Abstract
Background and Aim
Polyethylene glycol (PEG) is the gold standard for fecal disimpaction in constipation. A regimen of PEG combined with the stimulant laxative sodium picosulphate (SPS) produced fecal disimpaction in chronically constipated children in the community, but it is unknown if it is effective for more severe constipation. To determine the stool output and effect of a combined PEG and SPS regimen on fecaloma in children with severe constipation and impaction.
Methods
Children with symptoms for a duration of ≥2 years, a palpable fecaloma, and enlarged rectum on X‐ray (rectal: pelvic ratio > 0.6) were recruited from a tertiary hospital. Daily diaries recorded laxative dose, stool frequency, volume, and consistency (Bristol stool scale, BSS). Abdominal X‐rays were taken on day 1 and day 8, and stool loading was assessed using the Leech score. Laxative doses were based on the child's age. The dose of PEG with electrolytes taken was 2–8 sachets (14.7 g/sachet) on days 1–2, reducing to 2–6 sachets on day 3. The SPS dose was 15–20 drops on days 2–3.
Results
Eighty‐nine children (4–18 years) produced a large volume of soft stool (median/inter‐quartile‐range: 2.2/1.6–3.1 L) over 7 days. Stool volume on X‐rays decreased significantly in the colon (P < 0.001). Fecalomas resolved in 40 of 89 children, while 49 needed a second high dose. Rectal:pelvic ratios did not change.
Conclusions
A combined high dose of PEG and SPS on days 1 and 2 was effective in removing the fecaloma in half of the children. Administering high doses for a longer period should be tested to provide outpatient disimpaction for severe fecalomas. Rectums remained flaccid after emptying.
Keywords: chronic constipation, laxatives, macrogol, polyethylene glycol, X‐rays
Introduction
Chronic constipation is common in pediatrics, representing 3–5% of general outpatient visits and up to 25% of gastroenterology consultations.1 The prevalence of constipation in children has been estimated to range between 1 and 30%.2, 3 Treatments include dietary, pharmacological approaches (laxatives, prokinetics), behavioral therapy, and, in extreme cases, surgery.4, 5, 6 Fecal impaction is defined as accumulation of hard stool in the anorectum.7, 8 Disimpaction, to remove the hard fecal mass, is required before commencing maintenance therapy for constipation.
Polyethylene glycol (PEG) 3350 is an osmotic laxative that draws water into the colon to soften the stool. It is an oxyethylene polymer (H[OCH2CH2]3350OH) that cannot be absorbed or metabolized by the body.9 PEG disimpacted 92% of children in randomized control trials (RCTs) and was significantly more effective than a placebo.10, 11 In these clinical trials, the maximum high dose for each age (1.5 g/kg/day) was given for 3–6 days, although many patients developed incontinence, suggesting that the dose was too high.
Bisacodyl and its derivative sodium picosulphate (SPS) are stimulant laxatives that are effective but produce cramping in some patients. As SPS is a liquid, it is more easily taken by children and is more easily titrated.12 SPS is a locally acting laxative of the triarylmethane group, which undergoes bacterial cleavage in the colon to produce a diphenol, which stimulates bowel motility. SPS and bisacodyl have a similar level of effectiveness and, alone, can produce disimpaction in 80% of children.12, 13
A large volume of PEG is required for disimpaction, and this can result in poor compliance, but adding a stimulant can halve the volume of PEG required and increase compliance.14, 15 PEG with a stimulant has also been used to completely empty the bowel prior to colonoscopy16 in individuals with normal bowel motility. These findings can translate into regimens for disimpaction. In this study, we tested a method of combining PEG with SPS for disimpaction in children,17 aiming to determine its effectiveness in children with severe, chronic constipation and a palpable fecaloma.
Methods
A total of 120 patients (4–18 years of age) from the community or those presenting to The Royal Children's Hospital Emergency Department with chronic constipation were enrolled. Patients were screened by history, and those who met the criteria were assessed by a Pediatric Gastroenterologist (ML) for a palpable fecaloma. If a fecaloma was present, they were X‐rayed and then educated on how to do the disimpaction and the dose of laxatives to take. Fecal disimpaction was performed by patients as the first step in a RCT testing another intervention. All patients underwent disimpaction followed by 3 weeks of education on diet, stress, when to toilet, and toilet posture. Patients completed a daily diary that was used to record laxative volume taken, stool volume produced, stool frequency, and consistency measured with the Bristol stool scale (BSS). Study data were collected and managed using the REDCap electronic data capture tools hosted at The Murdoch Children's Research Institute.18 Research Electronic Data Capture (REDCap) is a secure, web‐based application designed to support data capture for research studies. This study is a review of the abdominal X‐rays and diary records of the first week. Data on symptoms have been previously reported in abstracts.19, 20 X‐rays were taken to determine fecal loading and the rectal:pelvic ratio before (day 1) and 1 week (day 8) after oral laxatives. Inclusion criteria were: ≥2 years’ chronic constipation defined by Rome III criteria21; palpable fecaloma on presentation, confirmed by palpation by an experienced clinician; an enlarged stool‐filled rectum on X‐ray; and a rectal:pelvic ratio above the normal range (>0.61).
