Abstract
Background and Aim
Bowel preparations with polyethylene glycol (PEG) and clear fluids are often poorly tolerated. We compared an innovative low‐residue White Diet and low‐volume, split‐dose Picosalax with the standard preparation at our institution of day‐before clear fluids and combination PEG plus sodium picosulfate/magnesium citrate (SPMC).
Methods
Adults undergoing morning colonoscopy were randomized to either the White Diet and split‐dose, two sachets of Picosalax (WD/PICO) or day‐before clear fluids and 1‐L PEG plus two sachets of SPMC (CF/PEG + SPMC). The primary endpoint was successful bowel preparation defined by an Ottawa bowel preparation score ≤ 6. An intention‐to‐treat analysis with a predefined non‐inferiority margin of 15% was used to compare efficacy.
Results
A total of 250 patients were randomized (125 WD/PICO and 125 CF/PEG + SPMC). WD/PICO was non‐inferior to CF/PEG + SPMC for successful bowel preparation by intention‐to‐treat analysis (58% WD/PICO vs 62% CF/PEG + SPMC, 95%CI: −14.2 to 6.2%) and per‐protocol analysis (64% WD/PICO vs 65% CF/PEG + SPMC, 95%CI: −11.3 to 9.4%). Patients in the WD/PICO group reported greater satisfaction with the diet (P < 0.001), greater ease of following the diet (P < 0.001), and improved experience compared with prior colonoscopy (P < 0.0001), less bloating (P = 0.02), less weakness (P = 0.046), less hunger (P < 0.0001), and less interference with daily activities (P = 0.001). Procedure/withdrawal times and adenoma detection rates were similar between groups.
Conclusion
Bowel preparation with the White Diet and low‐volume, split‐dose Picosalax was preferred and better tolerated without detriment to bowel preparation success compared with clear fluids and combination PEG plus SPMC for morning colonoscopy.
Keywords: adenoma, bowel preparation, colonoscopy
Introduction
Colonoscopy has been shown to reduce colorectal cancer (CRC) morbidity and mortality,1, 2, 3 but the procedure requires high‐quality bowel preparation. Many standard bowel preparations consist of large volumes of poorly palatable laxatives which many patients are unable to tolerate due to nausea, vomiting, bloating, and headache. Poor tolerance of the bowel preparation may reduce compliance and completion of the bowel preparation and adversely affect the final bowel cleanliness. To improve tolerability and compliance, low‐volume bowel preparations such as Picosalax are increasingly used with improved tolerability and comparable efficacy to polyethylene glycol (PEG) bowel preparation.4, 5, 6
Sodium picosulfate/magnesium citrate (SPMC) preparations contain two active ingredients with different mechanisms of action; sodium picosulfate is a stimulant laxative and magnesium oxide combined with citric acid acts as an osmotic laxative.7 Combination preparations with PEG plus SPMC may be better tolerated than high‐volume PEG alone8 and are standard practice in many Australian hospitals and endoscopy centers. Several randomized controlled trials and a meta‐analysis confirm that splitting the bowel preparation dose between the day prior and the day of the procedure results in both improved bowel cleansing and patient tolerance compared with day‐prior regimens.9, 10, 11, 12
In addition to the type and timing of the cleansing agent used, diet may influence the tolerability and quality of bowel preparation. Typically, patients are instructed to take a clear fluid diet the day prior to colonoscopy because high‐fiber foods may impair bowel preparation. Recent randomized trials, however, suggest that a low‐fiber diet13 or even regular diet14 the day before colonoscopy with split‐dose bowel preparation is associated with better tolerance of the preparation and comparable or better colon cleansing compared with a clear fluid‐only diet. The White Diet is a novel, low‐residue diet of white‐colored foods, which is better tolerated without detriment to bowel preparation quality compared to a clear fluid diet in patients undergoing colonoscopy.15
As part of the implementation of “state‐of‐the‐art” bowel preparation strategy at our institution, the aim of this prospective, single‐blinded, randomized, non‐inferiority trial was to determine whether an innovative low‐residue White Diet and low‐volume, split‐dose Picosalax is better tolerated, but with comparable bowel preparation quality, to the standard preparation at our institution of day‐before clear fluids and combination PEG plus SPMC for morning colonoscopy.
