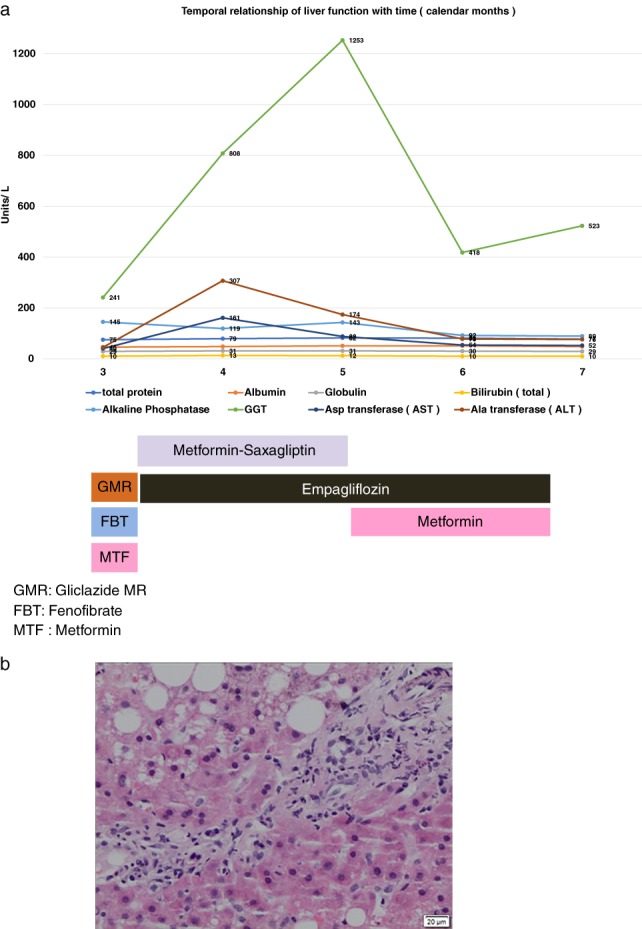
(a) Temporal relationship of liver function with commencement and withdrawal of Kombiglyze XR. ( ) Bilirubin (total), (
) Bilirubin (total), ( ) Alkaline Phosphatase, (
) Alkaline Phosphatase, ( ) GGT, (
) GGT, ( ) Asp transferase (AST), and (
) Asp transferase (AST), and ( ) Ala transferase (ALT). (b) Light microscopic findings of liver biopsy showing portal tract inflammation [H&E stain: ×20 objective].
) Ala transferase (ALT). (b) Light microscopic findings of liver biopsy showing portal tract inflammation [H&E stain: ×20 objective].
Following the results of the liver histology, a clinical diagnosis of drug‐induced liver injury (cholestasis), in the background of NASH, was made. A decision was made to discontinue Kombiglyze XR. Metformin was restarted at 850 mg twice daily, and empagliflozin was maintained at 25 mg daily. His LFTs continued to improve into the third week (calendar month 6) and subsequently in to the seventh week (calendar month 7) from date of Kombiglyze withdrawal. (Please refer to Fig. 1a).
Discussion
Several drugs involved in the management of the metabolic syndrome have the potential to cause hepatotoxicity. HMG‐CoA reductase inhibitors (statins) are widely prescribed for patients with hyperlipidemia and are generally well tolerated. Mild elevations in serum aminotransferases are demonstrated in up to 3% of treated patients, but clinically apparent drug‐induced liver injuries are rare. As shown in a prospective registry by the U.S. Drug Induced Liver Injury Network, which enrolled cases between 2004 and 2012, among 1188 cases of drug‐induced liver injury, only 22 were attributed to a statin (0.018%).6 With regard to fibrates, it was found that LFT improved with fibrates due to the reduction of fatty acid oxidation and inflammation.7 Fenofibrate may very rarely instigate an autoimmune hepatitis‐type reaction with resultant ductopenia, chronic hepatitis, and fibrosis, especially when used in combination with statin medications,8 a situation not encountered in our case. The same study also postulated that the rates of all adverse effect reactions (AERs) and muscle toxicity without rhabdomyolysis were modestly higher with gemfibrozil relative to fenofibrate; the rates of gemfibrozil‐associated rhabdomyolysis were approximately 10‐fold higher.
Metformin would have been the least likely culprit drug because it plays a role in improving NAFLD phenotypes by improving hepatic steatosis and suppressing liver inflammation. Metformin does not undergo hepatic metabolism (no metabolites have been identified in humans) or biliary excretion. Thus, metformin is considered safe from a hepatic standpoint.9 Our decision to continue empagliflozin was based on evidence from multiple studies to date that effectively rule out empagliflozin being implicated in causing hepatotoxicity.10, 11, 12, 13
A Pubmed search was conducted with a word search builder with “DILI,” “adult humans” and “saxagliptin,” resulting in 141 studies (2008 to December 2017), with all studies accounting for serious adverse events such as hypoglycemia, heart failure, renal impairment, and acute pancreatitis. To date, hepatotoxicity as a possible adverse event has not been reported. In the absence of any identifiable trigger of cholestatic hepatitis and considering the temporal sequence of events, saxagliptin therapy was highly suspected to be the triggering factor.