The laxatives given included PEG plus electrolytes (Movicol, Norgine, 14.7 g/sachet) and SPS (Dulcolax SP drops, Boehringer, 7.5 mg/mL, 0.5 mg/drop).22 The prescribed dose is shown in Table 1. The maximum PEG dose/day approved by Therapeutic Goods Association, TGA, Australia varies by age (4 sachets for 4 to 5 years, 6 sachets for 6 to 11 years and 8 sachets for 12 years and older). Patients were prescribed the maximum dose for their age on day 1, reducing on day 2 and 3, and then again to 1 sachet on day 4–7. Each sachet was dissolved in 125 mL water plus an equal volume of juice/milk. Children drank 125–250 mL per half hour over the morning, making it easier to drink small volumes each hour and spreading the fluid intake. They took 10–20 drops of SPS (dose dependent on age) in a drink at night, as described previously, starting on day 2, with the highest dose given on day 3. SPS was not started on day 1 to avoid producing pain by using a stimulant while the fecaloma was still hard. Delaying SPS by a day gave the PEG a chance to soften the stool. If disimpaction was not achieved, a second round of high‐dose laxatives was given on days 8–10.
Table 1.
Prescribed dose of Movicol (polyethylene glycol [PEG] and electrolytes 14.7 g/sachet) and Dulcolax SPS (drops 0.5 mg/drop)
| Age (years) | Day 1 | Day 2 | Day 3 | Day 4 | |
|---|---|---|---|---|---|
| Movicol sachets | 4–5 | 4 | 2–3 | 1–2 | 1 |
| 6–11 | 6 | 5–6 | 2–3 | 1 | |
| 12–18 | 6–8 | 4–8 | 2–3 | 1 | |
| SPS drops | 4–11 | — | 12 | 15 | 10 |
| 12–18 | — | 15 | 20 | 15 |
Stool loading on X‐rays was measured by three observers using the Leech scoring method.23 The Leech method divides the colon into three regions (right, left, and rectosigmoid/rectum), and each region is scored as follows: 0, no feces; 1, scant feces; 2, mild fecal loading; 3, moderate fecal loading; 4, severe fecal loading; and 5, severe fecal loading with bowel dilatation. Stool volume on X‐rays was estimated by outlining segments of colon and measuring the length and radius; then, volume was calculated using the following equation for a cylinder, where “r” is the radius of the colon (half the width) and “L” is the length of the colon segment: Volume of a cylinder = πr 2 × L. The volume of multiple segments was summed to obtain the total colon volume.
Ethical approval was obtained from the Murdoch Children's Research Institute (MCRI) and The Royal Children's Hospital (RCH), Melbourne, Australia (HREC 32014, 33132, 35151). The institutional ethics committee approved the study in accordance with international standards (including the Declaration of Helsinki). All participants gave informed consent prior to their inclusion in the study. The study was supported by the National Health and Medical Research Council (NHMRC), Project Grant (1025726) 2012–2015; clinical trial registration—ACTRN12612001009808.
Results
A total of 120 patients were enrolled from April 2013 to May 2015. Ninety‐nine commenced disimpaction. Six patients completed diary entries for fewer than 5 days in the disimpaction week, and so, their data were not analyzed. Ninety‐four patients filled in diaries for 6 or 7 days, but data were available for both X‐rays from only 89 patients (43 male, median age 8 years [range 4–18]). All patients had constipation and treatment for ≥2 years (2–10 years). Sixteen patients also had diary data collected for the 2 weeks before treatment. The frequency of bowel motions was a median of 6.5 (IQR 3, 11) per week before treatment, and median stool volume produced was 1.0 (IQR 0.73, 1.63) L/week. None of the patients had <3 bowel movements/week, with the lowest being 3.
All patients had a palpable fecaloma confirmed by two study clinicians and a rectal diameter:pelvic internal diameter (R:P) ratio above the normal range (0.63–0.96, median 0.84). The extent of hard stool was estimated by palpation. On day 1, most patients had hard stool palpable beyond the rectum, three quarters had hard stool in the sigmoid, one third into the descending colon, and 1 had hard stool all the way to the transverse colon. The volume of stool in the colon was estimated from the X‐rays and was between 0.05 and 1.7 L on day 1.
