Abstract
Background and Aim
Esophageal involvement in tuberculosis (TB) is rare and is usually secondary. Data on esophageal TB are scarce. We aimed to analyze clinical and endoscopic features and outcomes of treatment in esophageal TB.
Methods
We retrospectively identified patients with esophageal TB from January 2014 to December 2016 at GB Pant Hospital. Well‐defined granuloma with or without caseation and/or acid‐fast bacilli on staining either from esophageal biopsy or the adjacent mediastinal lymph node fine‐needle aspiration cytology (FNAC) specimen, along with clinical features and response to antitubercular therapy (ATT), were collectively considered to diagnose definite TB. Treatment received and response to therapy were documented and analyzed.
Results
A total of 19 patients had definite esophageal TB, and the median age of patients was 39 years (14–65 years) and 10 (52.6%) patients were female. The most common presenting symptom was dysphagia (n = 16, 84%) followed by odynophagia (n = 8, 42%). On endoscopy, the mid‐esophagus was the most common site of involvement, and findings included ulcers (n = 17), elevated lesions (n = 9), and fistulae (n = 4) in patients. The mediastinal lymphadenopathy was present in all patients, with parenchymal lesions seen in three patients. The endoscopic mucosal biopsies were diagnostic in 11 patients, and in the remaining 8 patients, endoscopic ultrasound‐guided FNAC from the mediastinal lymph nodes was diagnostic. A total of 18 patients completely responded to ATT, and 1 patient had partial response with persistent fistulae requiring additional treatment.
Conclusion
Esophagus involvement is rare in TB; endoscopic mucosal biopsy and EUS‐guided FNAC is diagnostic, and the response to ATT is excellent.
Keywords: endoscopic ultrasound, endoscopy: upper gastrointestinal, esophagus, fistula, tuberculosis
Introduction
Tuberculosis (TB) is aptly called the “Captain among these men of death”.1 The incidence of TB has declined since the late 19th century, but it remains a major public health problem. Esophageal involvement of TB is rare, and two types have been described: primary and secondary.2, 3 The secondary type is more common as primary involvement is extremely rare due to the intrinsic protective properties of esophagus.4 Esophageal TB is often misdiagnosed as malignancy or is diagnosed late in the course of the disease.5 Endoscopy and, nowadays, endoscopic ultrasound (EUS) have facilitated the early diagnosis of esophageal TB.6, 7 Esophageal involvement does not affect the treatment, and antitubercular therapy (ATT) is highly effective5, 8; these days, surgery is reserved for managing complications only.9
The epidemiological data are scarce on esophageal TB; so, we aimed to evaluate clinical, radiological, and endoscopic features of esophageal TB and also the response to standard ATT.
Methods
The data were collected retrospectively from January 2014 to December 2016 at the Department of Gastroenterology, GIPMER, New Delhi, India, for patients who were treated for esophageal TB. The data were collected systematically from records documenting clinical, radiological, endoscopic, and histopathological features in detail. All symptoms were documented; dysphagia was further graded when present.10 A definite diagnosis of TB was made if there were well‐defined epitheloid granuloma with or without caseation and/or Ziehl–Neelsen (ZN) stain positive for acid‐fast bacilli (AFB) on histopathology. Complete blood count, liver function tests, renal function, tuberculin test, and chest X‐ray were conducted for all patients. If contrast‐enhanced computed tomography (CECT) of chest was performed, the findings noted included parenchymal lesions, mediastinal lymphadenopathy (location, size, and necrosis), and esophageal involvement if any. The esophagogastroscopy procedure was performed on all patients under conscious sedation after informed consent. In the esophagogastroscopy site of esophageal involvement, mucosal abnormalities were noted, and biopsies were taken from abnormal mucosal areas and were processed for histopathology and ZN staining for AFB.
An EUS was performed if mucosal biopsies were inconclusive or if esophagogastroscopy extrinsic impression with normal overlying mucosa was seen. The EUS was performed under conscious sedation in left lateral position using Olympus 180 scope (Olympus Corporation, Tokyo, Japan). Fine‐needle aspiration cytology (FNAC) was performed on enlarged mediastinal lymph nodes or abnormal areas on EUS. The FNAC was performed using a 19G FNAC needle (Wilson Cook, USA), and the adequacy of the sample was checked immediately postprocedure by an expert pathologist who was present during the procedure.
Patients with a definite diagnosis of TB were treated as per standard World Health Organization (WHO) guidelines.11 Patients were followed weekly for the first 4 weeks and then monthly until the completion of therapy. The response and side effects of ATT were noted during the follow‐up visit. The radiological and endoscopic investigations were performed on follow up as and when required. Ethical approval for the current study was waived by the institutional review board.