This case report has also highlighted that drug‐induced liver injury should be clinically suspected in patients with NAFLD based on certain patterns of the LFT. Hepatotoxicity in a patient with NASH can be difficult to diagnose based on laboratory parameters alone. The phenotypes of liver injury are categorized according to the R value, defined as the ALT/ULN:ALP/ULN ratio. In our case, the R value pointed toward a mixed hepatocellular/ cholestatic type of liver injury (R = 3.203). In routine clinical practice, a predominant cholestatic pattern with markedly raised alanine and aspartate aminotransferase is rarely seen in NASH alone. The Rousal Uclaf Causality Assessment Method (RUCAM) is a scoring method used to determine the probability of drug‐induced liver injury. The scoring process uses descriptives including definite, highly likely, probable, possible, or unlikely. In our case study, the RUCAM score (calculated online) was 6 (probable adverse drug reaction). The diagnosis of DILI on top of NASH was finally clinched with a liver biopsy, which was essential in this case. There have been isolated reports of liver injury in patients treated with other DPP4 inhibitors, such as alogliptin,14 sitagliptin,15 and linagliptin.16 Liver injury because of these agents appears to be rare and generally mild.17
Based on a previous study on the relationship between a compound's metabolism profile and risk of hepatic adverse events, it was found that compounds with significant hepatic metabolism were significantly more hepatotoxic than compounds belonging to other groups.18 Comparing saxagliptin with other DPP4 inhibitors, saxagliptin has 51% hepatic metabolism compared to sitagliptin (21%), vildagliptin (2%), linagliptin (10%), and alogliptin (10%).19 Could this directly link saxagliptin use with a higher propensity for hepatotoxic injury? This remains to be further investigated in the future. For now, because this drug is increasingly used in T2DM, which often coexists with NAFLD, clinicians will need to be vigilant about this potentially serious complication.
References
- 1. Targher G, Bertolini L, Padovani R et al Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007; 30: 1212–8. [DOI] [PubMed] [Google Scholar]
- 2. Loria P, Lonardo A, Anania F. Liver and diabetes. A vicious circle. Hepatol. Res. 2013; 43: 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scheen AJ. Dipeptidylpeptidase‐4 inhibitors (gliptins): focus on drug‐drug interactions. Clin. Pharmacokinet. 2010; 49: 573–88. [DOI] [PubMed] [Google Scholar]
- 4. Rosenstock J, Gross JL, Aguilar‐Salinas C et al Long‐term 4‐year safety of saxagliptin in drug‐naive and metformin‐treated patients with type 2 diabetes. Diabet. Med. 2013; 30: 1472–6. [DOI] [PubMed] [Google Scholar]
- 5. Russo MW, Hoofnagle JH, Gu J et al The spectrum of statin hepatotoxicity: experience of the drug induced liver injury network. Hepatology. 2014. Aug; 60: 679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gandhi N, Lenton R, Bhartia M, Abbas A, Raju J, Ramachandran S. Effect of fibrate treatment on liver function tests in patients with the metabolic syndrome. Springerplus. 2014; 3: 14 Published online 2014 Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alsheikh‐Ali AA, Kuvin JT, Karas RH. Risk of adverse events with fibrates. Am. J. Cardiol. 2004; 94: 935–8. [DOI] [PubMed] [Google Scholar]
- 8. Graham GG, Punt J, Arora M et al Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011; 50: 81–98. [DOI] [PubMed] [Google Scholar]
- 9. Roden M, Weng J, Eilbracht J et al Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013; 1: 208–19. [DOI] [PubMed] [Google Scholar]
- 10. Rosenstock J, Seman LJ, Jelaska A et al Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add‐on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes. Metab. 2013; 15: 1154–60. [DOI] [PubMed] [Google Scholar]
- 11. Häring HU, Merker L, Seewaldt‐Becker E et al Empagliflozin as add‐on to metformin in patients with type 2 diabetes: a 24‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Care. 2014; 37: 1650–9. [DOI] [PubMed] [Google Scholar]
- 12. Jahagirdar V, Barnett AH. Empagliflozin for the treatment of type 2 diabetes. Expert Opin. Pharmacother. 2014; 15: 2429–41. [DOI] [PubMed] [Google Scholar]
- 13. Danan G, Benichou C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug‐induced liver injuries. J. Clin. Epidemiol. 1993; 46: 1323–30. [DOI] [PubMed] [Google Scholar]
- 14. Gooßen K, Graber S. Longer term safety of dipeptidyl peptidase‐4 inhibitors in patients with type 2 diabetes mellitus: systematic review and meta‐analysis. Diabetes Obes. Metab. 2012; 14: 1061–72. [DOI] [PubMed] [Google Scholar]
- 15. Toyoda‐Akui M, Yokomori H, Kaneko F et al A case of drug‐induced hepatic injury associated with sitagliptin. Intern. Med. 2011; 50: 1015–20. [DOI] [PubMed] [Google Scholar]
- 16. Kutoh E. Probable linagliptin‐induced liver toxicity: a case report. Diabetes Metab. 2014. Feb; 40: 82–4. [DOI] [PubMed] [Google Scholar]
- 17. US National Library of Medicine. National Institute of Diabetes and Digestive and Kidney Disease . Dipeptidyl peptidase‐4 inhibitors. LiverTox: clinical and research information on drug‐induced liver injury; 2014.
- 18. Lammert C, Bjornsson E, Niklasson A, Chalasani N. Oral medications with significant hepatic metabolism at higher risk for hepatic adverse events. Hepatology. 2010; 51: 615–20. [DOI] [PubMed] [Google Scholar]
- 19. Davis TME. Dipeptidyl peptidase‐4 inhibitors: pharmacokinetics, efficacy, tolerability and safety in renal impairment. Diabetes Obes. Metab. 2014; 16: 10891–9. [DOI] [PubMed] [Google Scholar]


