Abstract
Elemental diets, dietary elimination, and steroid therapies are the most common therapies in the clinical trials for eosinophilic esophagitis (EoE). Histological findings (usually reported as eosinophils per microscopic high‐powered field [hpf]) remain the most common end‐point used to define response. Yet, the threshold for defining “response” and “remission” are ill‐defined among consensus guidelines and may vary from study to study. We conducted a systematic literature review of articles on eosinophilic esophagitis, published between January 2007 and November 2017, considering histological remission as the primary outcome. We abstracted treatment information and definitions of histological remission or response. A comparison of definitions of histological remission across and within institutions was performed. A total of 61 articles were included in this review, with approximately 60% of the studies published from centers in the United States. Histological definitions of remission of EoE ranged from 0 to ≤20 eosinophils/hpf. The most stringent criteria, ranging from 0 to ≤5 eosinophils/hpf, were commonly used in interventional trial studies that examined the effects of new treatments. We found remarkable variability in definitions between studies, treatment types, and regions. Age or epidemiological distribution of study subjects did not influence the criteria for histological remission. Clinical and histological improvements are important measures of the effects of treatment. Histological findings, the most objective measure of treatment, should provide an optimal method for comparing the effectiveness of various treatments. Yet, our findings suggest a lack of consistent remission criteria in published studies. Considering these inconsistencies, it is difficult to compare the effectiveness of various treatments.
Keywords: eosinophilic esophagitis, histological, improvement, remission, response, treatment, trial
Introduction
Eosinophilic esophagitis (EoE) is a chronic allergic disorder that affects the esophagus with distinctive eosinophilic inflammation of the mucosa walls.1, 2 Consensus recommendation by interdisciplinary experts, in 2007, describes EoE as a clinical and histopathological entity characterized by an eosinophil count of 15 or more eosinophils per high power field. The goal of the recommendation was to increase diagnostic prudence to aid the evaluation and treatment of children and adults with suspected EoE.3 The consensus recommendation updated in 2010 defines EoE as a “chronic immune/antigen‐mediated disease characterized by symptoms related to esophageal dysfunction and histologically by eosinophil‐predominant inflammation.” Availability of these consensus recommendations has helped health professionals improve their understanding and diagnosis of EoE.2
The clinical manifestation of EoE varies across age groups. In children, commonly reported symptoms include feeding difficulties, vomiting, and failure to thrive.4, 5, 6, 7, 8, 9, 10, 11 Among the adolescent and adult populations with EoE, dysphagia, vomiting, and chest pain are the common presenting symptoms. There is no single treatment option that has been shown to be the most sufficient for the treatment of EoE. Physicians have widely adopted elemental diets, dietary elimination, and steroid therapies as the most common therapies for the management of EoE in both the children and adult populations.3, 12, 13 Monitoring of responses to treatments and defining the remission of EoE is mostly based on the patients’ experience of persistent symptom‐free state and normal endoscopic findings, as documented in most studies. Still, histological findings usually reported as eosinophils per microscopic high‐powered field (hpf)) remain the most common end‐point used to define treatment response. Generally, the threshold number of eosinophils per hpf considered “response” or “remission” is ill‐defined and varies from study to study. Agreement on the definition of histological remission of EoE is important to support interstudy comparison and allow a uniform treatment end‐point in future studies.
Despite the fact that there have been advances in the understanding, treatment, and research on EoE, the question about the most appropriate definition of histological remission is still not addressed.14, 15 The primary objective of this study is to systematically examine how clinicians and researchers define histological remission or responses to treatment among EoE subjects. The secondary objective is to compare whether the definition of histological remission is dependent on the characteristics of study population or the types of treatment and to propose the need for potential consensus recommendations for the histological definition of remission in patients with EoE.
Methods
A search of the literature was conducted in two phases over 2 years. We conducted our initial search in October 2015 and then a second search in November of 2017 to update our database. PubMed, Embase, Scopus, GoogleScholar, and http://clinicaltrials.gov were searched for randomized controlled trials and observational studies from January 2007 to December 2010 and then from January 2011 to November of 2017; the date range was chosen to reflect the increased consistency in the terminology of the definition of EoE before and after the updated consensus recommendation guideline for the diagnosis of EoE in children and adults. Reference lists of articles and reviews were searched to locate additional controlled trials and observational studies. In addition, we hand‐searched tables of contents for recent issues of relevant journals. Articles written in languages other than English were excluded. A detailed search strategy and list of keywords can be found in the supplemental materials (Table S2). The key search words using medical subject headings included “Eosinophilic esophagitis,” “treatment,” “trial,” “outcome,” “remission,” “response,” “Histology,” and “histopathology,” and we combined all possible subheadings. Reference lists of articles were scanned for additional items, and releases of key journals were reviewed for recently published studies. The search string used for the identification of articles is found in Table S2.
Eligibility criteria
We included publications that at least investigated the effect of any treatment formula or combination of treatment formulas, including proton pump inhibitors (PPI), elemental diets, dietary elimination (e.g. six or four food elimination), steroid therapies, and monoclonal antibodies. The studies were included if they mentioned the terms remission, response, or improvement of EoE in either the text or abstract of the articles. As we were interested in capturing articles that provided a histological definition of remission, improvement, or response, we excluded articles that did not provide adequate information on the cut‐off values used to define histological remission. We also excluded articles written in languages other than English. Furthermore, review articles in which the authors summarized multiple studies were excluded because detailed methods were not presented.