Results
Clinical and epidemiological data
A total of 24 patients were suspected to have esophageal TB, of whom 19 patients were diagnosed with definite TB. The median age of patients was 39 years (range: 14–65 years), and 10 (52.6%) patients were female. The median duration of symptoms at presentation was 8 weeks (range: 2–34 weeks). The most common presenting symptoms were dysphagia (n = 16, 79%) and odynophagia (n = 8, 42%), and both were diagnosed in patients (n = 6, 31.5%). Cough on swallowing, suggestive of fistulae, was present in four patients, while two patients had hematemesis. The constitutional symptoms seen were anorexia (n = 17, 89%), weight loss (n = 12, 63%), and fever (n = 9, 47%). Dysphagia was grade II in five patients, grade III in seven patients, and grade IV in three patients.
Biochemical and radiological investigations
The median hemoglobin was 11.2 g/dL, total leukocyte count was 7700 cells/μL, platelets were 2.20 platelets/μL, and erythrocyte sedimentation rate was 68 mm/hr. The Mantoux test was strongly positive (≥15 mm induration) in 16 patients and positive (10–15 mm induration) in 3 patients. Human immunodeficiency virus (HIV) serology was positive in one patient, and his CD4 count was 368 cells/μL at the time of diagnosis.
Chest X‐ray was normal in 13 patients and abnormal in 6 patients (mediastinal widening n = 3, consolidation n = 2, cavity n = 1). CECT scan was performed in all patients, and findings included mediastinal lymphadenopathy (n = 19); parenchymal lesions were also seen in three patients. On CECT, mediastinal lymph nodes compressing the esophageal wall were seen in 17 patients. Barium swallow was done in eight patients, which revealed fistulae in two and extrinsic impression with mucosal irregularity in five patients (Fig. 1).
Figure 1.
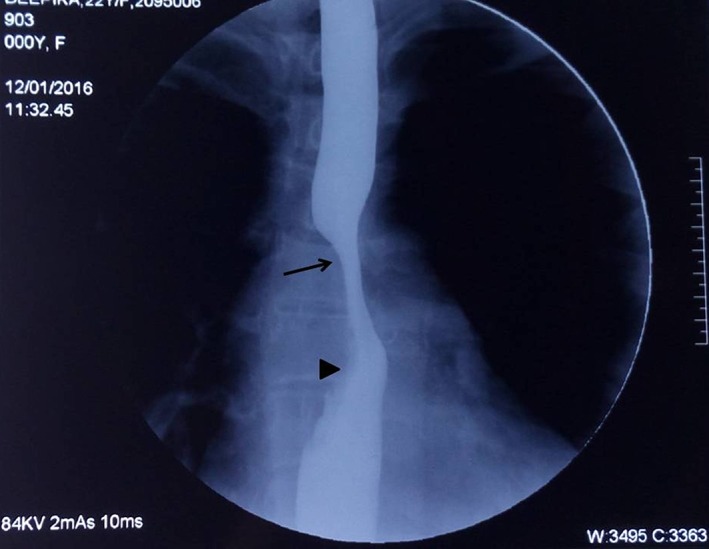
Barium swallow depicting extrinsic compression in mid‐esophagus (arrow) with mucosal irregularities in lower part (arrow head).
Endoscopic and histopathological features
The most common site of involvement was the thoracic esophagus (n = 17) followed by cervical esophagus in one patient, while one patient demonstrated the involvement of the lower esophagus. The endoscopic findings included ulcer (n = 17, single ulcer in 16, multiple in 1, size: 7–25 mm), extrinsic impression with ulcer at the center (n = 8) (Fig. 2), and fistula (n = 4). The fistula was located in the mid‐esophagus in three patients and at the lower end of the esophagus within diverticulum in one patient (Fig. 3); one patient with a fistula had active blood ooze from the fistula site (Tables 1,2).
Figure 2.