Methods
Study design
This was a prospective, randomized, single‐blinded, non‐inferiority trial comparing bowel preparation with the White Diet and low‐volume, split‐dose Picosalax with day‐before clear fluids and combination PEG plus SPMC for morning colonoscopy. The study was carried out at a single tertiary referral hospital (The Alfred Hospital, Melbourne, Australia). The Human Ethics Committee at The Alfred Hospital approved the study protocol and all subjects gave written informed consent. The study was prospectively registered with the Australian New Zealand Clinical Trials Registry (ACTRN12613000765729).
Study population
Consecutive patients having outpatient morning colonoscopy were invited to take part in the study from April 2013 to November 2014. Inclusion criteria were adult patients (aged ≥18 years) undergoing colonoscopy for clinically accepted indications. Exclusion criteria included severe renal impairment (estimated glomerular filtration rate < 30), severe heart failure (New York Heart Association Class III or IV), and conditions considered to be contraindications to colonoscopy such as suspected bowel perforation, gastric outlet obstruction, toxic megacolon, severe colitis, pregnancy, or lactation. Patients with hypersensitivity to PEG or SPMC including patients with phenylketonuria or glucose‐6‐phosphate dehydrogenase deficiency, due to the presence of aspartame or ascorbic acid in the bowel preparation, were excluded.
Study protocol
Eligible patients were randomized to one of the two treatment arms on a 1:1 basis using a computer generated block randomization list. Patients randomized to the intervention arm (White Diet and low‐volume Picosalax—WD/PICO) received the White Diet for 2 days prior to the procedure, then split‐dose Picosalax (consisting of 10 mg sodium picosulfate, 3.5 g magnesium oxide, and 2 g citric acid ; Ferring Pharmaceuticals, Melbourne, Australia) consisting of two sachets of SPMC taken at 21:00 h the day before and at 04:00 h on the day of the procedure with 200 mL of water per hour allowed until 06:00 h. As described previously,15 the White Diet comprises white‐colored foods of low residue (Table 1).
Table 1.
Patient instructions for food and fluid permitted in the White Diet
| 2 Days before your colonoscopy only consume foods and fluids permitted in the White Diet |
|---|
| White Diet foods and fluids permitted |
| Milk (regular, low fat, skim), water, lemonade, soda or mineral water, clear (not colored) Gatorade, or other sports drinks |
| Regular white bread/toast, rice bubbles, white rice, regular pasta, potatoes (peeled), rice noodles, plain rice crackers, white flour, sugar |
| Eggs, chicken breast (no skin), white fish fillet (no skin) |
| Plain cream cheese, cheddar cheese, ricotta, feta, cottage, parmesan or mozzarella cheese, white sauce |
| White‐colored yoghurt (no added fruit or inulin), mayonnaise, cream, sour cream, butter and margarine, oil for cooking |
| White chocolate, vanilla ice cream, lemonade icy‐pole, clear jelly, custard, “milk bottles” (white confectionery) |
| Foods to be excluded (not allowed) |
| Anything not listed above |
| Other white foods including pears, parsnip, cauliflower, onion, high‐fiber white breads, tofu, coconut, porridge, banana, mushrooms, semolina, couscous, popcorn |
Patients assigned to the standard preparation at our institution (CF/PEG + SPMC) received day‐before light breakfast, then clear fluids only, and combination 1 L PEG (Glycoprep‐C, consisting of macrogol 3350, sodium sulfate, sodium chloride, and potassium chloride; Fresenius Kabi Pty Ltd, Pymble, Australia) taken at 18:00 h the day before plus two sachets of SPMC (PicoPrep, consisting of 10 mg sodium picosulfate, 3.5 magnesium oxide, 12.0 g citric acid, and 36 mg aspartame; Fresenius Kabi Pty Ltd) taken at 17:00 and 19:00 h the day before the procedure with fasting from midnight. All participants received a single‐page handout with standardized instructions for the allocated bowel preparation and diet. Participants were asked to complete a food diary for 2 days prior to their colonoscopy and a questionnaire on the acceptance and tolerability of the bowel preparation. Endoscopists were blinded to the type of bowel preparation taken by the patient and completed a datasheet following the procedure.