Study selection and data extraction
The selection of studies was strongly influenced by the ‘Strengthening the Reporting of Observational studies in Epidemiology’ (STROBE) guidelines for reporting observational (cohort, case–control, and cross‐sectional) epidemiological studies.16 Three authors (RE, TL, and AW) independently reviewed the titles, index terms, and abstracts of the identified references and rated each paper as “potentially relevant” or “not relevant” using a screening algorithm based on study type, study design, subjects, setting, and intervention. Three authors (RE, TL, and AW) then independently reviewed the full texts of the selected articles and again rated each paper as “potentially relevant” or “not relevant” using the screening algorithm. Finally, two authors (RE and TL) independently applied the full set of inclusion and exclusion criteria to the potentially relevant studies to select the final set of included studies. Disagreements between reviewers were resolved by discussion (RE, JM, AL, and TL). We then assessed the completeness of data by comparing, in detail, the research objectives, study population, sample size, study design, treatment, and end‐points between the studies and determining the level of missing information in each study. For ease of description of the spectrum of definitions of histological remission of EoE, we classified the histological definitions into three categories: 0 to ≤5, 6 to ≤10, and 11 to ≤15. We excluded two studies with definitions outside these three categories.17, 18 As the majority of studies were conducted in the United States and Europe, we classified these studies into two regions to examine variability within and between regions.
Measurement of quality
The tool used for the methodological quality assessment was adapted from the Study Quality Assessment Tools provided on the National Institute of Health website (NIH – National Heart, Lung and Blood institute).19 We used the following tools: (i) Quality Assessment of Controlled Intervention Studies (14 items) and (ii) Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies (14 items). Two reviewers (TT and TL) independently reviewed and scored the eligible studies for methodological quality. The reviewers then discussed the scores and resolved any disagreement that existed. Depending on the study type, the quality items were scored as 1 or 0 based on whether the study satisfied the itemized quality parameters or not (Table S3). We then calculated the percentage scores for each study based on the proportion of quality measures satisfied (Table S4). We further classified the methodological quality as high or low quality depending on whether the study had a score ≥ 70% or less than 70%, respectively.
Classification by study designs
The study designs of eligible studies were reviewed and classified into two broad categories: observational studies and intervention trials. Observational studies measured the extent of the reduction of the eosinophil count based on retrospective, prospective, and cross‐sectional hospital record reviews of study participants, and there was no random assignment of treatment or intervention. Intervention trials measure the change in eosinophil counts before and after instituting a treatment formula to reduce EoE disease activity. Intervention trials include (i) randomized controlled trials (RCT) with a comparison group in which no new treatment formula was introduced during the study period and (ii) trials with historical controls that compared the eosinophil counts before the introduction of a treatment formula with the counts in the same group after the treatment formula; an example is the compassionate study.
This review is organized by the definitions of histological remission in the literature, definitions of histological remission between studies, definitions of histological remission between centers, definitions of histological remission within centers, and a discussion on the concerns of definition inconsistencies.
Results
Between 2007 and 2017, we identified 5498 potentially relevant articles related to EoE. Based on the relevance of the titles and abstract, we conducted a full‐text screening of 642 articles. We excluded 524 articles that had limited information on the outcome of interest or nonoriginal studies. The final analyses included a total of 61 articles that met the eligibility criteria set for this study (Fig. 1). Among the eligible studies, 47 were observational studies (2 cross‐sectional, 25 prospective, and 20 retrospective studies), while 15 studies were interventional trials (see references in Table S1). Fifty‐two studies were published after the 2010 publication of updated consensus recommendation guidelines for the diagnosis of EoE in children and adults. Over 50% of the studies (38) were conducted in the United States and about 40% in Europe (including 12 studies in Spain and 6 in Switzerland). The studies’ sample sizes ranged from 11 to 513. About 50% of the studies (30 studies) were conducted using an adult population with EoE, 20 studies using children, and 12 studies had heterogenous population (adult and children). Almost all studies indicated obtaining esophageal biopsy specimens from study subjects for the histological determination of esophageal peak eosinophil counts in one hpf (X400 magnification). Broadly, based on the classification of the definition criteria into three groups, about 41% of the studies defined histological remission within the range of 0–5 eosinophils/hpf, and about 37% defined remission within the range of 11–15 eosinophils/hpf (Table 1).
Figure 1.
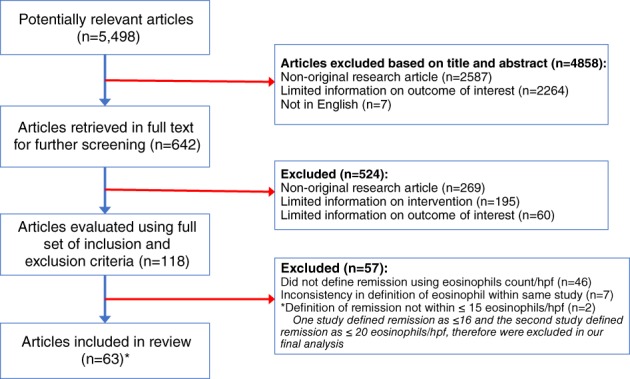
Flow diagram of article selection process.
Table 1.
Summary of definitions of histological remission in reviewed studies (N = 63)
| Definition | Number of studies n (%) |
|---|---|
| 0–5 eos/hpf | 26 (41) |
| 6–10 eos/hpf | 12 (19) |
| 11–15 eos/hpf | 23 (37) |
| 16–20 eos/hpf | 2 (3)† |
Excluded in our final analyses because the values are within the range of diagnostic criteria for EoE based on consensus definition criteria.
eos, eosinophils; hpf, high power field.