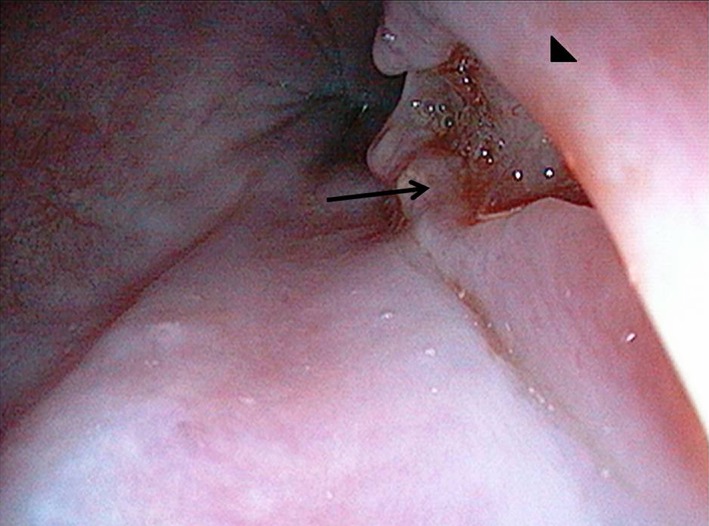
Endoscopic picture showing bulge (arrowhead) with overlying ulcer (arrow) in mid‐esophagus.
Figure 3.

Esophagopulmonary fistulae (long arrow, diverticulum with fistula within; arrowhead, esophageal opening; short arrow, diverticulum).
Table 1.
Endoscopic features of esophageal tuberculosis.
| Endoscopic findings | Number of patients |
|---|---|
| Esophageal ulcer only | 6 |
| Extrinsic bulge/impression only | 1 |
| Extrinsic bulge with ulcer | 8 |
| Fistula with ulcer | 3 |
| Fistula with diverticulum | 1 |
Table 2.
Diagnosis and histopathological features.
| Mode of sample acquisition | Done in | Caseating granuloma | Non‐caseating granuloma | Nonspecific | AFB POS |
|---|---|---|---|---|---|
| Endoscopic biopsy | 18 | 5 | 6 | 7 | 2 |
| EUS FNAC | 8 | 5 | 2 | 1 | 4 |
| Cervical LN FNAC | 3 | 2 | 0 | 1 | 2 |
AFB POS, acid‐fast bacilli stain positive; EUS, endoscopic ultrasound; FNAC, fine‐needle aspiration cytology; LN, lymph node.
EUS was performed in eight patients; all eight patients had subcarinal and paraesophageal lymphadenopathy, and lymph nodes were matted in five patients and discrete in three patients (Fig. 4).
Figure 4.

Endoscopic ultrasound showing subcarinal lymph node (arrow, lymph node; arrowhead, fine‐needle aspiration cytology).
Three patients with cervical lymphadenopathy underwent percutaneous FNAC; two patients had caseating granuloma with ZN stain positive in both, while one patient had inconclusive report. Esophageal biopsy was performed in 18 patients, of whom it was diagnostic in 11 patients (caseating granuloma n = 5, noncaseating epitheloid granuloma n = 6, ZN stain positive n = 2; Fig. 5). EUS with fine‐needle aspiration from enlarged nodes was performed in eight patients. Of these, five had caseating granuloma on FNAC along with ZN stain positivity in three; two had noncaseating granuloma, while one had nonspecific caseating material but was AFB positive. GeneXpert was conducted on one esophageal biopsy specimen, and three EUS‐guided FNAC aspirates. GeneXpert was positive in the esophageal biopsy, and in two EUS‐guided FNAC, all samples were sensitive to rifampicin.
Figure 5.

HE stain at 20× magnification showing stratified squamous epithelium with caseating well‐defined epitheloid granuloma.
Follow up and response to ATT
All 19 patients were treated with standard weight‐based ATT; 18 patients completed the treatment and 1 patient was lost to follow up after 3 months of treatment. ATT led to clinical improvement in 18 patients, but 1 patient with an esophagopulmonary fistula had persistent fistula after completion of ATT. The median time interval in significant improvement of dysphagia was 3 weeks (range: 2–8 weeks), and median time interval for the resolution of constitutional symptoms was 4 weeks (2–10 weeks). Out of four patients with fistulae, three patients had complete clinical and endoscopic response; one patient had the partial clinical response and repeat esophagogastroscopy revealed persistent fistula. The patient with persistent fistula underwent feeding jejunostomy, and later on, over‐the‐scope clip (OTSC) placement was performed for the closure of fistula. After 4 months of ATT and OTSC placement, the size of the fistula decreased with the improvement of symptoms, and the patient was able to consume solids but still had an occasional cough with liquids. Follow‐up esophagogastroscopy was performed in 10 patients, and complete healing of lesions was noted in all patients.