The primary outcome was successful bowel preparation as defined by an Ottawa bowel preparation score ≤ 6.16 The Ottawa scale (0–14 points) combines the preparation score (0 = excellent, 1 = good, 2 = fair, 3 = poor, and 4 = inadequate) of three bowel segments (left colon, transverse colon, and right colon) and the amount of fluid in the entire colon (0 = low, 1 = moderate, and 2 = large). Secondary outcomes included tolerability, acceptance, and compliance with the allocated bowel preparation regimen and colonoscopy outcomes such as adenoma detection and withdrawal time.
Sample size calculation and statistical analysis
The sample size was calculated assuming a 75% bowel preparation success rate with clear fluids and a non‐inferiority margin of 15% consistent with previous non‐inferiority trials for bowel preparation.17, 18 To be adequately powered with 80% power at a one‐sided alpha level of 5%, 104 patients were required in each group. An intention‐to‐treat (ITT) analysis was used to compare efficacy for the primary outcome with non‐inferiority established if the lower confidence limit for the difference in effect was above −15%. A per‐protocol analysis was also carried out for the primary efficacy endpoint, in which patients with major protocol violations were excluded. Comparisons of secondary outcomes were performed using the Student's t‐test for normally distributed continuous variables, Wilcoxon rank‐sum test for non‐normally distributed continuous variables, and chi‐square or Fisher's exact test as appropriate for categorical variables. Two‐sided P‐values <0.05 indicated statistical significance. Statistical analyses were performed with Stata software version 14 (StataCorp, TX, USA).
Results
Two hundred and fifty patients were randomized and, after exclusions, 112 patients were included in the WD/PICO group and 118 patients in the CF/PEG + SPMC group (Fig. 1). Patient demographics and clinical characteristics were similar between groups (Table 2).
Figure 1.
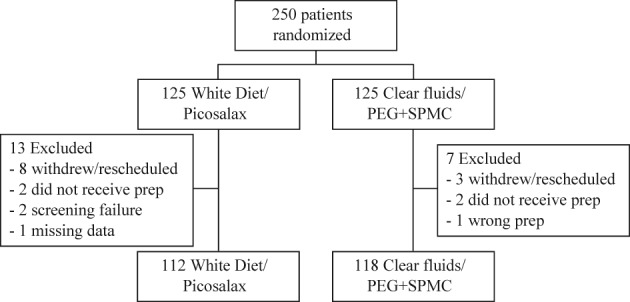
Enrollment flow chart. PEG, polyethylene glycol; SPMC, sodium picosulfate/magnesium citrate.
Table 2.
Baseline demographic and clinical characteristics of patients
| Type of preparation | P‐value | ||
|---|---|---|---|
| WD/PICO (n = 125) | CF/PEG + SPMC (n = 125) | ||
| Age (year), mean ± SD | 54.5 ± 13.4 | 54.0 ± 13.1 | 0.81 |
| Male gender, n (%) | 70 (56) | 65 (52) | 0.53 |
| Weight (kg), mean ± SD | 79.2 ± 15.7 (n = 94) |
77.3 ± 19.4 (n = 99) |
0.45 |
| Height (cm), mean ± SD | 171.4 ± 10.3 (n = 91) |
169.8 ± 14.4 (n = 99) |
0.36 |
| Diabetes, n (%) | 6 (5.6) (n = 107) |
5 (4.4) (n = 113) |
0.69 |
| Opioids, n (%) | 16 (15.2) (n = 105) |
14 (12.5) (n = 112) |
0.56 |
| Laxative use, n (%) | 11 (10.3) (n = 107) |
15 (13.4) (n = 112) |
0.48 |
CF, clear fluid; PEG, polyethylene glycol; PICO, picosalax; SPMC, sodium picosulfate/magnesium citrate; WD, White Diet.
