Abstract
Background and Aim
There is increasing prevalence of inflammatory bowel disease (IBD) in Asia, but Sri Lankan data on the state of epidemiology and clinical course of IBD are scarce.
Methods
A hospital‐based study was done by recruiting IBD patients who permanently reside in the Central Province (population 2.57 million, 12.6% of Sri Lankan population) of Sri Lanka. Cases were confirmed by standard criteria and data were collected from health records and patient interviews at clinic visits and hospital admissions.
Results
There were 200 cases of IBD; (ulcerative colitis [UC]—140, Crohn's disease [CD]—60, microscopic colitis—7). The crude prevalence rate of UC was 5.44/100 000 (95% CI 5.41–5.47/100 000) and CD was 2.33/100 000 (95% CI 2.31–2.35/100 000). Female to male ratios were 1:0.8 for UC but 1:1.5 for CD.
Mean age at diagnosis was 42.0 and 31.9 years for UC and CD, respectively. One UC and one CD patient had positive family history of IBD. Among the UC patients, 60.7%, 24.3%, and 15% had proctitis, left sided, and extensive disease, respectively. At presentation, 62.1% of the UC patients have had moderately severe disease. 60% of the CD patients had only large bowel involvement and 80% had nonstricturing and nonpenetrating disease. Extra intestinal manifestations were present in 45.7% and 60.0% of UC and CD patients, respectively, in which peripheral arthralgia and arthritis being the commonest. 6.4% of UC and 23.3% of the CD patients (total of 23) required infliximab for induction of remission.
Conclusion
The prevalence of IBD in the Central Province of Sri Lanka is lower than other Asian and Western populations. There is a predominance of male gender and isolated colonic disease in CD patients. UC patients have an equal gender distribution and a higher proportion of proctitis. CD needed induction with infliximab than UC.
Keywords: clinical course, disease characteristics, induction of remission, prevalence of ibd, Sri Lanka
Introduction
Crohn's disease (CD) and ulcerative colitis (UC) are chronic inflammatory bowel diseases (IBD) occurring due to a dysregulated mucosal immune response to antigenic stimulation on a background of genetic susceptibility. IBD has been widely discussed as a disease primarily occurring in Western countries and considered as a rare disease in Asia. The incidence and prevalence of UC in Asia range from 0.28 to 2.22 per 100 0001 and 5.3 to 63.6 per 100 000 persons,2, 3 whereas in the West they range from 8 to 14 per 100 000 and 120 to 200 per 100 000 persons, respectively.4 The incidence and prevalence of CD in Asia range from 0.07 to1.3 per 100 0001 and 1.2 to 21.1 per 100 000 persons,2, 3 but in the West they range from 6 to 15 per 100 000 and 50 to 200 per 100 000 persons, respectively.4 The prevalence and incidence of IBD are increasing in Asian countries, especially in East Asian countries, including Hong Kong, Korea, and Japan, although the exact etiology and mechanisms for this trend are not clear. There are very few population‐based studies of IBD in Asia, and in most studies that were carried out, the incidence and/or prevalence rates have been derived from a hospital database using the total population of the area that the hospital serves as a denominator. This study was aimed at investigating the prevalence and clinical course of IBD in the Central Province of Sri Lanka. The Central Province of Sri Lanka has a population of 2.57 million, which comprises 12.6% of Sri Lankan population (Fig. 1). As Sri Lanka still does not have national level studies and available studies are limited to a few centers in the Western province, this study, which covered the Central province, would take us one step closer to the national level study.
Figure 1.
Map of Sri Lanka with the location of secondary and tertiary care institutions of the Central Province in which IBD patients are followed up.
Methods
Study design
This study was a hospital‐based retrospective study.
Study setting
Curative care for IBD patients in the Central Province is provided by 7 secondary care and 3 tertiary care institutions under the government and 17 private hospitals (Fig. 1).
The seven secondary care institutions in the Central Province are namely DGH Nuwaraeliya, DGH Matale, DGH Nawalapitiya, DBH Gampola, DBH Dambulla, DBH Dickoya, and DBH Rikillagaskada. The research team visited the medical, surgical, and pediatric clinics of the above‐mentioned institutions over a period of 6 months. The screening for IBD patients was done at gastroenterology and hepatology clinics and gastroenterology surgical clinics in addition to medical, surgical, and pediatric clinics in three tertiary‐care hospitals, TH Kandy, TH Peradeniya, and Srimawo Bandaranaike Children's Hospital. IBD patients who were exclusively treated in the private sector in the Kandy district were directed to gastroenterology and hepatology clinic of TH Kandy by the consultants treating them for data collection and clinic registration.