The prescribed dose of Movicol was based on the age of the patients (Table 1). Some did not take the prescribed dose. The median dose of Movicol and Dulcolax taken each day is shown in Figure 1a,b. Patients took 2–8 sachets of PEG on day 1, 2–6 sachets on day 2, and 1–6 sachets on day 3. They then commenced a maintenance dose on days 4–7, with the dose starting at 1 sachet and ranging from 0 to 6 sachets (Fig. 1a). Patients were prescribed 0 drops of SPS on day 1. Most patients took no SPS on day 1 (but 11 patients took 10–20 drops), 15 drops on day 2, and 15–20 drops on day 3 and then 10 drops on days 4–7 (Fig. 1b). The variation in dose on days 4–7 indicates patients increasing or decreasing the dose if they felt they needed to.
Figure 1.
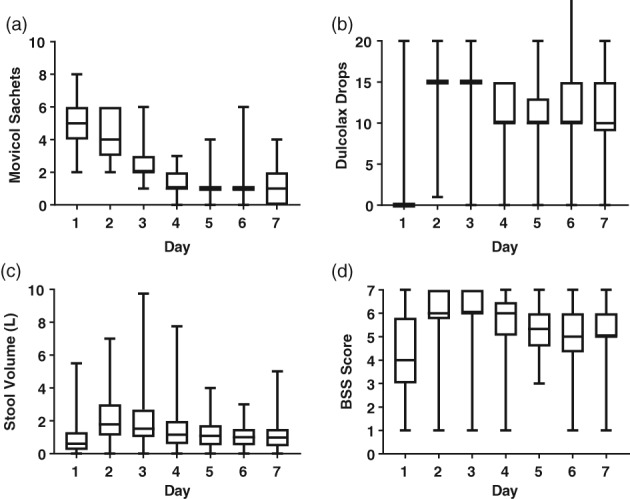
Dose of laxatives and stool output per day. (a) Dose of polyethylene glycol (PEG) plus electrolytes taken. One sachet =14.7 g. (b) Dose of stimulant sodium picosulphate (SPS) drops taken. (c) Volume of stool (liters) produced. Stool volume was estimated by patients/parents. (d) Softness of stool estimated using the Bristol Stool Scale (BSS), where 1 is very hard and 7 is very soft. (n = 89, median, range).
Prior to treatment, patients were taking a range of laxatives, including Osmolax, Movicol, Dulcolax, Lactulose, Parachoc, and Coloxyl. Despite this, they were heavily impacted. Most patients produced <1 cup (250 mL) of stool on day 1 (median 160 mL, Fig. 1c), with stool volumes peaking on day 2 and day 3. The stool volume then decreased on day 4, and patients continued to produce stool over 7 days. Stool consistency became softer (median BSS 4–6, P < 0.001). The increase in stool volume and softness occurred on day 2, the day after the first large dose of PEG and before any SPS was taken (on the evening of day 2). Over 7 days, they produced a median of 2.2 (IQR 1.6, 3.1) L of stool. In the 16 patients with baseline data, median stool frequency increased from 7 per the week before to 19 (IQR 13, 30)/week during the disimpaction week. Twenty‐eight (of 89) patients produced <0.5 L of stool over the 7 days, and 17 (61%) of these required a second disimpaction.
Soiling is commonly a problem in children with chronic constipation and is a common worry for patients using disimpaction doses of PEG. This regimen did not produce much soiling, with patients averaging less than 1 day of soiling in the week (data not shown).
Data for X‐ray measures and all days of the diaries were available for 89 patients. Day 1 X‐rays confirmed large stool masses in the rectosigmoid (stool volume in X‐ray, median 0.28 L IQR 0.19, 0.36, Fig. 2a,b), with a Leech score indicating significant fecal loading (median 4.0, IQR 3.4, 4.3, Fig. 2c,d and mean ± SEM rectal:pelvic ratio of 0.84 ± 0.01, Fig. 3). Three people independently scored the fecal loading using the Leech scoring system.23 Intraobserver consistency was very good to excellent [Intraclass Correlation Coefficient (ICC) 0.82–0.96], and interobserver agreement was fair to good (ICC 0.54). There was a significant reduction in the volume of stool in the right colon, left colon, rectosigmoid, and in the whole colon both by volume estimate (Fig. 2a,b) and by the Leech score (Fig. 2c,d). The Leech score and volume of stool on X‐ray showed a positive nonlinear relationship (Fig. 2e,f).
Figure 2.