Methodological quality
In Table S4, we present the methodological scoring of included studies. The scores ranged from 50% 20 to 100%.21 Approximately 50% of the studies were of high quality, having quality measures scores of 70% or above. Of 15 intervention trials 17, 22, 2 were rated as low‐quality studies (score of less than 70%), while nearly 60% of the observational studies were rated low quality. There were no significant dropouts reported in the different studies; however, most of the studies did not adequately document sample size justification in their report.
Variation of histological remission between study designs
We observed a wide band of reported histological definitions of remission from the eligible studies, ranging from a peak esophageal eosinophil count of 0 eosinophils per hpf to ≤20 eosinophils/hpf. However, for this systematic review, we included only studies that reported remission as an eosinophil count of 15 eosinophils/hpf or lower (Fig. 2). Except for one study, the randomized trial studies used more rigorous criteria to describe histological remission among trial subjects (range 0 to ≤5 eosinophils/hpf). Two cross‐sectional studies included in the analysis also considered patients with less than 5 eosinophils/hpf as experiencing histological remission. However, one of the cross‐sectional studies examined the treatment effect of proton pump inhibitors on an adult population with EoE, while the second study investigated the effectiveness of the PedsQL™ EoE Module in the evaluation of pediatric EoE disease‐specific health‐related quality of life (HRQOL) in clinical research and practice.23 Notably, we observed a high variability in the definition of histological remission among studies that had either a retrospective or prospective design. In addition, most of these studies (retrospective and prospective studies) used less stringent definitions, between 11 and ≤15 eosinophils/ hpf, as criteria for the remission of EoE among study subjects.
Figure 2.
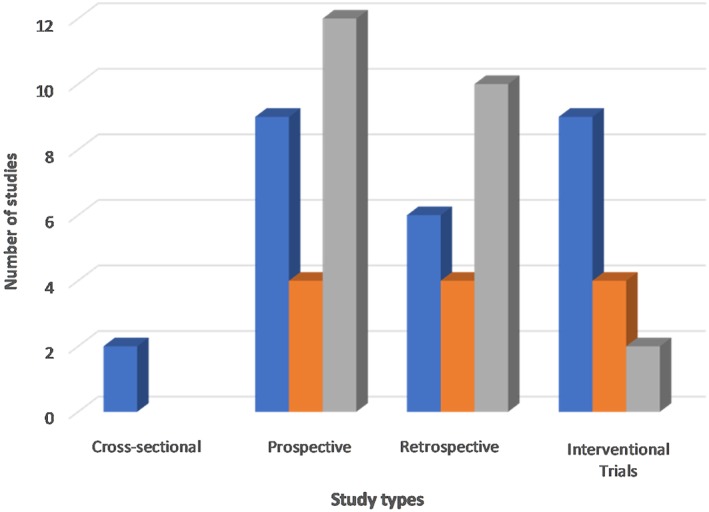
Definitions of histological remission of EoE by study types.
Variation of histological remission between treatment types
Treatment options for the management of EoE varied among studies and includes oral or swallowed steroids (budesonide, fluticasone), proton pump inhibitors (PPI), humanized anti‐interleukin‐5 monoclonal antibody (Mepolizumab, Reslizumab), diet elimination (Six‐food (SFED), four food (FFED)), diet restriction, or avoidance and elemental diet (ELED) (Table 2 ). Eight studies (13%) used a combination of any two or more treatment types described above. Overall, our findings demonstrated a high variability in the definition of histological remission constructed on the choice of EoE treatment type. Among the interventional trials (randomized controlled trial studies [RCTs] or trials with historical controls), 80 (80%) compared treatment effect using histological remission as values within the range of 0 to ≤5 eosinophils/hpf. Among studies that examined the effectiveness of monoclonal antibody treatment in EoE subjects, 80% defined histological remission as peak esophageal eosinophil counts within the range of 0 to ≤5.24, 25, 26, 27, 28 Approximately 26% (N = 15) of the eligible studies examined remission among EoE patients who received only the diet elimination treatment formula (SFED, FFGED, ELED) compared to other forms of treatment, with the most common definitions falling within 0 to ≤5 eosinophils/hpf 29, 30, 31, 32, 33, 34 or 11 to ≤15 eosinophils/hpf.35, 36, 37, 38, 39, 40 Interestingly, even among the SFED studies (N = 9), five studies defined remission within the range of 0 to ≤5 eosinophils/hpf, 29, 30, 31, 32, 34 and two studies considered histological remission as <15 eosinophils/hpf.38, 41
Table 2.
Comparing definitions of histological remission according to treatment types
| Treatment type | Definition of remission (eos/hpf†) | ||
|---|---|---|---|
| 0 to ≤5 n (%) | 6 to ≤10 n (%) | 11 to ≤15 n (%) | |
| Diet elimination (N = 16) | 7 (44) | 2 (12) | 7 (44) |
| Steroid (N = 27) | 9 (33) | 9 (33) | 9 (33) |
| Monoclonal antibody (N = 5) | 4 (80) | — | 1 (20) |
| Proton pump inhibitor (N = 9) | 4 (44) | — | 5 (56) |
| Other‡ (N = 8) | 4 (50) | 2 (25) | 2 (25) |
Eosinophil/high power field.
Other includes restricted diet (n = 1), elemental diet (n = 2), unspecified (n = 1), or treatment combination, for example, PPI + steroid + food elimination + avoidance (n = 4).
