Abstract
Background and Aim
Currently available staging systems for cholangiocarcinoma (CCA) are not applicable to patients with unresectable stage. A new clinical staging system for perihilar CCA (pCCA) subtype has been recently developed in a US cohort, with a good performance in predicting survival of all pCCA patients. We aimed to determine outcomes of pCCA patients and evaluate predictive performance of this staging system in an Asian population.
Methods
All 141 patients diagnosed with pCCA between 2003 and 2012 were identified. Clinical information was retrospectively abstracted. Patients were classified into four stages based on the new staging system. Survival predictors were analyzed using the Cox proportional hazard analysis.
Results
Of the 141 pCCA patients, 38 (27%), 101 (72%), and 2 (1%) received resection, palliative biliary drainage ± chemotherapy, and best supportive care, respectively. Survival predictors included resectable disease, tumor size, distant metastasis, and cancer antigen 19‐9 ≥ 1000 U/mL. When classified by clinical stages, 13, 4, 99, and 25 patients were in stages I, II, III, and IV, with median survivals of 18.4, 7.3, 6.3, and 2.6 months; and hazard ratio (95% confidence interval) of 1.0 (reference), 1.7 (0.5–5.5), 3.2 (1.5–6.7), and 10.8 (4.6–25.0), respectively.
Conclusion
The clinical staging system has a limited performance in differentiating stage II pCCA patients from stage III patients in the Thai cohort. This can be due to differences in patient characteristics and treatment modalities between the Asian and White pCCA populations. However, the median survivals of patients with other stages are significantly different.
Keywords: bile duct cancer, cholangiocarcinoma, prognosis, staging system, treatment outcome
Introduction
Cholangiocarcinoma (CCA), arising from epithelial lining of biliary tract, is a devastating cancer, with a 5‐year survival of only 10%.1 It accounts for 3% of all gastrointestinal tumors and is the second most common primary liver tumor after hepatocellular carcinoma. The worldwide incidence of CCA appears to be increasing.2, 3 CCA is generally classified based on anatomic location into three subtypes as intrahepatic, perihilar, and distal CCA.1 Each subtype is different in molecular pathogenesis, clinical presentation as well as treatment outcome; therefore, each requires their own prognostic staging system.4, 5
Perihilar CCA (pCCA), arising from the secondary branch of right and left hepatic ducts extending to just above the insertion of cystic duct, represents the most common subtype of CCA, that is 50%.5, 6 Surgical resection and liver transplantation with neoadjuvant chemotherapy are the only curative treatment options. Unfortunately, only 10% of patients present with early stage disease, which is amenable for curative treatment. For the majority of the patients who are not eligible for surgery or transplantation, the mainstay of treatment is palliative chemotherapy with cisplatin and gemcitabine, which prolongs median survival from 8 to 11 months.7, 8, 9
There are currently three established staging systems for pCCA, including Bismuth–Corlette classification, the American Joint Committee on Cancer/Union for International Cancer Control TNM staging system and the Memorial Sloan Kettering Cancer Center (MSKCC) staging system. The Bismuth–Corlette system is primarily aimed to guide surgical strategy based on anatomic consideration and does not yield prognostic value.10 The TNM staging system is mainly used for predicting prognosis after surgical resection or transplantation as it requires information on pathology.11 Nonetheless, previous studies showed that TNM was not associated with survival of pCCA patients undergoing surgery.12 The MSKCC is aimed to predict resectability before operation.13 Accordingly, there is no well‐established staging system for predicting prognosis of patients with unresectable disease.
Due to the limitations of the three staging systems,14 a new clinical staging system for pCCA was recently proposed and showed promising prognostic value for both unresectable and resectable disease.15 However, the major population of this development cohort was White (87.7%) and the major cause of pCCA in Western countries was primary sclerosing cholangitis (PSC). By contrast, the major cause of pCCA in Asian countries is liver fluke infestation (Opisthorchis viverrini and Clonorchis sinensis) that might pose different pathogenesis of pCCA as compared to PSC.16
Initially, we aimed to determine the performance of the new clinical staging system of pCCA in Asian population.15 Then, we sought to determine factors associated with survival of pCCA patients in Asian population.