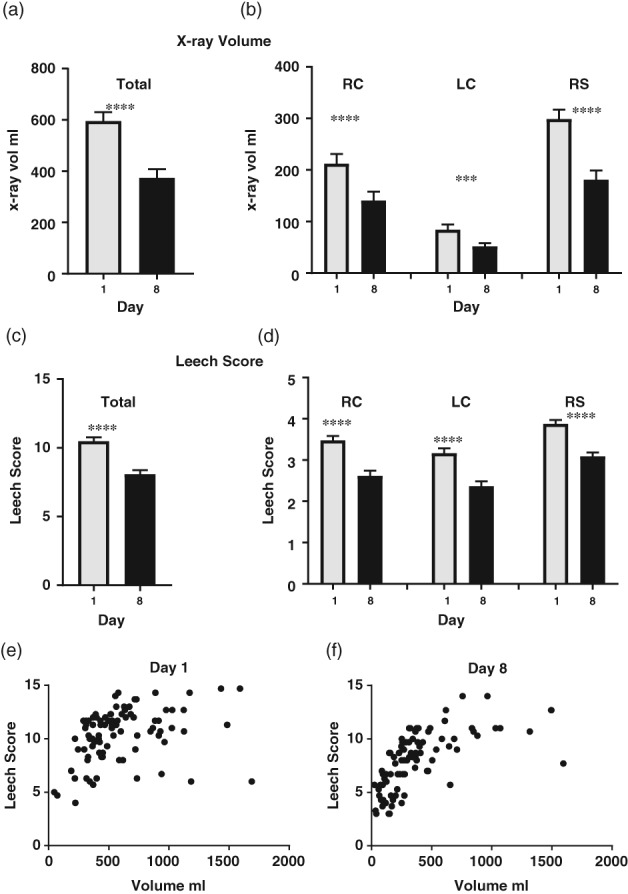
Volume of stool on X‐rays. Volume of stool measured in X‐rays in (a) total colon and (b) each region: right colon (RC), left colon (LC); rectosigmoid (RS). Stool loading in X‐ray using the Leech Score in the (c) total colon and (d) each region. ****P < 0.0001, ***P < 0.001. Relationship of stool volume on X‐ray to Leech Score on (e) day 1 and (f) day 8. (n = 89, mean, SEM).
Figure 3.
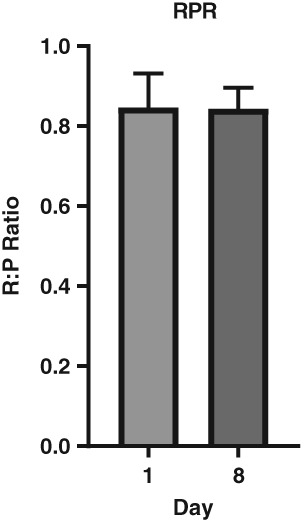
Rectal:Plevic ratio on day 1 and day 8. (n = 89, mean, SEM).
Forty patients had no palpable fecaloma on day 8 and were classified as achieving disimpaction. Forty‐nine patients required a second round of high‐dose laxatives for 2–3 days after day 8 and then achieved disimpaction. There was a weak positive relationship between stool volume on X‐ray and volume of stool produced (Fig. 4). There was little relationship between stool volume in the X‐ray and PEG dose (Fig. 5c,d).
Figure 4.
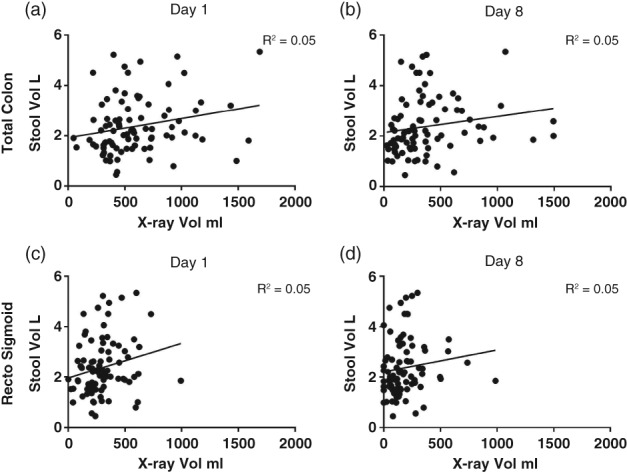
Relationship of X‐ray stool volume and stool output over 7 days. For total colon on (a) day 1 and (b) day 8. For rectosigmoid on (c) day 1 and (d) day 8.
Figure 5.