Among 10 studies that examined the effectiveness of PPI in the management of subjects with EoE, there were no consistent criteria for defining histological remission, and the values ranged from peak esophageal eosinophils count of 0 to ≤20 eosinophils/hpf. Nonetheless, four studies considered remission within the range of 0 to ≤5 eosinophils/hpf, 42, 43, 44, 45 and five studies considered remission as having ≤15 eosinophils/hpf.46, 47, 48, 49, 50
Variation of histological remission between and within regions
Most of the publications used for this analysis were either from the United States (62%) or Europe (39%) (Fig. 3). Others included a prospective open clinical trial conducted in Australia and an international multicenter randomized control trial.27, 43 Among published studies from the United States, the definition of remission ranged from <1 eosinophil/hpf to ≤15 eosinophils/hpf; 44% of the studies from the United States considered histological remission within the range of 0 to ≤5 eosinophils/hpf, while about 30% considered values within the range of 11 to ≤15 eosinophils/hpf. Six studies were published from centers in the United States, and the definitions provided include ≤5, ≤ 10, and ≤ 15 eosinophils/hpf. Notably, practically similar treatment options were used in the management of EoE subjects at the center.31, 34, 40, 41, 51 Two studies published from another US center, although with different treatment options and study design, used two different criteria to define histological remission.26, 52
Figure 3.
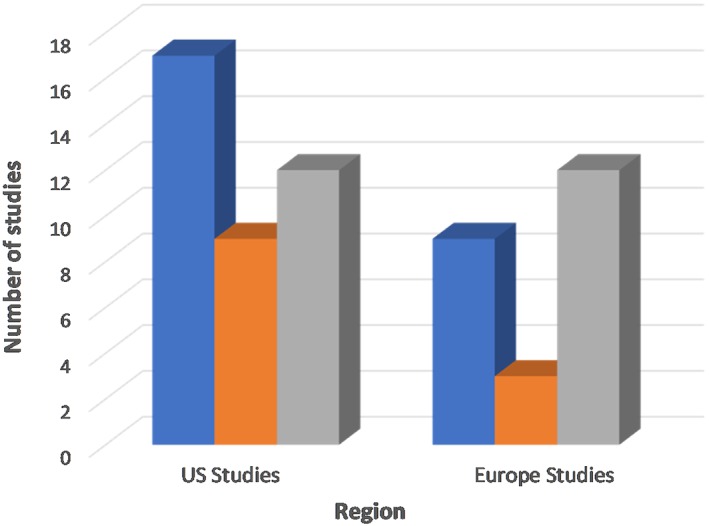
Comparing variation in the definition of histological remission of EoE between studies from the United States versus European regions.
Similarly, there was a remarkable variation in the definition of histological remission of EoE among studies published from European centers, including Spain, Germany, and Switzerland. Of the 22 studies selected from European centers, 12 studies (55%) considered values within the range 11 to ≤15 eosinophils/hpf 17, 36, 37, 38, 39, 46, 47, 53, 54, 55, 56, 57, 58 as histological remission, and 4 studies (23%) defined histological remission as less than 5 or ≤ 5 eosinophils/hpf.30, 33, 42, 45 Strikingly, all studies conducted in Switzerland used a more stringent and consistent end‐point of <5 eosinophils /hpf to identify cases of histological remission.28, 59, 60
Discussion
Advances in the understanding of the etiology, progression, and treatment of EoE have increased the demands for relevant standards or guidelines for the recognition of clinical status and improvement of patients with this condition. The 2007 consensus recommendation provides clarity regarding the clinical and histological criteria for the diagnosis and treatment of patient with EoE.3 This recommendation also provides insight into the clinicopathological entity of EoE, making the identification of esophageal eosinophilia a necessity in the assessment of individuals with EoE. Furthermore, the consensus provides a threshold cut‐off of 15 or more eosinophils per hpf in one or more esophageal biopsy specimens as one of the criteria for the diagnosis of EoE. In 2011, based on the evolution of published studies associating the effect of immune or antigenic response in EoE patients, the updated consensus recommendation expanded the definition of EoE to include an “immune/antigen‐mediated esophageal disease characterized by symptoms related to esophageal dysfunction and histologically by eosinophil‐predominant inflammation”.2 When comparing the number of published treatment trial studies that have demonstrated efficacy in the management of EoE, the conceptual basis for choosing a cut‐off value for the number of eosinophils per hpf to distinguish patients with historical remission is, to some degree, arbitrary. For many gastroenterologists and other health‐care researchers, the most important questions about remission of EoE relates to the degree to which it is associated with a decrease in eosinophil counts per hpf.
