Abstract
Recent advancement in the understanding of the pathophysiology of inflammatory bowel disease has seen an expansion in therapeutic options. Vedolizumab, a selective α4β7 inhibitor, and ustekinumab, an IL 12/23 p40 inhibitor, have provided the much‐awaited out‐of‐class alternatives for patients who have failed or who are intolerant to anti‐Tumor Necrosis Factor (TNF) therapy. However, questions remain as to how we may best use these novel therapeutic agents. We evaluate the evidence available from randomized controlled trials and postmarketing cohort studies and discuss their safety, efficacy, and limitations, in relation to anti‐TNF therapy, in optimizing the treatment outcomes.
Keywords: anti‐TNF, inflammatory bowel disease, ustekinumab, vedolizumab
Introduction
Inflammatory bowel disease (IBD) is a chronic idiopathic inflammatory condition of the intestines of a relapsing and remitting nature. IBD is often associated with high morbidity due to both disease‐ and treatment‐related complications. Up to 50% of patients with Crohn's disease (CD) and 30% of patients with ulcerative colitis (UC) may require surgery in their life time.1 The addition of anti‐TNF therapies to the treatment armamentarium since 1997 was a paradigm shift, especially in the setting of fistulizing CD and acute severe colitis.2 This is the first medication class that changes natural history by decreasing the risk of surgery and hospitalization, especially when used early in the treatment course.3
However, up to one‐third of patients do not respond to anti‐TNF agents.2 For patients who respond initially, approximately 40% ultimately stop responding due to subtherapeutic drug levels, the development of antidrug antibodies, or because of mechanistic escape where another cytokine may become more important in disease pathogenesis.4 Furthermore, a small minority of patients may require change in treatment class due to adverse effects, for example, drug‐induced lupus, psoriasis, or demyelinating disease.
Vedolizumab, an anti‐integrin α4β7 antibody, and ustekinumab, an anti IL12/23 p40 antibody, now offer alternatives to patients in whom a change in treatment class is needed. In this article, we review the efficacy and safety data of vedolizumab and ustekinumab, as well as their limitations in guiding clinical decision on choices of biologics.
Vedolizumab
Efficacy
Registration trials and postmarketing reports
Vedolizumab is the first gut‐selective biological agent. It inhibits integrin α4β7, thus inhibiting lymphocyte trafficking from the blood vessel to the intestine. Its efficacy in induction and maintenance of remission for CD and UC, both in anti‐TNF‐naïve and anti‐TNF‐experienced patients, is well documented in registration trials and postmarketing cohort studies (Table 1). The efficacy of vedolizumab in CD at the induction phase was, however, modest in GEMINI 2. Although the vedolizumab group achieved a modest but significantly higher remission rate than the placebo, (14.5% vs 6.8%, respectively, P = 0.02), the CDAI‐100 response rate was not significant.5 This is likely because of CD being a transmural disease and the slow inductive effect of vedolizumab. Rather than having an end‐point at week 6, an end‐point at week 14 may be more appropriate, where a peak effect would be expected.6 Furthermore, up to 50% of the study cohort did not receive concomitant corticosteroids, which could have improved the response rate.7 Although there appears to be a lower percentage point difference with the placebo in CD than in UC during the induction phase, the percentage point difference with the placebo at week 52 appears to be comparable for both diseases.8, 9
Table 1.