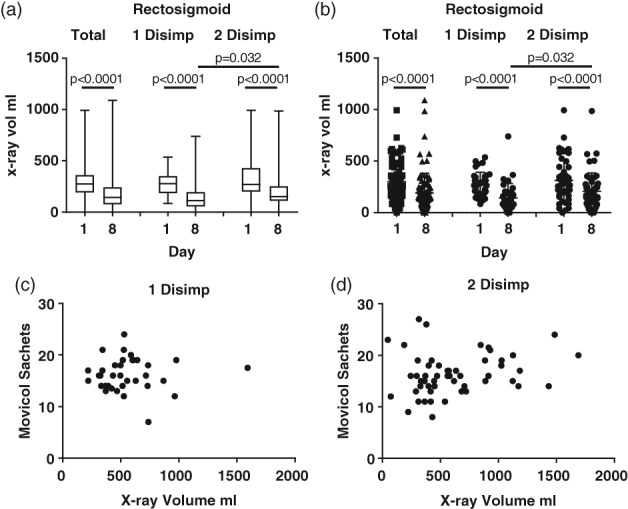
Volume of stool on X‐ray in patients requiring one or two rounds of high‐dose laxatives to achieve disimpaction. (a,b) volume of stool in X‐ray in rectosigmoid (RS) for whole group (total) and for group disimpacted after one round of high‐dose laxatives (1 disimp, n = 40) or two rounds of high‐dose laxatives (2 disimp, n = 49). A: Median, range. (c,d) comparison of stool volume on X‐ray on day 1 and dose of Movicol taken over 7 days. (c) Group requiring one round of disimpaction and (d) group requiring two rounds of disimpaction.
We expected that the patients requiring a second disimpaction might have more stool to move. To determine if there was a difference in the X‐ray volume of the two groups (one round of laxatives versus two rounds of laxatives), we compared the volume of stool in the rectosigmoid (Fig. 5). As expected, there was a significantly higher volume of stool in the rectosigmoid on day 8 in the group that required a second dose of laxatives for disimpaction (Fig. 5a,b). However, the volume range of the two groups overlapped both before and after the disimpaction, with only seven patients in the group requiring a second high dose of laxatives having a really high stool volume (>1 L) in the day 8 X‐ray (Fig. 5c,d).
Although the total stool volume in the whole colon was reduced, the R:P ratios (Fig. 3) did not change after disimpaction.
Discussion
PEG is the gold‐standard laxative treatment for constipation as it is well investigated,24, 25 safe and effective, and suitable for chronic use. The effect is dose dependent,26 with the main side effect being abdominal bloating24 due to distension of the bowel because of water binding.27 Previous studies used maximal doses for 3 or 6 days.10, 11 SPS is also effective for disimpaction, and studies for colonoscopy bowel preparation have shown that adding a stimulant laxative allows the volume of PEG to be reduced. We previously tested combined PEG and SPS for disimpaction in children in a suburban clinic.17 The aim of the current study was to determine the effect of the same treatment in children with more severe constipation and fecal impaction (symptoms for ≥2 years, palpable fecaloma with enlarged rectum proven by abdominal X‐ray, rectal:pelvic ratio of 0.62–0.96).28 Patients received a high dose of PEG and SPS over 3 days, and this resulted in increased stool frequency (from 7/week before to 19/week), volume, and improved stool softness. The hard fecaloma was completely removed in half of the patients.
PEG with electrolytes may be difficult to drink due to the large volume, salty taste, or the gritty texture.29 Kierkus et al. found that children were more likely to require insertion of a nasogastric tube if they were required to drink large volumes of PEG solution, compared to low‐volume PEG plus a stimulant laxative.30 We developed a method to overcome many of the compliance issues (add the child's favorite drink to cover the flavor, divide the dose into 250 mL aliquots, use small glasses, and make it a fun race).
A recent study compared two doses of PEG for chronic constipation (high 0.7 g/kg and low 0.3 g/kg over 6 weeks).31 Both were effective, although the low dose needed more adjustment to therapy, and more children experienced painful defecation.31 Children in the current study were given 2–8 sachets of PEG (0.9–1.5 g/kg on day 1–2, reducing to 0.3 g/kg on day 3). The treatment produced large volumes of stool and reduced fecal load, but half of the patients required a second round of disimpaction, suggesting inadequate dosing. Rather than reducing the dose each day, it may be necessary to keep the dose of PEG high until a defined end‐point of stool volume or wetness is reached. We previously reported this method for treating patients attending a suburban clinic where successful disimpaction was higher. The difference in results is likely to be due to the patients in the current study having more severe symptoms (and strict entry criteria).
Using plain abdominal radiography in diagnosing constipation is contentious, with no association between clinical symptoms of constipation and fecal loading in the radiograph.32, 33 The degree of fecal loading in abdominal X‐rays is affected by recent bowel movements emptying the rectum and fluctuations in diet.34 NASPGHAN guidelines recommend an abdominal X‐ray if there is fecal impaction.35 As the inclusion criteria for this study required that all patients have a palpable fecaloma (i.e. were impacted), it was felt it was appropriate to use radiography to objectively measure fecal loading in these patients.
There are three published scoring methods for evaluating fecal loading in children using abdominal radiography. Barr et al. used the quantity and quality of stool in the colon to score the fecal load.36 Blethyn et al. graded constipation solely based on the distribution of stool through the colon.37 Leech et al.23 (used in this study) divided the colon into three regions and scored each region separately from 0 to 5 based on the visible fecal load. All methods demonstrated high intraobserver correlation, while the Leech method has the strongest interobserver correlation.23, 38, 39, 40 High correlations between the Leech score and BSS, colonic transit time, and gastrointestinal symptoms have been demonstrated in constipated children.38 In this study, there was excellent correlation for intraobserver scoring, and the interobserver ICC was fair to moderate, suggesting that the use of the Leech system was valid.