In addition to the resolution of clinical symptoms related to EoE, the histological determination of the peak number of esophageal eosinophils per hpf is an important outcome domain that is necessary to evaluate patients’ disease experience.2, 61 To date, while data have suggested arbitrary histological end‐points, there is no compelling empirical evidence to use as a guide for the appropriate definition of histological remission of patients with EoE. Intuitively, based on the consensus guidelines for the diagnosis of EoE with the presence of ≥15 eosinophils/hpf, it is not uncommon to find published studies using the cut‐off value of ≤15 eosinophils/hpf as a definition of the histological improvement of the disease. This review aims to highlight the common values used to define histological remission or response to treatment by most trials or studies. This systematic review of literature found remarkable variation in the cut‐off values of peak eosinophil counts per hpf considered to be the histological resolution of EoE. The peak cut‐off values varied from 0 to ≤15 eosinophils/hpf, and the variability in definition was not associated with age or distribution of the study population. In addition, the definition of remission varied irrespective of study sample size. This inconsistency constitutes a serious problem in identifying actual improvement in patients with EoE. As there is no consensus in the literature about what should be considered histological remission, it is not unusual to find researchers using an arbitrary end‐point that is most suitable for their study expectations. Additional monitoring of improvement in other histological parameters suggestive of eosinophilic inflammation, such as eosinophilic microabscesses, surface layering of eosinophils, extracellular eosinophil granules, and basal cell hyperplasia, would help to adequately discriminate treatment success and nonsuccess. The presence of these histological features could indicate strong evidence of clinically unresolved EoE, even with low esophageal eosinophil counts after treatment.2, 62, 63, 64 Even though results from a recently published systematic review of disease activity indices in EoE, Warners and colleagues (2017),15 suggest that there is dissociation between symptomatic and histological improvement of EoE after treatment, Martin et al., using the EoE clinical symptomology instrument (Pediatric Eosinophilic Esophagitis Symptom Scores [PEESS v2.0]), had demonstrated that esophageal activity measured by eosinophil peroxidase (EPX) immunohistochemical staining is significantly correlated with dysphagia symptoms in pediatric patients with EoE.65
Although there were no common criteria to define histological remission in the studies reviewed, a majority of the randomized controlled trial studies used more stringent values ranging from 0 to ≤5 eosinophils/hpf to define histological remission or resolution.22, 26, 66, 67, 68 Compared to dietary treatment studies (e.g. diet elimination or restriction), we observed that medication trial studies (e.g. steroids, monoclonal antibody) used more rigorous criteria in their definition of histological remission in study subjects with EoE.21, 26, 27, 35, 53, 60 It is possible that different cut‐off values of peak eosinophil counts per hpf are appropriate for the definition of histological improvement because of the variations in the trial methodology (treatment, subjects’ characteristics). However, findings from our review did not suggest any correlation between variation in the definitions of histological remission and characteristics of the study population (children or adults) in the different studies. Comparing studies published from the US and European centers, our review showed a remarkable variation in the definition of histological remission across and within regions/institutions. However, four published studies from Switzerland59, 60, 69, 70 consistently used a peak value of <5 eosinophils/hpf to classify cases with histological remission of EoE. Although the studies did not provide a clear justification for choosing different cut‐off points to define histological remission, it could be deduced that two key factors could have influenced their options regarding histological remission. First, some studies probably used less than 15 eosinophil /hpf as a cut‐off to reflect the histological definition of EoE per consensus recommendation.2 In this review, approximately 34% of the studies defined histological remission as either less than 15 or ≤ 15 eosinophil/hpf. This was the most common criteria among observational studies. On the contrary, the second possible reason for choosing a more stringent cut‐off of ≤5 eosinophils/hpf, observed in most interventional studies, could be to ensure rigor in the assessment and proof of the therapeutic efficacy of trials.
Based on the findings of the present review, it could be argued that a stringent cut‐off value within the range of 0 to ≤5 eosinophil/hpf, as observed in most of the interventional trial studies, presents a parsimonious model with the most stringent criteria that could be adopted for investigation of the effectiveness of treatment formulas among EoE subjects, including children and adults. Alternatively, it can also be argued that a cut‐off of <15 eosinophil/hpf, representing the value below that recommended for the diagnosis of EoE, could be used to define histological remission. This would probably ease the criteria in most studies and allow for some presence of eosinophils due to the possible coexistence of gastro‐esophageal reflux.71, 72, 73, 74 In the absence of data arguing otherwise, we believe that it is important to consider a consensus recommendation for the definition of histological end‐point to enhance standardized goals and the effective management of patients with EoE.
This review has some possible limitations because we restricted our database search exclusively to only articles published in English and articles that had our key search terms—Eosinophilic esophagitis,” “treatment,” “trial,” “outcome,” “remission,” “response,” “Histology,” and “histopathology” in the title or abstract. This could potentially lead to less representative findings. Although broadening our search criteria and databases might have increased the generalizability of our results, we would likely have found even more variability in the definitions of histological definitions of EoE. Still, the major strengths of our study include capturing data from major clinical, gastrointestinal, and allergy journals. Furthermore, we thoroughly screened all articles from across different regions and compared the different study methodologies.
Conclusion
Significant strides have been made regarding the treatment and care of individuals with EoE. Several published treatment trial studies have demonstrated efficacy in the management of EoE; however, evidence from our review show that inconsistency in the definition of histological remission remains a major issue. Most of the studies, especially interventional studies, used a count of <5 eosinophils/hpf as criteria for histological remission. Recommending a clearly defined end‐point for histological remission is warranted to support claims of efficacy and comparison of therapies for EoE.
Acknowledgement
We thank Andy Jan for his assistance with literature review.
Supporting information
Table S1 List of eligible studies included in the review analysis.
Table S2 Article search algorithm.
Table S3 Description of Quality Assessment Tool adapted from National Institute of Health website (NIH – National Heart, Lung and Blood institute).
Table S4 Quality Assessment of eligible studies.
Disclosure statement: We have no disclosure.