ITT analysis of the primary efficacy endpoint showed that successful bowel preparation (defined by an Ottawa bowel preparation score ≤ 6) was 57.6% in the WD/PICO group and 61.6% in the CF/PEG + SPMC group (Table 3). The difference between groups was −4.0% (95% CI: −14.2 to 6.2%), suggesting non‐inferiority between groups. Per‐protocol analysis showed that successful bowel preparation was 64.3% in the WD/PICO group and 65.3% in the CF/PEG + SPMC group (difference: −1%, 95% CI: −11.3 to 9.4%) indicating non‐inferiority. Colonoscopy outcomes are shown in Table 4. There were no significant differences between groups with regard to insertion time, withdrawal time, cecal intubation rate, polyps removed, or detection of CRC.
Table 3.
ITT and per‐protocol analyses of the primary efficacy endpoint
| Total | Type of preparation | ||
|---|---|---|---|
| WD/PICO | CF/PEG + SPMC | ||
| ITT analysis | |||
| Number of patients in ITT analysis | 250 | 125 | 125 |
| Successful bowel preparation, n (%) | 149 (59.6) |
72 (57.6) |
77 (61.6) |
| Difference between groups (one‐sided 95% CI) | −4.0% (−14.2 to 6.2%) | ||
| Per‐protocol analysis | |||
| Number of patients in per‐protocol analysis | 230 | 112 | 118 |
| Successful bowel preparation, n (%) | 149 (64.8) |
72 (64.3) |
77 (65.3) |
| Difference between groups (one‐sided 95% CI) | −1.0% (−11.3 to 9.4%) | ||
CF, clear fluid; ITT, intention to treat; PEG, polyethylene glycol; PICO, picosalax; SPMC, sodium picosulfate/magnesium citrate; WD, White Diet.
Table 4.
Colonoscopy outcomes
| Type of preparation | P‐value | ||
|---|---|---|---|
| WD/PICO (n = 125) | CF/PEG + SPMC (n = 125) | ||
| Past colonic resection, n (%) | 4 (3.6) (n = 110) |
6 (5.2) (n = 116) |
0.75 |
| Cecal intubation, n (%) | 110 (98.2) (n = 112) |
116 (98.3) (n = 118) |
1.00 |
| TI intubation, n (%) | 89 (79.5) (n = 112) |
97 (82.2) (n = 116) |
0.60 |
| Repeat scope due to poor preparation, n (%) | 5 (4.5) (n = 111) |
4 (3.4) (n = 118) |
0.74 |
| Insertion time (min), median (IQR) | 9 (6–14) (n = 107) |
9 (5–14) (n = 115) |
0.95 |
| Withdrawal time (min), median (IQR) | 10 (7–14) (n = 95) |
10 (8–15) (n = 87) |
0.43 |
| Total time (min), median (IQR) | 20 (15–26) (n = 95) |
20 (16–26) (n = 87) |
0.74 |
| Polyps removed, n (%) | 34 (30.4) (n = 112) |
38 (32.8) (n = 116) |
0.70 |
| Adenoma detection rate, n (%) | 24 (21.4) (n = 112) |
29 (24.6) (n = 118) |
0.57 |
| Colorectal cancer, n (%) | 0 (0) (n = 107) |
2 (1.7) (n = 115) |
0.50 |
CF, clear fluid; IQR, interquartile range; PEG, polyethylene glycol; PICO, picosalax; SPMC, sodium picosulfate/magnesium citrate; TI, terminal ileum; WD, White Diet.
Patient‐reported satisfaction scores and tolerance to bowel preparation are shown in Tables 5 and 6, respectively. Patients in the WD/PICO group reported significantly higher satisfaction with the diet and greater ease in following the diet compared with the CF/PEG + SPMC group (P < 0.001). Patients in the WD/PICO group who had undergone previous colonoscopy reported significantly higher diet satisfaction and improved overall experience compared with their previous bowel preparation (P < 0.0001). Patients in the WD/PICO group also reported less bloating (P = 0.02), weakness (P = 0.046), hunger (P < 0.0001), and less interference to daily activities (P = 0.001).
Table 5.