It is well established that children with chronic constipation develop an enlarged rectum. However, the relationship between constipation and megarectum is still disputed, and the presence of an enlarged rectum reveals nothing about the pathophysiology of the constipation.28 Although the disimpaction method was able to empty the rectum, there was no change in the rectal:pelvic ratio, suggesting that acute disimpaction did not result in a toned rectum. This is consistent with the current literature proposing that reversion of the rectum back to its normal size takes months or years, and the flaccid rectum refills immediately. This is likely to be an ongoing problem and suggests that further interventions that target the rectal tone are needed to produce a strong and narrower section of bowel.
It is interesting that the stool frequency started initially above two defecations per week. Patients were on laxatives and were still impacted. Dinning et al.41 also reported that adults with chronic constipation take laxatives and have >2 defecations/week.
The study was limited by no comparator group because patients were part of a RCT where all patients had disimpaction before being randomized to two treatment groups. Pre‐disimpaction diary data were only available for a subset of patients as many wanted to start disimpaction straight away and did not fill diaries before disimpaction. The Leech measure of volume of stool in X‐rays is not validated, and clinicians disagree on the usefulness of X‐rays to measure stool and how to identify the outline of the empty bowel. All the patients in this study had impacted rectums, which made it easier to define the outline of the bowel, but many images were hard to identify. The volume of laxatives taken and stool output volumes were estimates by patients/parents who were shown a small adult fist as an example of a “cup” size (250 mL). It is difficult to estimate a volume for very watery stool.
In conclusion, disimpaction is difficult in patients with an impacted rectum and chronic constipation. Using PEG at the maximum dose for age on day 1 and reducing the dose over days 2 and 3, combined with SPS, produced a large volume of stool on days 2–3 and resolved fecalomas in half of the patients, suggesting that the method is effective for some, but a longer period on the high dose of PEG is needed for some patients with severe impaction. The method resulted in high levels of compliance, but maintaining higher doses for a longer period should be tested in these difficult patients. Effective disimpaction improves symptoms but does not reduce rectal diameter.
Acknowledgments
The authors thank Emily Dobson for secretarial support and Dr Lefteris Stathopoulos for discussions of study design.
Declaration of conflict of interest: None of the authors have pecuniary interests in Norgine (manufacturer of Movicol) or Boeringher (manufacturer of Dulcolax SPS drops) or received funding from either company. The Movicol and Dulcolax SPS drops were provided at a reduced price.
Financial support: This study was funded by the Australian National Health and Medical Research Council (NHMRC), Project Grant (1025726) 2012–2016 and was supported by the “Victorian Government Operational Infrastructural Support Program” and the Senior Research Fellowship (BS, 607396). The trial was registered for clinical trial registration‐ACTRN12612001009808. http://www.anzctr.org.au/TrialSearch.aspx?searchTxt=ACTRN12612001009808.&isBasic=True.
The study was presented to the Gastroenterology Society of Australia Annual meeting (Australian Gastro Week) in August 2017 and published in the abstracts of the Journal of Gastroenterology and Hepatology Volume 32, Issue S2, August 2017, Pages: 190–197, Version of Record online: 17 August 2017, DOI: https://doi.org/10.1111/jgh.13900. Data on symptom changes in subsets of the patients were presented at the American Gastroenterology Association Annual meeting (Digestive Diseases Week, 2015 (Southwell BR, Dughetti LD, Jordan‐Ely JA, Dobson KM, Stathopolous L, Leal MC, et al. Su2051 Combined Polyethylene Glycol and Sodium Picosulphate for Disimpaction in Children With Chronic Constipation and Palpable Faecaloma. Gastroenterology 2015;148 (4):S‐585) and the Gastroenterology Society of Australia Annual Meeting (Australian Gastro Week) in 2016 (Jordan‐Ely J, Dughetti L, Dobson K, Stathopoulos L, Lamanna A, Leal M, et al. Disimpaction for children with palpable faecalomas using polyethelene glycol and sodium picosulphate. J Gastroenterol Hepatol. 2016;31:178–9).