Efficacy data of vedolizumab in registration trials and postmarketing cohort studies
| Study | Design | Patients | Anti‐TNF experienced | Efficacy |
|---|---|---|---|---|
| Registration trials | ||||
| Feagan et al.9 (GEMINI 1) | Randomized controlled trial, phase 3 | UC: 895 | TNF failure: 48% | At W6: |
| Clinical response: 47.1% (VDZ) vs 25.5% (PBO), P < 0.001 | ||||
| At W52: | ||||
| Clinical remission: 41.8% (VDZ q8w) vs 44.8% (VDZ q4w) vs 24.8% (PBO), P < 0.001 | ||||
| Sandborn et al.5 (GEMINI 2) | Randomized controlled trial, phase 3 | CD: 1115 | TNF failure: 63.8% | At W6: |
| Clinical remission: 14.5% (VDZ) vs 6.8% (PBO), P = 0.02 | ||||
| CDAI‐100 response: 31.4% (VDZ) vs 25.7% (PBO), P = 0.23 | ||||
| Sands et al.80 (GEMINI 3) | Randomized controlled trial, phase 3 | CD: 416 | TNF failure: 75.7% | At W6: |
| Clinical remission: 15.2% (VDZ) vs 12.1% (PBO), P = NS | ||||
| At W10: | ||||
| Clinical remission: 26.6% (VDZ) vs 12.1%, P = 0.001 | ||||
| Sands et al.8 | GEMINI 2 and 3 trials (post hoc analysis) | CD: 1476 | TNF naïve: 35% | Clinical remission at W52: |
| TNF naïve: 48.9% (vs 26.8% PBO) | ||||
| TNF failure: 65% | ||||
| TNF failure: 27.7% (vs 12.8% PBO) | ||||
| Noman et al.81 (GEMINI LTS) | Single arm, open label, phase 3 | CD: 24 | TNF failure: 100% | Mucosal healing: |
| UC: 34 | ||||
| CD: 29% (median 33 months) | ||||
| UC: 50% (median 31 months) | ||||
| Postmarketing cohort studies | ||||
| Allegretti et al.17 | Retrospective multicenter | CD: 96 UC: 40 |
Not clear | At W54: |
| CD: Clinical response/remission: 52% | ||||
| UC: Clinical response/remission: 65% | ||||
| Amiot et al.82 | Retrospective multicenter | CD: 173 UC: 121 |
TNF failure: 99% | At W14: |
| CD: SFCR: 31% Clinical response: 51% | ||||
| UC: SFCR: 36% Clinical response: 50% | ||||
| Amiot et al.83 | Prospective multicenter | CD: 161 UC: 111 |
TNF failure: 99% | At W54 (SFCR): |
| CD: 27.2% UC: 40.5% | ||||
| Baumgart et al.84 | Prospective multicenter | CD: 97 UC: 115 |
TNF failure: | At W14 (clinical remission): |
| CD: 94.8% UC: 75.7% | ||||
| CD: 23.7% UC: 23.5% | ||||
| Dulai et al.12 (US VICTORY) | Retrospective multicenter | CD: 212 | TNF failure: 90% | At W52: |
| Clinical remission: 35% Mucosal healing: 63% Deep remission†: 26% | ||||
| Eriksson et al.85 (SWIBREG) | Prospective multicenter national registry | CD: 147 UC: 92 IBD‐U: 7 |
TNF failure: 86% | At median follow up of 17 (IQR: 14–20) months: |
| CD: 54% (clinical remission) UC: 64% (clinical remission) | ||||
| Kopylov et al.86 | Prospective multicenter | CD: 130 UC: 69 |
TNF failure: 92.6% | At W14: |
| CD: Clinical remission: 34.6% SFCR: 29.2% | ||||
| UC: Clinical remission 28.4% SFCR: 24.3% | ||||
| Shelton et al.87 | Prospective multicenter | CD: 107 UC: 59 IBD‐U: 6 |
TNF failure: 97.1% | At W14: |
| CD: Clinical response: 48.9% Clinical remission: 23.9% | ||||
| UC: Clinical response: 53.9% Clinical remission: 29.3% | ||||
| Singh et al.88 | Retrospective multicenter | CD: 30 UC: 22 |
TNF failure: 90% | At W14: |
| CD: Clinical remission:42% | ||||
| UC: Clinical remission: 76% | ||||
| Stallmach et al.89 | Prospective multicenter | CD: 67 UC: 60 |
TNF failure: | At W54 (clinical remission): |
| CD: 91% UC: 81.7% | ||||
| CD: 21% UC: 25% | ||||
| Vivio et al.90 | Prospective single center | CD: 30 UC: 21 |
TNF failure: | At W14: |
| CD: 96.7% UC: 76.2% | ||||
| CD: improvement in CDAI score (mean 35 points, P = 0.04) UC: clinical remission (55%) | ||||
Deep remission: clinical remission + mucosal healing.
MH, mucosal healing; PBO, placebo; SFCR, steroid‐free clinical remission.