Study population and recruitment
As there was no registry for IBD patients in the Central Province of Sri Lanka, the patients were identified after screening the clinic attendees. The patients’ demographic and clinical details were obtained via interviewer‐administered questionnaires. All patients who were diagnosed with UC, CD, or microscopic colitis and were permanent residents in the Central province were recruited. The diagnosis of IBD was taken into account if they satisfied accepted clinical, radiological, and endoscopic criteria with histological confirmation. To assess the current severity of the disease, biochemical and hematological investigations as well as colonoscopy and endoscopy were arranged. Patients with suspected concomitant tuberculosis infection were excluded from the study.
Patient identities were cross‐checked with their identity card numbers if available and, if not, comparison with several data including name, age, address, etc. was done to avoid replication. Clinical information was obtained by both case record reviewing and patient interviews after informed written consent of the patient or from the guardian of children where necessary.
Data collection and analysis
The data collected in the data collection sheets were transferred to SPSS IBM statistics version 20, which was used for carrying out the analysis. The population figures and other demographic data for the Central Province were obtained from the Department of Census and Statistics of Sri Lanka.5 The crude prevalence rates of UC and CD were calculated using the total number of residents in the Central Province (catchment area) as the denominator and the total number of patients with UC and CD, respectively, as the numerator. The rates were expressed as the number of patients per 100 000 persons.
Results
There were 200 cases of IBD; (ulcerative colitis [UC]—140, Crohn's disease [CD]—60). The crude prevalence rate of UC was 5.44/100 000 (95% CI 5.41–5.47/100 000) and CD was 2.33/100 000 (95% CI 2.31–2.35/100 000). Age‐related prevalence of UC and CD is presented in Figure 2. Female to male ratios were 1:0.8 for UC but 1:1.6 for CD (Table 1). Mean age at diagnosis was (males and females) 41.7 and 42.0 years for UC and 31.5 and 31.8 years for CD. The prevalence of IBD was highest in Sinhalese at 9.8 (UC—7.0, CD—2.8) per 100 000, and the lowest in Tamil at 4.1 (UC—1.3, CD—1.1) per 100 000 (Fig. 3).
Figure 2.
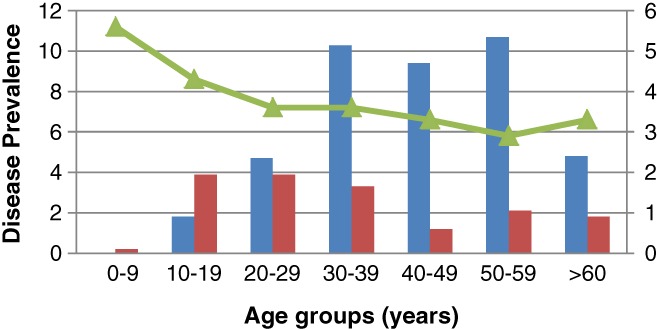
Age‐adjusted prevalence of IBD in the Central Province of Sri Lanka. Prevalence of UC is always higher than CD except in the age group of 10 to 19 years.  , Prevalence UC per 100 000;
, Prevalence UC per 100 000;  , Prevalence CD per 100 000;
, Prevalence CD per 100 000;  , Population (× 100 000).
, Population (× 100 000).
Table 1.
Clinical and demographic profiles of the IBD patients in the Central Province of Sri Lanka
| Ulcerative colitis (n = 140) | Crohn's disease (n = 60) | ||
|---|---|---|---|
| Sex | Male | 65 (46.4%) | 36 (60.0%) |
| Female | 75 (53.6%) | 24 (40.0%) | |
| Mean Age At diagnosis | Male | 42.00 | 31.96 |
| Female | 42.04 | 31.88 | |
| Ethnicity | Sinhala | 118 (84.3%) | 47 (78.3%) |
| Tamil | 9 (6.4%) | 7 (11.7%) | |
| Muslim | 13 (9.3%) | 6 (10.0%) | |
| Severity of disease at the time of diagnosis (UC) | Mild | 26 (18.6%) | |
| Moderate | 87 (62.1%) | ||
| Severe | 27 (19.3%) | ||
| Severity of disease at the time of interview (CD) | Asymptomatic remission | 27 (45%) | |
| Moderately to severely active disease | 20 (33.3%) | ||
| Severely active to fulminant disease | 13 (21.7%) | ||
| Presence of extra intestinal manifestations | Arthritis and arthralgia | 60 (42.9%) | 36 (60.0%) |
| Ocular disease | 2 (1.4%) | 3 (5.0%) | |
| Primary sclerosing cholangitis | 1 (0.7%) | 1 (1.7%) | |
| Skin rashes | 1 (0.7%) | 1 (1.7%) | |
| Thromboembolism | 2 (1.4%) | 0 | |
| Positive family history | 1 (0.7%) | 1 (1.7%) | |
| Surgical interventions | 2 (1.4%) | 10 (16.7%) | |
| Maintenance treatment | Topical aminosalicylate | 9 (6.4%) | 0 |
| Oral aminosalicylate | 92 (65.7) | 12 (20%) | |
| Oral azathioprine | 6 (4.3%) | 13 (21.6%) | |
| Oral aminosalicylate plus | 33 (23.6%) | 33 (55%) | |
| Azathioprine | |||
| Azathioprine plus infliximab | 0 | 2 (3.4%) | |
Figure 3.