Methods
Study cohort
We conducted a retrospective cohort study of pCCA patients seen at King Chulalongkorn Memorial Hospital, Bangkok, Thailand, between 2001 and 2012. A total of 141 patients were identified using the International Classification of Diseases, Tenth Revision, Thai Modification (ICD‐10‐TM) codes of C22.1 and C24. Inclusion criteria were newly diagnosed pCCA patients who were aged over 18 years. Patients who underwent surgical resection or received chemotherapy prior to the first visit at our hospital were excluded. Diagnosis of pCCA was made based on the criteria previously published.15 Briefly, (i) surgical histopathology from resected tumor (n = 28); (ii) histology from intraluminal endoscopic core biopsy, percutaneous needle biopsy, or intraoperative biopsy (n = 40); (iii) intraluminal brush cytology (n = 1); (iv) a CA (cancer antigen) 19‐9 level > 100 U/mL in the setting of a malignant‐appearing biliary tract stricture demonstrated by radiologic imaging and without bacterial cholangitis (n = 47); or (v) a perihilar mass lesion with a malignant‐appearing biliary tract stricture on cross‐sectional imaging studies with evidence of malignant clinical progression during the follow‐up period (n = 25).
Data collection
Demographic and clinical information, including underlying diseases, Eastern Cooperative Oncology Group (ECOG) status, tumor size and number, lymph node and distant metastases, vascular encasement, treatment received, and CA 19‐9 level at the time of pCCA diagnosis was abstracted from medical records. All‐cause mortality defined as any death was obtained from death certificate.
Categorization of patients by Mayo Clinic clinical staging system
All patients were classified into four stages by the new clinical staging system that was recently developed.15 Briefly, this staging system relied on five variables obtained preoperatively, including tumor size and number, vascular encasement, presence of intrahepatic or distant metastasis, ECOG status, and CA 19‐9 level (Table 1).
Table 1.
Mayo Clinic clinical staging system for pCCA
| Variables | Stage I | Stage II | Stage III | Stage IV |
|---|---|---|---|---|
| Mass lesion | Unicentric ≤ 3 cm | Unicentric ≤ 3 cm | Unicentric > 3 cm or multicentric | NA |
| Vascular encasement | No | Yes | NA | NA |
| Metastasis | No | No | Lymph node metastasis | Peritoneal or other organ metastasis |
| ECOG status | 0 | 1–2 | 0–2 | 3–4 |
| CA 19‐9 level (U/mL) | <1000 | <1000 | ≥1000 | NA |
CA, cancer antigen; ECOG, Eastern Cooperative Oncology Group; NA, not applicable, pCCA, perihilar cholangiocarcinoma.
Statistical analysis
Continuous variables were expressed as mean ± SD or median, interquartile range (IQR), while categorical variables were presented as number (%). Factors associated with survival were identified using Cox proportional hazards regression analysis and presented as hazard ratio (HR) along with 95% confidence interval (CI). Subsequently, patients were classified into four clinical stages15 and survivals of patients with different stages were estimated using Kaplan–Meier curve and compared using the log‐rank test. Data analysis was performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA). P‐value of <0.05 was determined as statistically significant.
Results
Baseline characteristics
Of the 141 pCCA patients, 77 (55%) were males and mean age was 58 years. Most patients had good ECOG performance status, that is 46 and 91 patients had ECOG 0 and 1, respectively. Two patients had ECOG 2, and only one patient had ECOG 3. None had PSC. The median of maximum tumor diameter was 3.6 cm. The median CA 19‐9 value was 674.8 U/mL. The proportion of patients with CA 19‐9 level ≥ 1000 U/mL was 40%. There were 38 (27%), 101 (72%), and 2 (1%) patients who were treated with resection, palliative biliary drainage ± systemic chemotherapy, and best supportive care (BSC), respectively. None underwent liver transplantation (Table 2).
Table 2.
Baseline characteristics
| Characteristics | N = 141 |
|---|---|
| Age (mean ± SD) (range), years | 58 ± 12 (16–87) |
| Male sex, n (%) | 77 (55%) |
| Comorbid and social conditions | |
| Cirrhosis, n (%) | 5 (4%) |
| HBV, n (%) | 13 (9%) |
| HCV, n (%) | 1 (1%) |
| DM, n (%) | 22 (16%) |
| HIV, n (%) | 1 (1%) |
| Smoking, n (%) | 47 (33%) |
| Alcohol drinking, n (%) | 54 (38%) |
| ECOG performance status, n (%)† | |
| 0 | 46 (33%) |
| ≥1 | 94 (67%) |
| Tumor location, n (%)‡ | |
| Common hepatic duct | 26 (18%) |
| Hepatic duct confluence | 40 (29%) |
| Right or left hepatic duct | 44 (31%) |
| Right and left hepatic duct | 28 (20%) |
| Maximum tumor diameter, median (range), cm | 3.6 (1.0–15.6) |
| Number of tumor, n (%) | |
| Non‐identified | 16 (11%) |
| Single | 104 (74%) |
| Two | 9 (6%) |
| Multiple | 12 (9%) |
| Vascular encasement, n (%) | 67 (48%) |
| Lymph node metastasis, n (%) | |
| No | 75 (53%) |
| Hilar | 14 (10%) |
| Distant | 52 (37%) |
| Other organ metastasis, n (%) | 37 (26%) |
| Median CA 19‐9,U/mL | 674.8 (0.6–6825) |
| CA 19‐9 level ≥ 1000 U/mL, n (%) | 56 (40%) |
One ECOG status missing.