Long‐term efficacy data of patients in the GEMINI 1 and two studies who received 152 weeks of continuous vedolizumab were reported in GEMINI LTS. These included patients who responded at week 6 of the GEMINI study and completed 1 year of maintenance vedolizumab (either every 8 weeks or every 4 weeks) before receiving open‐label vedolizumab every 4 weeks in GEMINI LTS. When data were analyzed conservatively, with incomplete data considered to represent treatment failures, the clinical remission for CD (GEMINI 2) was 71, 69, and 43% at weeks 52, 104, and 152, respectively.10 For UC (GEMINI 1), clinical remission was 74, 78, and 46% at week 52, 104, and 152, respectively.11 Anti‐TNF‐naïve patients responded better to vedolizumab compared with anti‐TNF‐experienced CD patients.8, 10 For UC, however, both groups appeared to have responded equally well.11 For patients where vedolizumab treatment was interrupted for medical or nonmedical reasons, retreatment was successful for both CD and UC patients with no increase in anti‐vedolizumab antibody regardless of the duration of time from last vedolizumab dose.10 Similar to anti‐TNF agents, a relatively lower vedolizumab level was noted in the secondary nonresponders in the GEMINI 2 cohort. Shortening the dosing interval to every 4 weeks could be considered salvage therapy, with up to 45% of the treatment response recaptured in the GEMINI LTS cohort.10
Mucosal healing
Significant mucosal healing in UC with vedolizumab was reported in the GEMINI 1 trial at week 6 [40.9% (vedolizumab) vs 24.8% with placebo, P = 0.001] and week 52 (56% [vedolizumab Q4W] vs 51.6% [vedolizumab Q8W] vs 19.8% [placebo], P < 0.001).9 While endoscopy data were not reported in the GEMINI 2 and 3 trials, GEMINI LTS has observed mucosal healing in 29% patients at a median of 33 months for CD.8 A postmarketing report observed mucosal healing in 63% of patients at week 52 for CD.12
Immunogenicity
Overall immunogenicity for vedolizumab appears to be low in comparison with conventional anti‐TNF agents. When patients who received/had not received concomitant immunosuppressant at baseline were compared, the risk of developing anti‐vedolizumab antibody was only marginally lower, 3% versus 4%.13
Combination therapy with immunomodulator
The superiority of combination therapy with an immunomodulator over monotherapy with use of biologics was first reported in SONIC (infliximab and azathioprine in CD) and UC SUCCESS (infliximab and azathioprine in UC).14, 15 The benefit of its use with vedolizumab is, however, less clear. Post hoc analyses from GEMINI 1 and 2 trials did not reveal any additional clinical benefit with combination therapy, both at week 6 and week 52.7, 16 A separate retrospective study of 136 patients observed higher clinical response or remission at week 54 for CD patients who received combination therapy but not patients with UC.17 Prospective data will be required to confirm any synergistic effect with combination therapy or whether the long‐term benefit is only a result of the reduced immunogenicity. With the coadministration of an immunomodulator, it should be considered that the benefit of gut‐selective immunosuppression with vedolizumab will be negated.
Role of therapeutic drug monitoring (TDM)
Based on the premise of the drug exposure–clinical efficacy relationship, the measurement of an active drug level and antidrug antibodies has been increasingly used, especially for anti‐TNF therapies. However, the utility of TDM for vedolizumab is unclear. To achieve clinical remission at week 6, post hoc analysis from GEMINI trials observed that a minimum serum vedolizumab trough of 17.1 μg/mL is required for UC and 16.0 μg/mL for CD.6 A separate prospective study observed that a median trough level of >18 μg/mL at week 6 is less likely to be associated with the need for further vedolizumab dose optimization within the first 6 months.18 This echoes the observation of further response recapturing when the vedolizumab administration interval is shortened from every 8 to every 4 weeks.10 While a higher vedolizumab trough level is beneficial, its relationship with clinical remission is not linear, especially for CD, as observed from the GEMINI trials.6 As little as 2 mg/kg of vedolizumab is sufficient to saturate most α4β7 integrin receptors. Furthermore, receptor occupancy does not correlate with treatment response.13, 19 It is unclear if this may be related to other unknown mechanisms of action that may benefit from a higher level of vedolizumab.19
Perianal CD
Prospective data on the efficacy of vedolizumab in perianal CD is currently limited. Post hoc analysis from the GEMINI 2 trial reported higher sustained perianal fistula closure at week 52 for patients who received vedolizumab Q8W versus placebo, 41.2% (95% CI: 18.4–67.1) versus 11.1% (95% CI: 1.4–34.7), respectively.20 A separate retrospective cohort study observed 34.3% complete remission of perianal disease at week 54.21 A specifically designed trial on the efficacy of vedolizumab on perianal fistulizing CD is currently in progress (http://clinicaltrials.gov Identifier: NCT02630966).