References
- 1. Liacouras CA, Spergel J, Gober LM. Eosinophilic esophagitis: Clinical presentation in children. Gastroenterol. Clin. North Am. 2014; 43: 219–29. [DOI] [PubMed] [Google Scholar]
- 2. Liacouras CA, Furuta GT, Hirano I et al Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J. Allergy Clin. Immunol. 2011; 128: 3–20. [DOI] [PubMed] [Google Scholar]
- 3. Furuta GT, Liacouras CA, Collins MH et al Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007; 133: 1342–63. [DOI] [PubMed] [Google Scholar]
- 4. Mukkada VA, Furuta GT. Management of refractory eosinophilic esophagitis. Dig. Dis. 2014; 32: 134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aceves SS. Unmet therapeutic needs in eosinophilic esophagitis. Dig. Dis. 2014; 32: 143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukkada VA, Haas A, Maune NC et al Feeding dysfunction in children with eosinophilic gastrointestinal diseases. Pediatrics. 2010; 126: e672–7. [DOI] [PubMed] [Google Scholar]
- 7. Levine J, Lai J, Edelman M, Schuval SJ. Conservative long‐term treatment of children with eosinophilic esophagitis. Ann. Allergy Asthma Immunol. 2012; 108: 363–6. [DOI] [PubMed] [Google Scholar]
- 8. Lai J, Levine J. Conservative long‐term treatment of children with eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 2010; 51: E17–18. [Google Scholar]
- 9. Ferreira CT, Goldani HA. Contribution of endoscopy in the management of eosinophilic esophagitis. World J. Gastrointest. Endosc. 2012; 4: 347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lai AL, Girgis S, Liang Y, Carr S, Huynh HQ. Diagnostic Criteria for Eosinophilic Esophagitis: A 5‐year Retrospective Review in a Pediatric Population. J. Pediatr. Gastroenterol. Nutr. 2009; 49: 63–70. [DOI] [PubMed] [Google Scholar]
- 11. Dalby K, Nielsen RG, Kruse‐Andersen S, Fenger C, Durup J, Husby S. Gastroesophageal reflux disease and eosinophilic esophagitis in infants and children. A study of esophageal pH, multiple intraluminal impedance and endoscopic ultrasound. Scand. J. Gastroenterol. 2010; 45: 1029–35. [DOI] [PubMed] [Google Scholar]
- 12. Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am. J. Gastroenterol. 2003; 98: 777–82. [DOI] [PubMed] [Google Scholar]
- 13. Contreras EM, Gupta SK. Steroids in pediatric eosinophilic esophagitis. Gastroenterol. Clin. North Am. 2014; 43: 345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eke R, Markowitz J. Inconsistent remission criteria is a consistent issue in therapeutic trials for eosinophilic esophagitis. JPGN. 2015; 61: S17. [Google Scholar]
- 15. Warners MJ, Hindryckx P, Levesque BG et al Systematic review: disease activity indices in eosinophilic esophagitis. Am. J. Gastroenterol. 2017; 112: 1658–69. 10.1038/ajg.2017.363. [DOI] [PubMed] [Google Scholar]
- 16. Vandenbroucke JP, von Elm E, Altman DG et al Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int. J. Surg. 2014; 12: 1500–24. [DOI] [PubMed] [Google Scholar]
- 17. Schlag C, Miehlke S, Heiseke A et al Peripheral blood eosinophils and other non‐invasive biomarkers can monitor treatment response in eosinophilic oesophagitis. Aliment. Pharmacol. Ther. 2015; 42: 1122–30. [DOI] [PubMed] [Google Scholar]
- 18.Safroneeva E, Straumann A, Coslovsky M et al Symptoms Have Modest Accuracy in Detecting Endoscopic and Histologic Remission in Adults with Eosinophilic Esophagitis. Gastroenterology. 2016; 150: 581–590e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. NIH (National Institute of Health) . Study Quality Assessment Tools Available from URL: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 20. Peterson KA, Byrne KR, Vinson LA et al Elemental diet induces histologic response in adult eosinophilic esophagitis. Am. J. Gastroenterol. 2013; 108: 759–66. [DOI] [PubMed] [Google Scholar]
- 21. Konikoff MR, Noel RJ, Blanchard C et al A randomized, double‐blind, placebo‐controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006; 131: 1381–91. [DOI] [PubMed] [Google Scholar]
- 22.Dellon ES, Sheikh A, Speck O et al Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology. 2012; 143: 321–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Franciosi JP, Hommel KA, Bendo CB et al PedsQL eosinophilic esophagitis module: feasibility, reliability, and validity. J. Pediatr. Gastroenterol. Nutr. 2013; 57: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Markowitz JE, Jobe L, Miller M, Frost C, Laney Z, Eke R. Safety and efficacy of reslizumab for children and adolescents with eosinophilic esophagitis treated over nine years. J. Pediatr. Gastroenterol. Nutr. 2017. [DOI] [PubMed] [Google Scholar]
- 25.Loizou D, Enav B, Komlodi‐Pasztor E et al A pilot study of omalizumab in eosinophilic esophagitis. PLoS One. 2015; 10: e0113483–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spergel JM, Rothenberg ME, Collins MH et al Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double‐blind, randomized, placebo‐controlled trial. J. Allergy Clin. Immunol. 2012; 129: 456–63. [DOI] [PubMed] [Google Scholar]
- 27.Assa'ad AH, Gupta SK, Collins MH et al An antibody against IL‐5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011; 141: 1593–604. [DOI] [PubMed] [Google Scholar]
- 28.Straumann A, Conus S, Grzonka P et al Anti‐interleukin‐5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: A randomised, placebo‐controlled, double‐blind trial. Gut. 2010; 59: 21–30. [DOI] [PubMed] [Google Scholar]