Three tumor location information missing.
CA, cancer antigen; DM, diabetes mellitus; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B viral infection; HCV, hepatitis C viral infection; HIV, human immunodeficiency viral infection.
Factors associated with survival of pCCA patients by clinical staging system
The overall median survival of the entire cohort was 5.6 months. The 1‐, 2‐, and 5‐year survival rates were 28%, 11%, and 4%, respectively. Factors associated with survival included resectable disease, tumor size, distant metastasis, and CA 19‐9 ≥ 1000 U/mL; with adjusted HRs (95% CI) of 0.28 (0.16–0.47), 1.11 (1.02–1.21), 2.47 (1.46–4.20), and 1.58 (1.10–2.28), respectively (P < 0.05 for all; Table 3).
Table 3.
Cox regression analysis for factors associated with survival of pCCA patients (n = 141)
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Age | 1.01 (0.99–1.02) | 0.28 | 1.02 (1.00–1.04) | 0.01 |
| Male sex | 0.99 (0.70–1.38) | 0.93 | 1.02 (0.71–1.47) | 0.91 |
| Comorbid conditions | ||||
| Cirrhosis | 1.19 (0.49–2.93) | 0.70 | ||
| HBV | 1.04 (0.57–1.88) | 0.90 | ||
| HCV | 2.33 (0.3216.86) | 0.40 | ||
| DM | 0.92 (0.58–1.46) | 0.74 | ||
| HIV | 1.75 (0.24–12.60) | 0.58 | ||
| Smoking | 1.14 (0.78–1.65) | 0.49 | ||
| Alcohol drinking | 1.12 (0.78–1.61) | 0.54 | ||
| ECOG performance status | ||||
| 0 | 1.00 (reference) | |||
| ≥ 1 | 1.32 (0.92–1.89) | 0.14 | ||
| Tumor location | ||||
| Common hepatic duct | 1.00 (reference) | |||
| Hepatic duct confluence | 0.96 (0.58–1.59) | 0.88 | ||
| Right or left hepatic duct | 0.77 (0.46–1.28) | 0.32 | ||
| Right and left hepatic duct | 1.59 (0.93–2.72) | 0.09 | ||
| Maximum tumor diameter | 1.15 (1.08–1.26) | <0.001 | 1.11 (1.02–1.21) | 0.01 |
| Number of tumor | ||||
| Non‐identified | 1.00 (reference) | 1.00 (reference) | ||
| Single | 1.13 (0.65–1.96) | 0.66 | 0.82 (0.42–1.60) | 0.57 |
| Multiple | 2.41 (1.22–4.74) | 0.01 | 0.64 (0.24–1.70) | 0.37 |
| Vascular encasement | 1.41 (1.00–1.96) | 0.05 | 1.03 (0.67–1.58) | 0.88 |
| Lymph node metastasis | ||||
| No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 2.02 (1.41–2.90) | <0.001 | 1.32 (0.87–2.01) | 0.19 |
| Other organ metastasis | 3.71 (2.44–5.63) | <0.001 | 2.47 (1.46–4.20) | 0.001 |
| CA 19‐9 level ≥ 1000 U/mL | 1.47 (1.04–2.09) | 0.03 | 1.58 (1.10–2.28) | 0.01 |
| Treatment mode | ||||
| Unresectable | 1.00 (reference) | 1.00 (reference) | ||
| Resectable | 0.26 (0.16–0.40) | <0.001 | 0.28 (0.16–0.47) | <0.001 |
CA, cancer antigen; CI, confidence interval; DM, diabetes mellitus; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B viral infection; HCV, hepatitis C viral infection; HIV, human immunodeficiency viral infection; HR, hazard ratio; pCCA, perihilar cholangiocarcinoma.