Primary sclerosing cholangitis
Due to the expression of MadCAM‐1 receptors in the liver, it was thought that the inhibition of α4β7 by vedolizumab may have a potential therapeutic role in patients with primary sclerosing cholangitis (PSC). However, evidence of clinical efficacy is lacking. Two abstracts were presented recently, with one reporting no change in liver chemistry, whereas the second abstract observed an increasing trend of serum alkaline phosphatase at the 30‐week follow up.22, 23
Safety
A recent systematic review of vedolizumab‐exposed patients from six registration trials (2830 patients) and six postmarketing cohort studies (1049 patients) demonstrated no increase in adverse events (AE) or serious adverse events (SAE) in the vedolizumab‐exposed group.24 Follow up ranged from 10 weeks to 46 months for the registration trials and 14 to 53 weeks for the cohort studies. Risk of SAE for the vedolizumab‐exposed group was 20.0/100 patient years (95% CI, 18.5–21.5) versus 28.3/100 patient years (95% CI, 20.6–35.9) for the placebo group.
Infection
Nasopharyngitis was the most common AE reported. This is, however, not higher as compared with placebo, 13.5/100 PY (95% CI, 12.3–14.7) versus 14.1/100 PY (95% CI, 8.8–19.3), respectively.24 All other infections were comparable between both cohorts. When assessed using the Cox proportional hazards model, independent risk factors of serious infection for UC included prior anti‐TNF therapy and narcotics use. For CD, younger age, concomitant steroid and narcotic use were predictors of serious infection.25
Unlike natalizumab, an anti‐α4 integrin antibody, patients administered vedolizumab do not develop progressive multifocal leukoencephalopathy (PML) from JC virus reactivation in the central nervous system (CNS). In addition to inhibiting the trafficking of gut‐specific lymphocytes, it is believed that natalizumab inhibits lymphocyte trafficking to the CNS by targeting integrin α4β1‐positive cells. A single dose of natalizumab has been observed to reverse the CD4/CD8 ratio in the cerebrospinal fluid.26 This has not been observed in patients who received vedolizumab.27 To date, there is no reported case of vedolizumab‐related PML.25
Malignancy
There has been a theoretical concern for GI malignancies due to reduced immunosurveillance in the gut. From the registration trials of 2830 patients, there were 18 malignancies reported, 6 of which were gastrointestinal. However, the risk of malignancies were not significantly higher when compared with anti‐TNF agents using placebos as common comparators (OR: 0.87; 95% CI, 0.26–2.88).28 In a postmarketing surveillance study, 25 malignancies were reported, half of which were gastrointestinal. Of note, this was not higher when compared with the background risk. Furthermore, most of the malignancies reported in the cohorts were patients with short vedolizumab exposure of less than 6 months, tobacco users, or those who had a previous history of malignancy prior to vedolizumab treatment.25 While vedolizumab is not thought to be associated with increased risk of malignancy, more long‐term data is required.
Safety of vedolizumab in special populations
Children
Children <18 years old were excluded from the registration trials. Postmarketing case series demonstrate that vedolizumab is safe in children.29 ESPGHAN recently published the largest multicenter case series of 64 children, mean 14.5 (range 2–18) years old and treated with vedolizumab. No serious drug‐related AEs were reported at a median follow up of 24 weeks (IQR 14–38, range 6–116).30
Elderly
Post hoc analysis of the GEMINI 1 and 2 trials was performed, with patients stratified by age into <35, 35–54, and > 55 years old.31 Of the entire cohort, 11% (220/2010) were > 55 years old. Safety profile was similar across all age groups for vedolizumab and placebo. A separate, single‐center, retrospective study showed that vedolizumab is safe and efficacious in 29 patients (10 UC and 19 CD) who were more than 60 years old.32 A total of 41% achieved clinical remission at week 52, while four patients (13.8%) developed pneumonia, clostridium difficile infection, and flu‐like symptoms.