Patient satisfaction with bowel preparation according to 5‐point visual analog scale
| Type of preparation | P‐value | ||
|---|---|---|---|
| WD/PICO (n = 125) | CF/PEG + SPMC (n = 125) | ||
| Understanding the diet, median (IQR) | 1 (1–2) (n = 100) |
1 (1–2) (n = 105) |
0.76 |
| Preparing food/fluids for the diet, median (IQR) | 1 (1–2) (n = 100) |
1 (1–2) (n = 104) |
0.39 |
| Sticking to/following the diet, median (IQR) | 1 (1–2) (n = 100) |
2 (1–3) (n = 102) |
<0.001 |
| Overall satisfaction with the diet, median (IQR) | 1 (1–2) (n = 98) |
2 (1–3) (n = 102) |
<0.001 |
| Previous colonoscopy, n (%) | 54 (54.0) (n = 100) |
65 (60.2) (n = 108) |
0.37 |
| Restricted to clear fluids last time, n (%) | 48 (90.6) (n = 53) |
54 (93.1) (n = 58) |
0.73 |
| Diet this time versus previous colonoscopy, median (IQR) | 1 (1–2) (n = 48) |
3 (3–3) (n = 53) |
<0.0001 |
| Overall experience this time versus previous colonoscopy, median (IQR) | 1 (1–3) (n = 50) |
3 (2–3) (n = 53) |
<0.0001 |
CF, clear fluid; IQR, interquartile range; PEG, polyethylene glycol; PICO, picosalax; SPMC, sodium picosulfate/magnesium citrate; WD, White Diet.
Table 6.
Patient tolerance to bowel preparation according to 5‐point visual analog scale
| Type of preparation | P‐value | ||
|---|---|---|---|
| WD/PICO (n = 125) | CF/PEG + SPMC (n = 125) | ||
| Bloating, median (IQR) | 1 (1–1) (n = 88) |
1 (1–2) (n = 100) |
0.02 |
| Abdominal cramping, median (IQR) | 1 (1–2) (n = 89) |
1 (1–2) (n = 101) |
0.28 |
| Nausea, median (IQR) | 1 (1–2) (n = 92) |
1 (1–3) (n = 101) |
0.22 |
| Headache, median (IQR) | 1 (1–2) (n = 95) |
2 (1–3) (n = 101) |
0.13 |
| Weakness, median (range) | 1 (1–4) (n = 91) |
1 (1–5) (n = 101) |
0.046 |
| Sleeping difficulty, median (IQR) | 1 (1–3) (n = 94) |
1 (1–3) (n = 101) |
0.42 |
| Hunger, median (IQR) | 1 (1–2) (n = 92) |
3 (2–4) (n = 104) |
<0.0001 |
| Interference with daily activities, median (IQR) | 2 (1–2) (n = 92) |
2 (1–3) (n = 104) |
0.001 |
| Vomiting post‐bowel preparation, n (%) | 5 (4.7) (n = 107) |
7 (6.4) (n = 110) |
0.59 |
CF, clear fluid; IQR, interquartile range; PEG, polyethylene glycol; PICO, picosalax; SPMC, sodium picosulfate/magnesium citrate; WD, White Diet.
Discussion
An adequate bowel preparation prior to colonoscopy is important as poor preparations are associated with missed adenomas,19 longer and more difficult procedures, higher rates of incomplete examinations and the need for repeat procedures or shorter surveillance intervals.20 The ideal bowel preparation would reliably cleanse the colon of all fecal material, be well tolerated by patients, be inexpensive, and have low risk of adverse events. Although a 4‐L split‐dose PEG preparation is considered the gold‐standard bowel preparation,21 PEG‐based preparations are often poorly tolerated, and side effects including bloating, nausea, and vomiting may lead to a failure to complete the preparation. Combination PEG and SPMC with a clear fluid diet the day before colonoscopy is the current standard bowel preparation at many Australian institutions and endoscopy centers. In this study, we performed a randomized, prospective, endoscopist‐blinded, non‐inferiority trial comparing a novel low‐residue White Diet with low‐volume, split‐dose Picosalax with a standard bowel preparation of day‐before clear fluids with combination PEG plus SPMC for morning colonoscopy. We found that low‐volume, split‐dose Picosalax with the White Diet was significantly better tolerated without detriment to bowel preparation success.