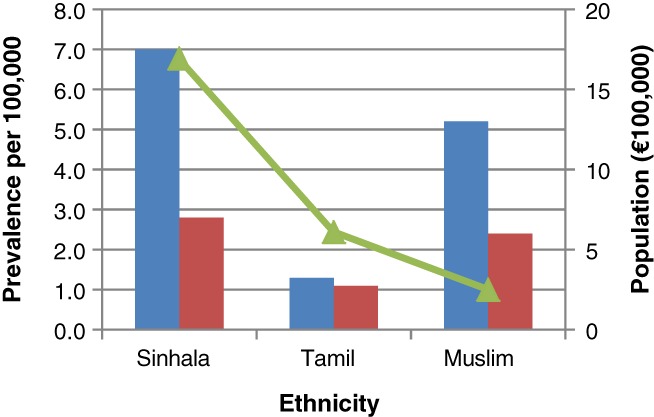
Prevalence of IBD in different ethnic groups in the Central Province of Sri Lanka. The prevalence of IBD was the highest in Sinhalese people 9.8 (UC—7.0, CD—2.8) per 100 000, and the lowest in Tamil people 4.1 (UC—1.3, CD—1.1) per 100 000.  , Prevalence of UC per 100 000;
, Prevalence of UC per 100 000;  , Prevalence of CD per 100 000;
, Prevalence of CD per 100 000;  , Population (× 100 000).
, Population (× 100 000).
To understand the disease phenotype of the patients, Montreal classification was used for both UC and CD.6, 7 Among the UC patients, 61.4% had proctitis (E1), 23.6% had left‐sided (E2), and 15% had extensive (E3) disease (Fig. 4). Symptoms at presentation were altered bowel habits (96%), rectal bleeding (88%), abdominal pain (58%), anemia (30%), and weight loss (62%). At the time of diagnosis, 61.4% of the UC patients have had a moderately severe disease and only 20% of the patients were found to have a severe disease according to Truelove and Witts classification of severity of UC. Severity of the disease was not significantly different among ethnic groups, age groups, or gender. Extra intestinal manifestations were present in 46.4% of the UC patients among whom arthritis and arthralgia were the commonest. Only one (0.7%) patient had a positive family history. Induction of remission in UC was achieved by medical treatments and nearly 99% were given oral 5ASA, while 68% of them were given combined treatment with oral steroids. Azathioprine was used in 15% of the patients (Fig. 5). Despite 61% of the UC patients having distal disease, local therapy with 5ASA or steroid enemas were used only in 11% of patients. About 6% of the nine UC patients were given biological therapy (infliximab) as the induction treatment, but all of them were initially treated with a combination of other medical treatments including steroids, 5ASA, and azathioprine. For maintenance, 71% of the UC patients were exclusively on oral 5ASA drugs and 24% were on combined therapy with oral 5ASA and azathioprine.
Figure 4.

Extent of the disease: IBD. Among UC patients, 61.4% had proctitis (E1), 23.6% had left‐sided (E2), and 15% had extensive (E3) disease.  , Distal disease;
, Distal disease;  , Left‐sided disease;
, Left‐sided disease;  , Extensive disease. Among CD patients 60% had only large bowel (L2) involvement followed by ileocolonic (L3) disease (23.3%) and ileal disease (L1) (16.7%).
, Extensive disease. Among CD patients 60% had only large bowel (L2) involvement followed by ileocolonic (L3) disease (23.3%) and ileal disease (L1) (16.7%).  , Only small intestinal involvement;
, Only small intestinal involvement;  , Only large intestinal involvement;
, Only large intestinal involvement;  , Large and small intestinal involvement;
, Large and small intestinal involvement;  , SI and upper GI;
, SI and upper GI;  , LI and upper GI.