Discussion
Esophageal TB is a rare entity. The first diagnosed case of esophageal TB was postmortem diagnosis and dates back to 1890, while the first antemortem case dates back to 1907.4 In 1913, an autopsy series demonstrated secondary involvement of the esophagus in only 25 of 16 489 (0.15%) patients who died of TB.2 Another autopsy series of TB patients from 1942 found esophageal TB in 0.14%.3
Modes of tubercular involvement of esophagus may include: (i) from swallowed sputum; (ii) direct spread from Potts spine, mediastinal lymph node, and tubercular lung cavity; (iii) retrograde from lymphatic drainage and (iv) blood borne. Esophageal tubercular involvement may be primary or secondary. Primary esophageal TB is rare and is defined as the involvement of esophagus without the involvement of any other site in the body. Explanations for the same include mucosal protecting factors, stratified squamous epithelium, esophageal peristalsis, mucus, saliva, and erect posture.12 Secondary esophageal TB is more common and is primarily the secondary involvement of esophagus due to an adjacent mediastinal lymph node, pulmonary parenchyma, or vertebral column involvement. Initial case reports of esophageal TB seemed to be advanced cases and consisted more of surgical reports. With the advent and availability of esophagogastroscopy, more and more medical case reports and series have been published.4, 13, 14 Now, with EUS availability, diagnostic abilities and yield have been enhanced, as are the number of cases. There is still a scarcity of published data considering that esophageal involvement is a rare phenomenon, although TB remains quite rampant.
Our report presents a detailed account of 19 patients with a definitive diagnosis of TB. The most common age group affected is the middle age group (median age = 39 years), although no age is barred. This is in accordance with the epidemiological spectrum of TB in developing overcrowded countries. In comparison, the other two recent publications also reported median age of 31 and 37 years.12, 13 Dysphagia is the most common symptom as mentioned in all publications,5, 7, 8, 9, 13, 14, 15, 16, 17, 18, 19 but uncommon presentation like hematemesis has also been reported in a few, and, in our series as well.5 Four of our patients also had fistula‐related symptoms. Constitutional symptoms were present in 48–89% patients. Our series had a slight female predominance in contrast to the other two studies from India.13, 14 Pulmonary lesions are uncommon, although it is worth mentioning in view of the ease of their detection on chest X‐ray. Mediastinal lymphadenopathy was present in all patients and correlated well with esophageal involvement adjacent to them in 12 patients. Three of our patients had esophageal involvement secondary to parenchymal pulmonary involvement, one had lung abscess with esophageal fistula, one had consolidation with esophageal fistula, and one had esophageal fistulous communication with lung cavity, although all of them had additional mediastinal lymphadenopathy. In total, all of our patients had secondary involvement of esophagus. Most other reports also demonstrated that secondary TB is the most common type, with the most common mode of esophageal involvement secondary to mediastinal lymph nodal disease,13, 14 and only one case series had predominant primary involvement of esophagus.16 In our study, the most common site of involvement was the mid‐esophagus, which correlated well with adjacent mediastinal lymphadenopathy.
Blood parameters did not reveal much except elevated ESR. Chest X‐ray was helpful in six patients. A report by Mokoena et al.5 demonstrated abnormal chest X‐ray in 74%, while another report by Devarbhavi et al.8demonstrated 50% patients with abnormal chest X‐ray. Report by Jain et al.16showed chest X‐ray abnormality in only one patient, which can be explained on the basis that the majority of them had primary esophageal involvement. On endoscopy, the ulcer was the most common finding; even though ulcers are not characteristic, in our opinion, summit ulcers with an underlying impression in the mid‐esophagus are highly suggestive of TB as seen in eight of our patients; similar findings were noted by another series by Rana et al.14 Esophageal biopsy taken from ulcers are useful as they were diagnostic in 57% cases in our series, while in remaining patients, EUS‐guided FNAC of mediastinal lymph node was diagnostic. Thus, esophageal biopsy is important in resource‐limited settings, and biopsy during esophagogastroscopy should be taken from abnormal mucosa, specifically ulcerated areas when there is suspicion of TB. EUS has already established its role in mediastinal lymph node FNAC and esophageal TB diagnosis and has excellent sensitivity and specificity.9, 13, 14, 19 Excellent response with ATT had been proven long ago and remains a mainstay of therapy.5 The difficult cases are the ones with fistulae, especially if they are long standing as they tend to epithelize and become resistant to healing. Even these cases merit ATT first, and if there is no healing with ATT, further management may include advanced endoscopic interventions and/or surgery.8 We had three patients with fistulae who had complete healing of fistulae with ATT alone, and only one patient needed endoscopic closure of the eight fistula.