The White Diet, recently described by Butt et al.,15 is a pre‐colonoscopy low‐residue diet of white or cream‐colored foods. In that randomized controlled trial of 226 patients, the White Diet in conjunction with a 2‐L PEG with ascorbate bowel preparation was preferred by patients with less hunger and interference to daily activities without detriment to bowel preparation quality or colonoscopy performance compared with a clear fluid diet.15 Other randomized trials of diet liberalization during bowel preparation suggest that either low‐fiber13 or regular diet14 the day before colonoscopy with split‐dose bowel preparation is associated with improved tolerance and comparable or better colon cleansing compared with a clear fluid diet. In our study, patients taking the White Diet for 2 days reported higher overall satisfaction with the diet and higher satisfaction with the diet compared with any previous colonoscopy (>90% had clear fluid diet at previous colonoscopy). There was also significantly less bloating, weakness, hunger, and interruption to daily activities with the White Diet. Although the duration of the dietary restriction was longer in the White Diet group, our group has previously reported that the daily median energy intake with the White Diet was twice that of a clear fluid diet15 which may contribute to the improved tolerance compared with a 24‐h clear fluid diet. Furthermore, the use of white color as a guide to choose food is a simple strategy which patients found significantly easier to follow and the flexibility of the White Diet allows patients to individualize the diet according to personal dietary preferences. Importantly, patients taking the White Diet reported significantly greater ease in following the diet compared with clear fluids suggesting successful implementation of the diet. Improved patient satisfaction and tolerance during bowel preparation has several potential benefits including increased bowel preparation completion rates which may improve bowel preparation quality and increased likelihood of patients returning for surveillance or screening procedures.
In this study, patients taking the White Diet took a low‐volume (two sachets), split‐dose Picosalax bowel preparation, which was better tolerated and resulted in non‐inferior bowel preparation compared with day‐before clear fluids and 1 L PEG and two sachets of SPMC. Low‐volume SPMC has been shown to have greater tolerability and equal or greater efficacy compared with sodium phosphate,22 2 L PEG and bisacodyl,5, 6 2 L PEG with ascorbic acid,23 3 L of sulfate‐free PEG,24 and 4 L PEG preparations.25 Non‐inferiority of SPMC to PEG has also recently been shown in a meta‐analysis.26 Splitting the dose of either PEG or SPMC is preferred by patients and increases the quality of the bowel preparation compared with day‐before regimens.12, 27 A split‐dose bowel preparation for all patients or same day preparation for afternoon colonoscopy has been recommended in recent guidelines.28 Although well tolerated and safe in most, there is a small increased risk of hyponatremia with SPMC in the elderly29 and, therefore, PEG‐based bowel preparation may be more appropriate in these patients.
There are some limitations to our study. We note the low bowel preparation success rates for both the WD/PICO (64.3% per protocol and 57.6% ITT) and CF/PEG + SPMC groups (65.3% per protocol and 61.6% ITT). Bowel preparation success in our study was defined by an Ottawa bowel preparation score ≤ 6 (range: 0–14), which may have been too stringent for determining adequacy of the bowel preparation. Recently, the US Multi‐Society Task Force on CRC defined adequate bowel preparation as one that enables the endoscopist to follow the recommended screening and surveillance guidelines and the ability to detect lesions >5 mm in size (target of ≥85%).30 Alternately, by including patients with risk factors for poor bowel preparation such as diabetes, opioid use, and laxative use (as a marker of constipation),31 bowel preparation success rates in our study may have been lowered. A limitation of this study was that we compared groups with different dietary regimens (White Diet vs clear fluids), bowel preparation types (low‐volume Picosalax vs combination PEG/SPMC), and bowel preparation dose timing (split‐dose vs day‐before) which makes it difficult to determine which of the study variables resulted in the improved tolerance of the bowel preparation found in the WD/PICO group. A further limitation of our study was that the bowel preparation success rate of 75% used in our sample size calculation was higher than the bowel preparation success rates found in our study which may have resulted in a smaller sample size and therefore reduced the power of our study. Finally, our study was a single‐center, non‐inferiority controlled trial and not powered to detect a significant difference in bowel preparation quality between groups.