When stratified by four clinical stages, 13, 4, 99, and 25 patients were in stages I, II, III, and IV, with median survivals of 18.4, 7.3, 6.3, and 2.6 months (P < 0.001), respectively. HRs (95% CI) were 1.0 (reference), 1.7 (0.5–5.5), 3.2 (1.5–6.7), and 10.8 (4.6–25.0), for stages I, II, III, and IV (P = 0.42, 0.002, and <0.001), respectively (Fig. 1 and Table 4).
Figure 1.
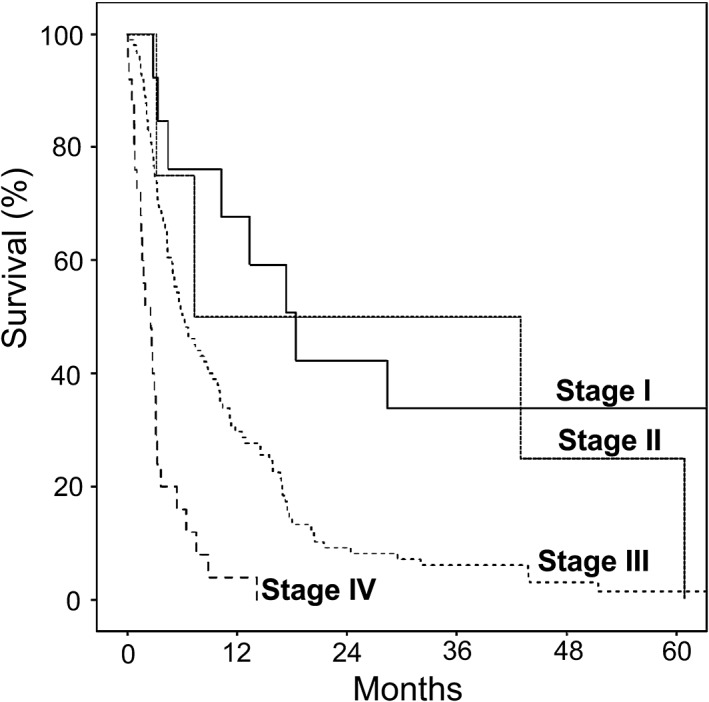
Survival curves of perihilar cholangiocarcinoma patients classified by clinical staging.
Table 4.
Median survival of patients by clinical staging (n = 141)
| Stage | Number of death/total patients | Median survival (months) | HR (95% CI) | P‐value |
|---|---|---|---|---|
| I | 11/13 | 18.4 | 1.0 (reference) |
— |
| II | 4/4 | 7.3 | 1.7 (0.5–5.5) |
0.42 |
| III | 96/99 | 6.3 | 3.2 (1.5–6.7) |
0.002 |
| IV | 25/25 | 2.6 | 10.8 (4.6–25.0) |
<0.001 |
CI, confidence interval; HR, hazard ratio.
Survival of pCCA patients classified by different treatment modalities
Patients who were treated with resection had significantly better survival than those treated with palliative biliary drainage ± systemic chemotherapy, that is 17.3 versus 4.1 months, respectively, P < 0.001. The median survival of BSC group was not able to be estimated as only two patients received BSC. The survivals of these two patients were 2 days and 4.3 months.
Of the 38 patients treated with resection, 6, 2, and 30 were classified as stages I, II, and III, respectively. Median survivals of stage I, II, and III patients receiving resection were 66.6, 7.3, and 16.7 months, respectively (P = 0.01; Fig. 2a). Patients who had free resected margin had longer survival than those who had R1 or R2 surgical margin (51.4 vs 18.0 months, P = 0.14). Patients who did not have lymph node metastasis had significantly longer survival than those with nodal metastasis, that is 28.4 versus 16.7 months (P = 0.01). Perineural invasion, vascular invasion, and histologic subtype did not have an impact on survival of resected patients in this cohort, that is 16.8 and 18.0 months for those with and without neural invasion; 17.5 and 17.5 months for those with and without vascular invasion; and 18.0 and 28.4 months for those with well‐differentiated and moderately‐poorly differentiated subtype (P = 0.90, 0.70, and 0.87), respectively.
Figure 2.