Our series had no mortality related or unrelated at 6 months follow up; earlier reports do mention deaths mostly related to bleeding, but now, with advanced imaging and therapeutic capability of endoscopy, it appears to be a rare phenomenon, reflected in a few of the latest reports where there was no mortality.5, 13, 14
Our study had few limitations. Our data are retrospective, and we did not perform mycobacterial cultures. EUS was performed in only eight patients.
In conclusion, the tubercular involvement of the esophagus is uncommon. With the advent of endoscopic techniques and EUS, diagnosis is now easier to make and ATT has excellent response.
Declaration of conflict of interest: None.
References
- 1. Rubin SA. Tuberculosis. Captain of all these men of death. Radiol. Clin. North Am. 1995; 33: 619–39. [PubMed] [Google Scholar]
- 2. Lockard LB. Esophageal tuberculosis. A critical review. Laryngoscope. 1913; 23: 561–84. [Google Scholar]
- 3. Carr DT, Spain DM. Tuberculosis in a carcinoma of the esophagus. Am. Rev. Tuberc. 1942; 46: 346–9. [Google Scholar]
- 4. Fahmy AR, Guindi R, Farid A. Tuberculosis of the oesophagus. Thorax. 1969; 24: 254–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mokoena T, Shama DM, Ngakane H, Bryer JV. Esophageal tuberculosis: a review of eleven cases. J. Postgrad. Med. 1992; 68: 110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon AH, Marshall JB. Esophageal tuberculosis: definite diagnosis by endoscopy. Am. J. Gastroenterol. 1990; 85: 174–7. [PubMed] [Google Scholar]
- 7. Han XM, Yang JM, Xu LH, Nie LM, Zhao ZS. Endoscopic ultrasonography in esophageal tuberculosis. Endoscopy. 2008; 40: 701–2. [DOI] [PubMed] [Google Scholar]
- 8. Devarbhavi HC, Alvares JF, Radhikadevi M. Esophageal tuberculosis associated with esophagotracheal or esophagomediastinal fistula: report of 10 cases. Gastrointest. Endosc. 2003; 57: 588–92. [DOI] [PubMed] [Google Scholar]
- 9. Ni B, Lu X, Gong Q et al Surgical outcome of esophageal tuberculosis secondary to mediastinal lymphadenitis in adults: experience from single center in China. J. Thorac. Dis. 2013; 5: 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knyrim K, Wagner HJ, Bethge N, Keymling M, Vakil N. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N. Engl. J. Med. 1993; 329: 1302–7. [DOI] [PubMed] [Google Scholar]
- 11. WHO . Treatment Tuberculosis Guidelines, 2010. Cited 20 Feb 2017. Available from URL: http://apps.who.int/iris/bitstream/10665/44165/1/9789241547833_eng.pdf?ua=1&ua=1
- 12. Gupta SP, Arora A, Bhargava DK. An unusual presentation of oesophageal tuberculosis. Tuber. Lung Dis. 1992; 73: 174–6. [DOI] [PubMed] [Google Scholar]
- 13. Puri R, Khaliq A, Kumar M, Sud R, Vasdev N. Esophageal tuberculosis: role of endoscopic ultrasound in diagnosis. Dis. Esophagus. 2012; 25: 102–6. [DOI] [PubMed] [Google Scholar]
- 14. Rana SS, Bhasin DK, Rao C, Srinivasan R, Singh K. Tuberculosis presenting as dysphagia: clinical, endoscopic, radiological and endosonographic features. Endosc. Ultrasound. 2013; 2: 92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dow CJ. Oesophageal tuberculosis: four cases. Gut. 1981; 22: 234–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jain SK, Jain S, Jain M, Yaduvanshi A. Esophageal tuberculosis: is it so rare? Report of 12 cases and review of the literature. Am. J. Gastroenterol. 2002; 97: 287–91. [DOI] [PubMed] [Google Scholar]
- 17. Abid S, Jafri W, Hamid S, Khan H, Hussainy A. Endoscopic features of esophageal tuberculosis. Gastrointest. Endosc. 2003; 57: 759–62. [DOI] [PubMed] [Google Scholar]
- 18. Baijal R, Agal S, Amarapurkar DN, Kumar P, Kotli N, Jain M. Esophageal tuberculosis: an analysis of fourteen cases. J. Dig. Endosc. 2010; 1: 14–8. [Google Scholar]
- 19. Fritscher‐Ravens A, Ghanbari A, Topalidis T et al Granulomatous mediastinal adenopathy: can endoscopic ultrasound‐guided fine‐needle aspiration differentiate between tuberculosis and sarcoidosis? Endoscopy. 2011; 43: 955–61. [DOI] [PubMed] [Google Scholar]