In conclusion, a novel bowel preparation with the White Diet and low‐volume, split‐dose Picosalax was non‐inferior for successful bowel preparation and better tolerated compared with a preparation of clear fluids and day‐before combination PEG/SPMC. By utilizing modern bowel preparation strategies prior to colonoscopy such as dietary liberalization with the White Diet, split‐dosing, and low‐volume regimens, bowel preparations will be better tolerated by patients without compromising cleansing quality.
Acknowledgment
The authors disclose that Picosalax was provided by the manufacturer Ferring Pharmaceuticals, Melbourne, Australia.
References
- 1. Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin. Gastroenterol. Hepatol. 2009; 7: 770–5. [DOI] [PubMed] [Google Scholar]
- 2. Winawer SJ, Zauber AG, Ho MN et al Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N. Engl. J. Med. 1993; 329: 1977–81. [DOI] [PubMed] [Google Scholar]
- 3. Zauber AG, Winawer SJ, O'Brien MJ et al Colonoscopic polypectomy and long‐term prevention of colorectal‐cancer deaths. N. Engl. J. Med. 2012; 366: 687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim HG, Huh KC, Koo HS et al Sodium picosulfate with magnesium citrate (SPMC) plus laxative is a good alternative to conventional large volume polyethylene glycol in bowel preparation: a multicenter randomized single‐blinded trial. Gut Liver. 2014; 9: 494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rex DK, Katz PO, Bertiger G et al Split‐dose administration of a dual‐action, low‐volume bowel cleanser for colonoscopy: the SEE CLEAR I study. Gastrointest. Endosc. 2013; 78: 132–41. [DOI] [PubMed] [Google Scholar]
- 6. Katz PO, Rex DK, Epstein M et al A dual‐action, low‐volume bowel cleanser administered the day before colonoscopy: results from the SEE CLEAR II study. Am. J. Gastroenterol. 2013; 108: 401–9. [DOI] [PubMed] [Google Scholar]
- 7. Hoy SM, Scott LJ, Wagstaff AJ. Sodium picosulfate/magnesium citrate: a review of its use as a colorectal cleanser. Drugs. 2009; 69: 123–36. [DOI] [PubMed] [Google Scholar]
- 8. Song KH, Suh WS, Jeong JS et al Effectiveness of sodium picosulfate/magnesium citrate (PICO) for colonoscopy preparation. Ann. Coloproctol. 2014; 30: 222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El Sayed AM, Kanafani ZA, Mourad FH et al A randomized single‐blind trial of whole versus split‐dose polyethylene glycol‐electrolyte solution for colonoscopy preparation. Gastrointest. Endosc. 2003; 58: 36–40. [DOI] [PubMed] [Google Scholar]
- 10. Marmo R, Rotondano G, Riccio G et al Effective bowel cleansing before colonoscopy: a randomized study of split‐dosage versus non‐split dosage regimens of high‐volume versus low‐volume polyethylene glycol solutions. Gastrointest. Endosc. 2010; 72: 313–20. [DOI] [PubMed] [Google Scholar]
- 11. Park JS, Sohn CI, Hwang SJ et al Quality and effect of single dose versus split dose of polyethylene glycol bowel preparation for early‐morning colonoscopy. Endoscopy. 2007; 39: 616–9. [DOI] [PubMed] [Google Scholar]
- 12. Martel M, Barkun AN, Menard C, Restellini S, Kherad O, Vanasse A. Split‐dose preparations are superior to day‐before bowel cleansing regimens: a meta‐analysis. Gastroenterology. 2015; 149: 79–88. [DOI] [PubMed] [Google Scholar]
- 13. Sipe BW, Fischer M, Baluyut AR et al A low‐residue diet improved patient satisfaction with split‐dose oral sulfate solution without impairing colonic preparation. Gastrointest. Endosc. 2013; 77: 932–6. [DOI] [PubMed] [Google Scholar]
- 14. Aoun E, Abdul‐Baki H, Azar C et al A randomized single‐blind trial of split‐dose PEG‐electrolyte solution without dietary restriction compared with whole dose PEG‐electrolyte solution with dietary restriction for colonoscopy preparation. Gastrointest. Endosc. 2005; 62: 213–8. [DOI] [PubMed] [Google Scholar]