- 29. Wolf WA, Jerath MR, Sperry SLW, Shaheen NJ, Dellon ES. Dietary elimination therapy is an effective option for adults with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2014; 12: 1272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Colson D, Kalach N, Soulaines P et al The impact of dietary therapy on clinical and biologic parameters of pediatric patients with eosinophilic esophagitis. J. Allergy Clin. Immunol. Pract. 2014; 2: 587–93. [DOI] [PubMed] [Google Scholar]
- 31.Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. et al Elimination diet effectively treats eosinophilic esophagitis in adults; Food reintroduction identifies causative factors. Gastroenterology. 2012; 142: 1451–1459. [DOI] [PubMed] [Google Scholar]
- 32. Henderson CJ, Abonia JP, King EC et al Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J. Allergy Clin. Immunol. 2012; 129: 1570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Molina‐Infante J, Martin‐Noguerol E, Alvarado‐Arenas M, Porcel‐Carreño SL, Jimenez‐Timon S, Hernandez‐Arbeiza FJ. Selective elimination diet based on skin testing has suboptimal efficacy for adult eosinophilic esophagitis. J. Allergy Clin. Immunol. 2012; 130: 1200–2. [DOI] [PubMed] [Google Scholar]
- 34. Kagalwalla AF, Sentongo TA, Ritz S et al Effect of six‐food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2006; 4: 1097–102. [DOI] [PubMed] [Google Scholar]
- 35. Reed CC, Fan C, Koutlas NT, Shaheen NJ, Dellon ES. Food elimination diets are effective for long‐term treatment of adults with eosinophilic oesophagitis. Aliment. Pharmacol. Ther. 2017; 46: 836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Molina‐Infante J, Arias A, Barrio J et al Four‐food group elimination diet for adult eosinophilic esophagitis: a prospective multicenter study. J. Allergy Clin. Immunol. 2014; 134: 1093–9. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez‐Sánchez J, Gómez Torrijos E, López Viedma B et al Efficacy of IgE‐targeted vs empiric six‐food elimination diets for adult eosinophilic oesophagitis. Allergy. 2014; 69: 936–42. [DOI] [PubMed] [Google Scholar]
- 38. Lucendo AJ, Arias Á, González‐Cervera J, Mota‐Huertas T, Yagüe‐Compadre JL. Tolerance of a cow's milk‐based hydrolyzed formula in patients with eosinophilic esophagitis triggered by milk. Allergy Eur. J. Allergy Clin. Immunol. 2013; 68: 1065–72. [DOI] [PubMed] [Google Scholar]
- 39. Lucendo AJ, Arias Á, González‐Cervera J et al Empiric 6‐food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J. Allergy Clin. Immunol. 2013; 131: 797–804. [DOI] [PubMed] [Google Scholar]
- 40. Kagalwalla AF, Amsden K, Shah A et al Cow's milk elimination: a novel dietary approach to treat eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 2012; 55: 711–16. [DOI] [PubMed] [Google Scholar]
- 41. Kagalwalla AF, Shah A, Li BUK et al Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J. Pediatr. Gastroenterol. Nutr. 2011; 53: 145–9. [DOI] [PubMed] [Google Scholar]
- 42. Gutiérrez‐Junquera C, Fernández‐Fernández S, Cilleruelo ML et al High prevalence of response to proton‐pump inhibitor treatment in children with esophageal eosinophilia. J. Pediatr. Gastroenterol. Nutr. 2016; 62: 704–10. [DOI] [PubMed] [Google Scholar]
- 43. Philpott H, Nandurkar S, Royce SG, Thien F, Gibson PR. A prospective open clinical trial of a proton pump inhibitor, elimination diet and/or budesonide for eosinophilic oesophagitis. Aliment. Pharmacol. Ther. 2016; 43: 985–93. [DOI] [PubMed] [Google Scholar]
- 44. Vazquez‐Elizondo G, Ngamruengphong S, Khrisna M, DeVault KR, Talley NJ, Achem SR. The outcome of patients with oesophageal eosinophilic infiltration after an eight‐week trial of a proton pump inhibitor. Aliment. Pharmacol. Ther. 2013; 38: 1312–19. [DOI] [PubMed] [Google Scholar]
- 45.Molina–Infante J, Ferrando–Lamana L, Ripoll C et al Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin. Gastroenterol. Hepatol. 2011; 9: 110–17. [DOI] [PubMed] [Google Scholar]
- 46.Gómez‐Torrijos E, García‐Rodríguez R, Castro‐Jiménez A, Rodríguez‐Sanchez J, Méndez Díaz Y, Molina‐Infante J. The efficacy of step‐down therapy in adult patients with proton pump inhibitor‐responsive oesophageal eosinophilia. Aliment. Pharmacol. Ther. 2016; 43: 534–40. [DOI] [PubMed] [Google Scholar]
- 47. Molina‐Infante J, Rodriguez‐Sanchez J, Martinek J et al Long‐term loss of response in proton pump inhibitor‐responsive esophageal eosinophilia is uncommon and influenced by CYP2C19 genotype and rhinoconjunctivitis. Am. J. Gastroenterol. 2015; 110: 1567–75. [DOI] [PubMed] [Google Scholar]
- 48. Molina‐Infante J, Katzka DA, Gisbert JP. Review article: Proton pump inhibitor therapy for suspected eosinophilic oesophagitis. Aliment. Pharmacol. Ther. 2013; 37: 1157–64. [DOI] [PubMed] [Google Scholar]
- 49. Moawad FJ, Schoepfer AM, Safroneeva E et al Eosinophilic oesophagitis and proton pump inhibitor‐responsive oesophageal eosinophilia have similar clinical, endoscopic and histological findings. Aliment. Pharmacol. Ther. 2014; 39: 603–8. [DOI] [PubMed] [Google Scholar]