References
- 1. van den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am. J. Gastroenterol. 2006; 101: 2401–9. [DOI] [PubMed] [Google Scholar]
- 2. Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract. Res. Clin. Gastroenterol. 2011; 25: 3–18. [DOI] [PubMed] [Google Scholar]
- 3. Quigley EMM. Definition and epidemiology In: Southwell BR, Hutson JM, eds. Constipation: Current and Emerging Treatments. London: Future Medicine, 2013; 7–15. [Google Scholar]
- 4. Tabbers MM, Dilorenzo C, Berger MY et al Evaluation and treatment of functional constipation in infants and children: evidence‐based recommendations from ESPGHAN and NASPGHAN. J. Pediatr. Gastroenterol. Nutr. 2014; 58: 265–81. [DOI] [PubMed] [Google Scholar]
- 5. Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013; 144: 211–7. [DOI] [PubMed] [Google Scholar]
- 6. Muller‐Lissner SA, Kamm MA, Scarpignato C, Wald A. Myths and misconceptions about chronic constipation. Am. J. Gastroenterol. 2005; 100: 232–42. [DOI] [PubMed] [Google Scholar]
- 7. Tobias N. Management principles of organic causes of childhood constipation. J. Pediatr. Health Care. 2008; 22: 398. [DOI] [PubMed] [Google Scholar]
- 8. Wald A. Management and prevention of fecal impaction. Curr. Gastroenterol. Rep. 2008; 10: 499–501. [DOI] [PubMed] [Google Scholar]
- 9.Public Assessment Report: Movicol Plain 2008. http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con031174.pdf
- 10. Nurko S, Youssef NN, Sabri M et al PEG3350 in the treatment of childhood constipation: a multicenter, double‐blinded, placebo‐controlled trial. J. Pediatr. 2008; 153: 254–61 61.e1. [DOI] [PubMed] [Google Scholar]
- 11. Thomson MA, Jenkins HR, Bisset WM et al Polyethylene glycol 3350 plus electrolytes for chronic constipation in children: a double blind, placebo controlled, crossover study. Arch. Dis. Child. 2007; 92: 996–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kienzle‐Horn S, Vix JM, Schuijt C, Peil H, Jordan CC, Kamm MA. Comparison of bisacodyl and sodium picosulphate in the treatment of chronic constipation. Curr. Med. Res. Opin. 2007; 23: 691–9. [DOI] [PubMed] [Google Scholar]
- 13. Wulkow R, Vix JM, Schuijt C, Peil H, Kamm MA, Jordan C. Randomised, placebo‐controlled, double‐blind study to investigate the efficacy and safety of the acute use of sodium picosulphate in patients with chronic constipation. Int. J. Clin. Pract. 2007; 61: 944–50. [DOI] [PubMed] [Google Scholar]
- 14. Dik VK, Moons LM, Huyuk M et al Predicting inadequate bowel preparation for colonoscopy in participants receiving split‐dose bowel preparation: development and validation of a prediction score. Gastrointest. Endosc. 2015; 81: 665–72. [DOI] [PubMed] [Google Scholar]
- 15. Martel M, Barkun AN, Menard C, Restellini S, Kherad O, Vanasse A. Split‐dose preparations are superior to day‐before bowel cleansing regimens: a meta‐analysis. Gastroenterology. 2015; 149: 79–88. [DOI] [PubMed] [Google Scholar]
- 16. Phatak UP, Johnson S, Husain SZ, Pashankar DS. Two‐day bowel preparation with polyethylene Glycol 3350 and bisacodyl: a new, safe, and effective regimen for colonoscopy in children. J. Pediatr. Gastroenterol. Nutr. 2011; 53: 71–4. 10.1097/MPG.0b013e318210807a. [DOI] [PubMed] [Google Scholar]
- 17. Jordan‐Ely J, Hutson JM, Southwell BR. Disimpaction of children with severe constipation in 3‐4 days in a suburban clinic using polyethylene glycol with electrolytes and sodium picosulphate. J. Paediatr. Child Health. 2015; 51: 1195–8. [DOI] [PubMed] [Google Scholar]
- 18. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Southwell BR, Dughetti LD, Jordan‐Ely JA et al Su2051 combined polyethylene glycol and sodium picosulphate for disimpaction in children with chronic constipation and palpable faecaloma. Gastroenterology. 2015; 148: S‐585. [Google Scholar]
- 20. Jordan‐Ely J, Dughetti L, Dobson K et al Disimpaction for children with palpable faecalomas using polyethelene glycol and sodium picosulphate. J. Gastroenterol. Hepatol. 2016; 31: 178–9. [Google Scholar]
- 21. Rome F. Guidelines—Rome III diagnostic criteria for functional gastrointestinal disorders. J. Gastrointestin. Liver Dis. 2006; 15: 307–12. [PubMed] [Google Scholar]
- 22. Jordan‐Ely J, Dobson K, Hutson J, Southwell B. Transcutaneous electrical stimulation combined with disimpaction, laxative and diet modification produced daily defecation in children with slow‐transit constipation and is more effective than Tes alone. J. Gastroenterol. Hepatol. 2013; 28: 130. [Google Scholar]