, LI and upper GI.
Figure 5.
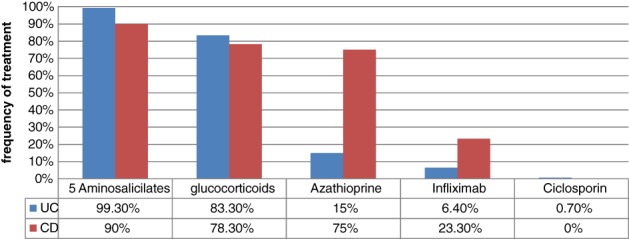
Drugs used in Induction of Remission in IBD. In UC, 99% were given oral 5ASA and 68% of them were given combined treatment with oral steroids. Azathioprine was used in 15% of patients and 6% of patients were given infliximab. 40% of the CD patients achieved remission with combined treatment with systemic steroids, 5ASA drugs, and azathioprine. 23% of the CD patients needed induction with infliximab.
Among the CD patients, 60% had only large bowel (L2) involvement followed by ileocolonic (L3) disease (23.3%) and ileal disease (L1) (16.7%) (Fig. 4). More patients had nonstricturing and nonpenetrating disease (B1) (78.3%) than penetrating (B2) (20.0%) or stricturing disease (B3) (1.7%). Peri‐anal disease occurred in 21.3% of our patients and 16.7% of CD patients had undergone surgery for complications. At the time of interview, 45% of the CD patients were in asymptomatic remission and 21% of the patients had a severely active to fulminant disease according to the Crohn's Disease Activity Index (CDAI). Extra intestinal manifestations were present in 60% of the CD patients. Only one (1.7%) patient had a positive family history for CD. About 40% of the CD patients achieved remission with combined treatment with systemic steroids, 5ASA drugs, and azathioprine (Fig. 5). 23% of the CD patients needed induction with infliximab after failing the above‐mentioned treatment. Maintenance of the remission was achieved in 96% of the CD patients with 5ASA and azathioprine either alone or in combination. Only two (3.4%) CD patients were on infliximab maintenance.
Discussion
In the Central Province of Sri Lanka, the crude prevalence rate of UC was 5.44/100 000 and CD was 2.33/100 000. Traditionally, the highest occurrence of both UC and CD was found in the developed and industrialized countries of North America and Europe.8 Although there is a relatively stable incidence in Europe, IBD has become more prevalent in previously low‐incidence areas including Asia.1 There are no national level studies in Sri Lanka on IBD, but the prevalence in the current study tallies with the previous hospital‐based study done in two districts of Sri Lanka 7 years ago, except for a slight increase in UC and CD prevalence.2 As there are not many population‐based studies done in Asian countries, exact prevalence rates for IBD are not yet known for Asia. Hospital‐based studies would detect only the treatment‐seeking IBD patients, which would leave out defaulted patients who are asymptomatic and those who take alternative (ayurvedic) medicine. The increasing prevalence of IBD in Asia could be partly due to physician and patient awareness, increased availability of better diagnostic facilities; however, environment and lifestyle changes could have contributed to this. Sri Lanka is undergoing socioeconomic change where hygiene is improving and dietary habits are changing. A study done in 2014 in the Asia Pacific region to find the environmental risk factors for IBD revealed a more “westernized diet” and smoking were associated with high IBD risk but inverse relationship with antibiotic use.9
The gender distribution of this study was similar to previous Asian studies done in China, Japan, Korea, and Hong Kong where the equal gender distribution for UC and male predominance for CD were found.10 This is in contrast to Western studies, which show equal or moderate female predominance for CD. Male predominance of CD may be partly due to the gender difference in smoking. However, more studies need to be done to confirm this.
The mean age at diagnosis of IBD in the study was similar to Western studies where CD is diagnosed most frequently in early thirties, but UC tends to be diagnosed most frequently in the 30–40‐year‐old age group.