- 15. Butt J, Bunn C, Paul E, Gibson PR, Brown G. The White Diet is preferred, better tolerated and non‐inferior to a clear‐fluid diet for bowel preparation: a randomised controlled trial. J. Gastroenterol. Hepatol. 2016; 31: 355–63. [DOI] [PubMed] [Google Scholar]
- 16. Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest. Endosc. 2004; 59: 482–6. [DOI] [PubMed] [Google Scholar]
- 17. Ell C, Fischbach W, Bronisch HJ et al Randomized trial of low‐volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am. J. Gastroenterol. 2008; 103: 883–93. [DOI] [PubMed] [Google Scholar]
- 18. Bitoun A, Ponchon T, Barthet M, Coffin B, Dugue C, Halphen M. Results of a prospective randomised multicentre controlled trial comparing a new 2‐L ascorbic acid plus polyethylene glycol and electrolyte solution vs. sodium phosphate solution in patients undergoing elective colonoscopy. Aliment. Pharmacol. Ther. 2006; 24: 1631–42. [DOI] [PubMed] [Google Scholar]
- 19. Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest. Endosc. 2012; 75: 1197–203. [DOI] [PubMed] [Google Scholar]
- 20. Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am. J. Gastroenterol. 2002; 97: 1696–700. [DOI] [PubMed] [Google Scholar]
- 21. Enestvedt BK, Tofani C, Laine LA, Tierney A, Fennerty MB. 4‐Liter split‐dose polyethylene glycol is superior to other bowel preparations, based on systematic review and meta‐analysis. Clin. Gastroenterol. Hepatol. 2012; 10: 1225–31. [DOI] [PubMed] [Google Scholar]
- 22. Hookey LC, Vanner SJ. Pico‐salax plus two‐day bisacodyl is superior to pico‐salax alone or oral sodium phosphate for colon cleansing before colonoscopy. Am. J. Gastroenterol. 2009; 104: 703–9. [DOI] [PubMed] [Google Scholar]
- 23. Yoo IK, Lee JS, Chun HJ et al A randomized, prospective trial on efficacy and tolerability of low‐volume bowel preparation methods for colonoscopy. Dig. Liver Dis. 2015; 47: 131–7. [DOI] [PubMed] [Google Scholar]
- 24. Regev A, Fraser G, Delpre G et al Comparison of two bowel preparations for colonoscopy: sodium picosulphate with magnesium citrate versus sulphate‐free polyethylene glycol lavage solution. Am. J. Gastroenterol. 1998; 93: 1478–82. [DOI] [PubMed] [Google Scholar]
- 25. Kao D, Lalor E, Sandha G et al A randomized controlled trial of four precolonoscopy bowel cleansing regimens. Can. J. Gastroenterol. 2011; 25: 657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jin Z, Lu Y, Zhou Y, Gong B. Systematic review and meta‐analysis: sodium picosulfate/magnesium citrate vs. polyethylene glycol for colonoscopy preparation. Eur. J. Clin. Pharmacol. 2016; 72: 523–32. [DOI] [PubMed] [Google Scholar]
- 27. Manes G, Repici A, Hassan C. Randomized controlled trial comparing efficacy and acceptability of split‐ and standard‐dose sodium picosulfate plus magnesium citrate for bowel cleansing prior to colonoscopy. Endoscopy. 2014; 46: 662–9. [DOI] [PubMed] [Google Scholar]
- 28. Saltzman JR, Cash BD, Pasha SF et al Bowel preparation before colonoscopy. Gastrointest. Endosc. 2015; 81: 781–94. [DOI] [PubMed] [Google Scholar]
- 29. Weir MA, Fleet JL, Vinden C et al Hyponatremia and sodium picosulfate bowel preparations in older adults. Am. J. Gastroenterol. 2014; 109: 686–94. [DOI] [PubMed] [Google Scholar]
- 30. Johnson DA, Barkun AN, Cohen LB et al Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the US Multi‐Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2014; 109: 1528–45. [DOI] [PubMed] [Google Scholar]
- 31. Dik VK, Moons LM, Huyuk M et al Predicting inadequate bowel preparation for colonoscopy in participants receiving split‐dose bowel preparation: development and validation of a prediction score. Gastrointest. Endosc. 2015; 81: 665–72. [DOI] [PubMed] [Google Scholar]