- 23. Leech SC, McHugh K, Sullivan PB. Evaluation of a method of assessing faecal loading on plain abdominal radiographs in children. Pediatr. Radiol. 1999; 29: 255–8. [DOI] [PubMed] [Google Scholar]
- 24. Attar A, Lemann M, Ferguson A et al Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut. 1999; 44: 226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DiPalma JA, Mv C, McGowan J, Herrera JL. A randomized, multicenter, placebo‐controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am. J. Gastroenterol. 2007; 102: 1436–41. [DOI] [PubMed] [Google Scholar]
- 26. Hammer HF, Santa Ana CA, Schiller LR, Fordtran JS. Studies of osmotic diarrhea induced in normal subjects by ingestion of polyethylene glycol and lactulose. J. Clin. Invest. 1989; 84: 1056–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muller‐Lissner S. Pharmacokinetic and pharmacodynamic considerations for the current chronic constipation treatments. Expert Opin. Drug Metab. Toxicol. 2013; 9: 391–401. [DOI] [PubMed] [Google Scholar]
- 28. van der Plas RN, Benninga MA, Staalman CR et al Megarectum in constipation. Arch. Dis. Child. 2000; 83: 52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alper A, Pashankar DS. Polyethylene glycol: a game‐changer laxative for children. J. Pediatr. Gastroenterol. Nutr. 2013; 57: 134–40. [DOI] [PubMed] [Google Scholar]
- 30. Kierkus J, Horvath A, Szychta M et al High‐ versus low‐volume polyethylene glycol plus laxative versus sennosides for colonoscopy preparation in children. J. Pediatr. Gastroenterol. Nutr. 2013; 57: 230–5. [DOI] [PubMed] [Google Scholar]
- 31. Dziechciarz P, Horvath A, Szajewska H. Polyethylene glycol 4000 for treatment of functional constipation in children. J. Pediatr. Gastroenterol. Nutr. 2015; 60: 65–8. [DOI] [PubMed] [Google Scholar]
- 32. Reuchlin‐Vroklage LM, Bierma‐Zeinstra S, Benninga MA, Berger MY. Diagnostic value of abdominal radiography in constipated children: a systematic review. Arch. Pediatr. Adolesc. Med. 2005; 159: 671–8. [DOI] [PubMed] [Google Scholar]
- 33. Berger MY, Tabbers MM, Kurver MJ, Boluyt N, Benninga MA. Value of abdominal radiography, colonic transit time, and rectal ultrasound scanning in the diagnosis of idiopathic constipation in children: a systematic review. J. Pediatr. 2012; 161: 44–50 e1–2. [DOI] [PubMed] [Google Scholar]
- 34. Moylan S, Armstrong J, Diaz‐Saldano D, Saker M, Yerkes EB, Lindgren BW. Are abdominal x‐rays a reliable way to assess for constipation? J. Urol. 2010; 184 (4 Suppl.): S1692–8. [DOI] [PubMed] [Google Scholar]
- 35. Tabbers MM, DiLorenzo C, Berger MY et al Evaluation and treatment of functional constipation in infants and children: evidence‐based recommendations from ESPGHAN and NASPGHAN. J. Pediatr. Gastroenterol. Nutr. 2014; 58: 258–74. [DOI] [PubMed] [Google Scholar]
- 36. Barr RG, Levine MD, Wilkinson RH, Mulvihill D. Chronic and occult stool retention: a clinical tool for its evaluation in school‐aged children. Clin. Pediatr. (Phila). 1979; 18: 674–86. [DOI] [PubMed] [Google Scholar]
- 37. Blethyn AJ, Verrier Jones K, Newcombe R, Roberts GM, Jenkins HR. Radiological assessment of constipation. Arch. Dis. Child. 1995; 73: 532–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koh H, Lee MJ, Kim MJ, Shin JI, Chung KS. Simple diagnostic approach to childhood fecal retention using the Leech score and Bristol stool form scale in medical practice. J. Gastroenterol. Hepatol. 2010; 25: 334–8. [DOI] [PubMed] [Google Scholar]
- 39. van den Bosch M, Graafmans D, Nievelstein R, Beek E. Systematic assessment of constipation on plain abdominal radiographs in children. Pediatr. Radiol. 2006; 36: 224–6. [DOI] [PubMed] [Google Scholar]
- 40. Pensabene L, Buonomo C, Fishman L, Chitkara D, Nurko S. Lack of utility of abdominal x‐rays in the evaluation of children with constipation: comparison of different scoring methods. J. Pediatr. Gastroenterol. Nutr. 2010; 51: 155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dinning PG, Hunt L, Lubowski DZ, Kalantar JS, Cook IJ, Jones MP. The impact of laxative use upon symptoms in patients with proven slow transit constipation. BMC Gastroenterol. 2011; 11: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]