The disease locations of UC and CD patients were generally similar to the Asian, Australian, and Western populations.1, 8 Earlier studies suggested a relatively benign disease course for UC and CD in Sri Lanka in relation to Western countries. In addition, this study had a high proportion of CD patients with nonstricturing and nonpenetrating disease in contrast to Chinese11 and European populations.8
The remission induction treatment for IBD was similar to Asian and European studies, however, in this study, all the patients were managed medically. This may be partly due to less disease severity compared to the West and treatment with biologics is the rescue treatment in severe diseases. The use of biological treatment for CD was 23% and, as a developing country, the use of biological treatment for IBD being broadly similar to the West (22%) was a striking finding in this study.12 But, surely it would have improved the outcome of the disease. The patients were given biological treatments free of charge by the Government of Sri Lanka. There is always a time gap between the request and supply of the drug to the patient as it has to be ordered specially for the patients. Until that time, all the patients were given other treatment combinations. Prospective randomized studies on biological treatments in our population should be carried out to explore the outcomes such as the relapse rate, disease severity, mortality and surgical morbidity, and cost–benefit analysis.13
During the maintenance of remission, steroids were not used. Despite the high proportion of proctitis patients, local therapy as enema was not widely practiced. Steroid or mesalazine enemas are not supplied free of charge by the Government Hospitals in Sri Lanka. The patients’ preference for oral drugs over enemas and having to purchase them by spending money out of their pockets could have made local therapy less popular.
Conclusion
The prevalence of IBD in the Central Province of Sri Lanka is lower than other Asian and Western populations. There is a predominance of male gender and isolated colonic disease in CD patients and they are diagnosed earlier than UC patients. UC patients have an equal gender distribution and a higher proportion of proctitis, similar to the West. There is less familial clustering in both UC and CD. The severity and the complications of IBD were lower but the use of biologics in CD patients was similar to the West. Prospective population‐based studies at a national level are needed to identify the environmental and genetic factors attributing to the IBD prevalence and the disease course with newer treatments specially biologics in Sri Lanka.
Acknowledgments
We thank the Physicians, Surgeons, and Pediatricians of the Secondary care Hospitals of the Central Province and their staff for their great support extended during the study.
Dr. Tharanga Liyanaarachchi is now working at Gastroenterology and Hepatology Unit, National Hospital of Sri Lanka, Colombo, Sri Lanka.
Declaration of conflict of interests: None.
References
- 1. Ng SC, Tang W, Ching JY et al Incidence and phenotype of inflammatory bowel disease based on results from the Asia‐pacific Crohn's and colitis epidemiology study. Gastroenterology. 2013; 145(1): 158–65 e2. [DOI] [PubMed] [Google Scholar]
- 2. Niriella MA, De Silva AP, Dayaratne AH et al Prevalence of inflammatory bowel disease in two districts of Sri Lanka: a hospital based survey. BMC Gastroenterol. 2010; 10: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asakura K, Nishiwaki Y, Inoue N, Hibi T, Watanabe M, Takebayashi T. Prevalence of ulcerative colitis and Crohn's disease in Japan. J. Gastroenterol. 2009; 44(7): 659–65. [DOI] [PubMed] [Google Scholar]
- 4. Cosnes J, Gower‐Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011; 140(6): 1785–94. [DOI] [PubMed] [Google Scholar]
- 5. http://www.statistics.gov.lk/page.asp?page=Population%20and%20Housing 2012. Cited 2017.
- 6. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006; 55(6): 749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dignass A, Eliakim R, Magro F et al Second European evidence‐based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J. Crohns Colitis. 2012; 6(10): 965–90. [DOI] [PubMed] [Google Scholar]
- 8. Burisch J, Jess T, Martinato M, Lakatos PL. The burden of inflammatory bowel disease in Europe. J. Crohn's Colitis. 2013; 7(4): 322–37. [DOI] [PubMed] [Google Scholar]
- 9. Ng SC, Tang W, Leong RW et al Environmental risk factors in inflammatory bowel disease: a population‐based case‐control study in Asia‐Pacific. Gut. 2015; 64(7): 1063–71. [DOI] [PubMed] [Google Scholar]
- 10. Thia KT, Loftus JEV, Sandborn WJ, Yang S‐K. An update on the epidemiology of inflammatory bowel disease in Asia. Am. J. Gastroenterol. 2008; 103(12): 3167–82. [DOI] [PubMed] [Google Scholar]
- 11. Leong RW, Lau JY, Sung JJ. The epidemiology and phenotype of Crohn's disease in the Chinese population. Inflamm. Bowel Dis. 2004; 10(5): 646–51. [DOI] [PubMed] [Google Scholar]
- 12. Burisch J, Pedersen N, Cukovic‐Cavka S et al East‐West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO‐EpiCom inception cohort. Gut. 2014; 63(4): 588–97. [DOI] [PubMed] [Google Scholar]
- 13. van der Valk ME, Mangen MJ, Leenders M et al Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti‐TNFalpha therapy: results from the COIN study. Gut. 2014; 63(1): 72–9. [DOI] [PubMed] [Google Scholar]