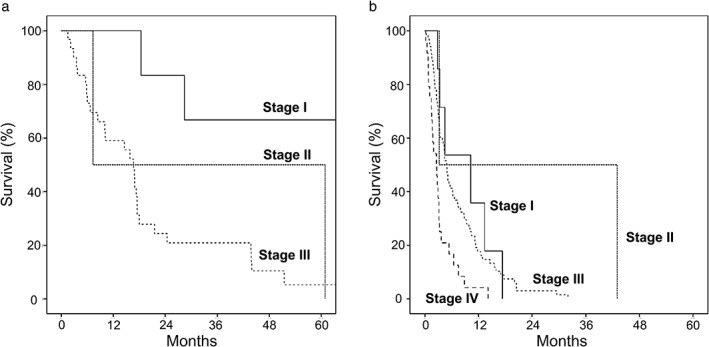
Survival curves of resectable patients (a) and patients receiving palliative biliary drainage ± systemic chemotherapy (b).
Of the 101 patients who underwent palliative biliary drainage ± systemic chemotherapy, 7, 2, 68, and 24 were classified as stages I, II, III, and IV, with median survivals of 10.3, 3.2, 4.9, and 2.6 months, respectively(P < 0.001; Fig. 2b).
Discussion
Our findings suggest that pCCA patients have poor prognosis and short survival, particularly those with unresectable disease. The identified outcome included tumor size, other organ metastasis, and CA 19‐9 ≥ 1000 U/mL. We found that the new clinically based staging system had a good performance in prognosticating outcomes of patients with early and advanced disease, but had a limited performance in differentiating outcomes of patients with intermediate stages.
Similar to the development cohort,15 tumor size, other organ metastasis, and CA 19‐9 ≥ 1000 U/mL were found to be significant predictors of survival. Treatment modality was also significantly related to survival outcome in our cohort and the development cohort. However, this factor was not included in the Mayo Clinic clinical staging system as the staging system was aimed to be applicable for all patients regardless of treatment received. Inconsistent with the findings reported in the development cohort,15 we did not observe the association between ECOG status and survival, likely due to the small number of patients with ECOG 2–4.
Like the development cohort,15 survivals of stage I, III, and IV patients were significantly different, that is 18.4, 6.3, and 2.6 months for stages I, III, and IV, respectively. However, due to the very small number of patients with stage II (n = 4), we were not able to determine the performance of the clinical staging system in predicting outcomes of these patients. Overall, the patients in our cohort had shorter survival than the patients in the development cohort, that is 5.6 versus 12.2 months. This is likely due to a couple of reasons. First, there were more patients who were classified as advanced stage in our cohort than the development cohort, that is 70% versus 42% for stage III, and 18% versus 15% for stage IV, respectively. The greater number of patients with advanced stage in our cohort could therefore contribute to the poorer prognosis. Second, curative treatment options are limited in our country. Liver transplantation is currently not available as a treatment option for CCA. We observed that patients with stage I disease in this cohort had much shorter survival than stage I patients in the development cohort, that is 18.4 versus 48.6 months, respectively. This discrepancy was most likely due to the difference in treatment modality given to early stage patients. Specifically, none of the patients in our study received liver transplantation while 43.4% of stage I patients in the previous study15 were treated with liver transplantation. Nonetheless, when comparing survivals of those receiving resection and palliative biliary stenting between the two cohorts, it appears that there was not much difference in survival of the present cohort and the development cohort, that is 17.3 versus 25.3 months for resection and 4.1 versus 5.8 months for stenting. We believe that, rather than the different etiologies of CCA, cancer stage at presentation and treatment received are major factors contributing to different prognosis.
Although efforts have been made to develop prognostic scores or predictors of outcomes that can be used for all pCCA patients, the currently available staging systems are not applicable to all pCCA patients. Very recently, survival nomogram for resectable pCCA was developed in a Chinese population and demonstrated higher accuracy to predict patient survival than the Mayo Clinic clinical staging system.17 This nomogram was based on age, lymph node metastasis, CA 19‐9 level, portal vein involvement, hepatic artery invasion, and surgical outcome (i.e. R0 vs R1/R2). Despite its high predictability, the use remained limited to patients with resectable disease as it required information on surgical pathology, specifically neural invasion, to stage patients. Several prognostic factors such as inflammatory biomarkers were also proposed. Unresectable pCCA patients with both neutrophil‐lymphocyte ratio (NLR) ≥5 and C‐reactive protein (CRP) >10 mg/L had poorer outcomes compared to patients without elevation of both biomarkers with median survivals of 3 and 12.8 months, respectively.18
The strength of this study is that this is the first study to validate the newly proposed staging system for resectable and unresectable pCCA. Unlike most previous studies, which mainly focused on patients with resectable disease, our study had a full breadth of patients with all stages of disease.17, 19 However, there were some limitations in our study. First, the majority of our patient cohort presented with advanced stage, and only four patients in stage II, which possibly limited us in assessing the performance of the staging system in predicting the outcome of stage II patients. Second, due to organ shortage, liver transplantation is currently not available as a treatment option for pCCA in Thailand. Consequently, no patients received liver transplantation in our study, which could lead to differences in survivals as compared to other cohorts that could be treated with transplantation. Third, CCA is relatively rare as compared to other types of cancer. Even in the small cohort, we could demonstrate the significant difference of median survival between stages. Nonetheless, a further cohort with large sample size is needed to validate the staging system.