- 50. Schroeder S, Capocelli KE, Masterson JC et al Effect of proton pump inhibitor on esophageal eosinophilia. J. Pediatr. Gastroenterol. Nutr. 2013; 56: 166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kagalwalla AF, Akhtar N, Woodruff SA et al Eosinophilic esophagitis: Epithelial mesenchymal transition contributes to esophageal remodeling and reverses with treatment. J. Allergy Clin. Immunol. 2012; 129: 1387–1396.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Spergel JM, Andrews T, Brown‐Whitehorn TF, Beausoleil JL, Liacouras CA. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann. Allergy Asthma Immunol. 2005; 95: 336–43. [DOI] [PubMed] [Google Scholar]
- 53. Warners MJ, Vlieg‐Boerstra BJ, Verheij J et al Elemental diet decreases inflammation and improves symptoms in adult eosinophilic oesophagitis patients. Aliment. Pharmacol. Ther. 2017; 45: 777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miehlke S, Hruz P, Vieth M et al A randomised, double‐blind trial comparing budesonide formulations and dosages for short‐term treatment of eosinophilic oesophagitis. Gut. 2016; 65: 390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nennstiel S, Bajbouj M, Becker V et al High‐resolution manometry in patients with eosinophilic esophagitis under topical steroid therapy‐a prospective observational study (HIMEOS‐study). Neurogastroenterol. Motil. 2016; 28: 599–607. [DOI] [PubMed] [Google Scholar]
- 56.Kakati B, Krishna S, McKnight W. Treatment of eosinophilic esophagitis: is budesonide better than fluticasone. Am. J. Gastroenterol. 2010; 105: S162–2. [Google Scholar]
- 57.Van Rhijn BD, Verheij J, van den Bergh Weerman MA, Verseijden C, Van Den Wijngaard RM, De Jonge WJ, Smout AJ, Bredenoord AJ. Histological response to fluticasone propionate in patients with eosinophilic esophagitis is associated with improved functional esophageal mucosal integrity. Am. J. Gastroenterol. 2015; 110: 1289–97. [DOI] [PubMed] [Google Scholar]
- 58. Molina‐Infante J, Gonzalez‐Cordero PL, Lucendo AJ. Proton pump inhibitor‐responsive esophageal eosinophilia: still a valid diagnosis? Curr. Opin. Gastroenterol. 2017; 33: 285–92. [DOI] [PubMed] [Google Scholar]
- 59. Straumann A, Conus S, Degen L et al Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010; 139: 1526–37. [DOI] [PubMed] [Google Scholar]
- 60.Straumann A, Conus S, Degen L et al Long‐term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2011; 9: 400–9. [DOI] [PubMed] [Google Scholar]
- 61. Dellon ES, Gonsalves N, Hirano I et al ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am. J. Gastroenterol. 2013; 108: 679–92. [DOI] [PubMed] [Google Scholar]
- 62.Collins MH, Martin LJ, Alexander ES et al Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis. Esophagus. 2017; 30: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collins MH, Martin LJ, Alexander ES et al Histology scoring system (HSS) is superior to peak eosinophil count (PEC) to identify treated vs untreated eosinophilic esophagitis (EOE) patients. J. Allergy Clin. Immunol. 2012; 129: AB96. [Google Scholar]
- 64. Rusin S, Covey S, Perjar I et al Determination of esophageal eosinophil counts and other histologic features of eosinophilic esophagitis by pathology trainees is highly accurate. Hum. Pathol. 2017; 62: 50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin LJ, Franciosi JP, Collins MH et al Pediatric Eosinophilic Esophagitis Symptom Scores (PEESS v2.0) identify histologic and molecular correlates of the key clinical features of disease. J. Allergy Clin. Immunol. 2015; 135: 1519–1528.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Peterson KA, Thomas KL, Hilden K, Emerson LL, Wills JC, Fang JC. Comparison of esomeprazole to aerosolized, swallowed fluticasone for eosinophilic esophagitis. Dig. Dis. Sci. 2010; 55: 1313–19. [DOI] [PubMed] [Google Scholar]
- 67.Butz BK, Wen T, Gleich GJ et al Efficacy, dose reduction, and resistance to high‐dose fluticasone in patients with eosinophilic esophagitis. Gastroenterology. 2014; 147, e5: 324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo‐controlled trial. Gastroenterology. 2010; 139: 418–29. [DOI] [PubMed] [Google Scholar]
- 69. Greuter T, Bussmann C, Safroneeva E et al Long‐term treatment of eosinophilic esophagitis with swallowed topical corticosteroids: development and evaluation of a therapeutic concept. Am. J. Gastroenterol. 2017; 112: 1527–35. [DOI] [PubMed] [Google Scholar]
- 70. Straumann A, Hoesli S, Bussmann C et al Anti‐eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013; 68: 375–85. [DOI] [PubMed] [Google Scholar]
- 71. Dellon ES, Gibbs WB, Fritchie KJ et al Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin. Gastroenterol. Hepatol. 2009; 7: 1305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bhattacharya B, Carlsten J, Sabo E et al Increased expression of eotaxin‐3 distinguishes between eosinophilic esophagitis and gastroesophageal reflux disease. Hum. Pathol. 2007; 38: 1744–53. [DOI] [PubMed] [Google Scholar]
- 73. Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am. J. Gastroenterol. 2007; 102: 1301–6. [DOI] [PubMed] [Google Scholar]
- 74. Katzka DA. The complex relationship between eosinophilic esophagitis and gastroesophageal reflux disease. Dig. Dis. 2014; 32: 93–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 List of eligible studies included in the review analysis.
Table S2 Article search algorithm.
Table S3 Description of Quality Assessment Tool adapted from National Institute of Health website (NIH – National Heart, Lung and Blood institute).
Table S4 Quality Assessment of eligible studies.