Conclusion
The new clinical staging system of pCCA is valid to predict treatment outcome of both resectable and unresectable pCCA in our cohort of Asian population. Tumor size, other organ metastasis, and CA 19‐9 ≥ 1000 U/mL are potential predicting factors in patients with pCCA. Further study in a larger Asian population is warranted to validate this new clinical staging system.
Declaration of conflict of interest: The authors declared no conflict of interest.
Funding support: Grant for International Research Integration: Chula Research Scholar, Ratchadaphiseksomphot Endowment Fund.
References
- 1. Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: liver, biliary tract, and pancreas. Gastroenterology. 2009; 136: 1134–44. [DOI] [PubMed] [Google Scholar]
- 2. Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract. Res. Clin. Gastroenterol. 2015; 29: 221–32. [DOI] [PubMed] [Google Scholar]
- 3. Khan SA, Davidson BR, Goldin RD et al Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012; 61: 1657–69. [DOI] [PubMed] [Google Scholar]
- 4. Hezel AF, Deshpande V, Zhu AX. Genetics of biliary tract cancers and emerging targeted therapies. J. Clin. Oncol. 2010; 28: 3531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakeeb A, Pitt HA, Sohn TA et al Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann. Surg. 1996; 224: 463–73; discussion 473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeOliveira ML, Cunningham SC, Cameron JL et al Cholangiocarcinoma: thirty‐one‐year experience with 564 patients at a single institution. Ann. Surg. 2007; 245: 755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rizvi S, Gores GJ. Current diagnostic and management options in perihilar cholangiocarcinoma. Digestion. 2014; 89: 216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valle J, Wasan H, Palmer DH et al Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010; 362: 1273–81. [DOI] [PubMed] [Google Scholar]
- 9. Zhu AX. Future directions in the treatment of cholangiocarcinoma. Best Pract. Res. Clin. Gastroenterol. 2015; 29: 355–61. [DOI] [PubMed] [Google Scholar]
- 10. Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg. Gynecol. Obstet. 1975; 140: 170–8. [PubMed] [Google Scholar]
- 11. National Comprehensive Cancer Network . NCCN Guidelines Version 1, 2013. Cited 15 November 2016. Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
- 12. Cho MS, Kim SH, Park SW et al Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10‐year single‐institution experience. J. Gastrointest. Surg.. 2012; 16: 1672–9. [DOI] [PubMed] [Google Scholar]
- 13. Jarnagin WR, Fong Y, DeMatteo RP et al Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann. Surg. 2001; 234: 507–17; discussion 517–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hiriart JB, Laurent C, Blanc JF. Perihilar cholangiocarcinoma, the need for a new staging system. Clin. Res. Hepatol. Gastroenterol. 2011; 35: 697–8. [DOI] [PubMed] [Google Scholar]
- 15. Chaiteerakij R, Harmsen WS, Marrero CR et al A new clinically based staging system for perihilar cholangiocarcinoma. Am. J. Gastroenterol. 2014; 109: 1881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sripa B, Kaewkes S, Sithithaworn P et al Liver fluke induces cholangiocarcinoma. PLoS Med. 2007; 4: e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen P, Li B, Zhu Y et al Establishment and validation of a prognostic nomogram for patients with resectable perihilar cholangiocarcinoma. Oncotarget. 2016; 7: 37319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okuno M, Ebata T, Yokoyama Y et al Appraisal of inflammation‐based prognostic scores in patients with unresectable perihilar cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2016; 23: 636–42. [DOI] [PubMed] [Google Scholar]
- 19. Groot Koerkamp B, Wiggers JK, Gonen M et al Survival after resection of perihilar cholangiocarcinoma‐development and external validation of a prognostic nomogram. Ann. Oncol. 2015; 26: 1930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