Pregnancy and lactation
Animal studies using supratherapeutic doses of vedolizumab did not report an increased risk of teratogenicity, still birth, impaired intrauterine growth, or postnatal physical development up to 6 months of age.33 Data from vedolizumab trials (pregnancies in 27 female participants, 19 with male partners exposed to vedolizumab) and postmarketing reports (81 pregnancies) were published recently.34 The exposure of female trial participants to vedolizumab was limited as vedolizumab was terminated once pregnancy was confirmed. In postmarketing reports, pregnancy outcomes were only available in 15 pregnancies (4 live birth and 11 spontaneous miscarriages), while remaining 66 had either ongoing pregnancies or undocumented outcomes.34
Vedolizumab was detected in the milk of lactating monkeys, but there is currently no human data available.33 A human lactation study on vedolizumab is currently in progress (http://clinicaltrials.gov identifier NCT02559713).
Perioperative setting
Safety data of vedolizumab in the perioperative setting have so far been conflicting. While vedolizumab's gut selectivity does not result in systemic immunosuppression, there are theoretical concerns that the inhibition of lymphocyte trafficking may impede intestinal healing (Table 2). Lightner et al. observed that perioperative vedolizumab use was associated with higher surgical site infection. However, their vedolizumab cohort was sicker, had higher median platelet levels, was more likely to be on a concomitant immunomodulator, and had less primary anastomosis and more ostomy. It is uncertain if these could have confounded the findings.35 A more recent study by Yamada et al. and a pediatric study from the Mayo Clinic, however, did not show an increased risk of infection with preoperative vedolizumab exposure.36, 37
Table 2.
Perioperative safety outcomes with vedolizumab
| Study | Design | Patient numbers | Types of IBD | Duration | Predictors of postop complication | Conclusion |
|---|---|---|---|---|---|---|
| Lightner et al.35 | Retrospective, single center (VDZ vs anti‐TNF/nonbiologics) | VDZ: 94 Anti‐TNF: 126 Nonbiologics: 172 |
CD and UC | 30 days | VDZ use | VDZ group has higher rates of postoperative infection: 53% vs 33% anti‐TNF, 28% non‐biologics, P < 0.001 |
| Lightner et al.(2)37 | Retrospective single center (VDZ vs anti TNF agents) *pediatric cohorts (≤18 years) |
VDZ: 13 Anti‐TNF: 36 |
CD and UC | 30 days | Age > 50 years | No increased risk with vedolizumab use |
| Yamada et al.36 | Retrospective single center (VDZ vs anti‐TNF/nonbiologics) | VDZ: 64 anti‐TNF: 129 Nonbiologics: 250 |
CD and UC | 30 days | Age > 65, low albumin | No increased risk with vedolizumab use |
VDZ, vedolizumab.
Pitfalls with vedolizumab
Slow onset of action
The clinical response with vedolizumab is slow in comparison with anti‐TNF therapies. While the clinical response in relation to placebo is reported to be significantly different at week 6, the peak effect may not be expected till weeks 10–14.6 This is not unexpected given vedolizumab's unique mechanism in inhibiting lymphocyte trafficking rather than directly inhibiting cytokine activity.
Post hoc analysis of the GEMINI 2 trial observed synergistic efficacy at week 6 for CD when corticosteroid was used in addition to vedolizumab. Patients who received corticosteroid with vedolizumab or a combination of corticosteroid, immunomodulator, and vedolizumab at baseline were more likely to be in clinical remission at week 6 in comparison with patients who received vedolizumab alone or vedolizumab in combination with immunomodulator.7 While this additional benefit was not observed in UC from the GEMINI 1 post hoc analysis, a prospective study will be required as GEMINI trials were not specifically designed to assess outcomes with combination therapy.16
Use of combination treatment with anti‐TNF therapy or tacrolimus (in the setting of acute severe UC) to overcome the delayed efficacy of vedolizumab has been reported.38, 39 Favorable long‐term efficacy and safety outcomes of up to 21 months of combination therapy with certolizumab (used off label) have been reported in UC.40 To confirm its utility, dedicated clinical trials are currently in progress. This includes the use of vedolizumab monotherapy versus combination therapy with tacrolimus for moderate‐to‐severe UC (http://clinicaltrials.gov Identifier: NCT02954159) and triple therapy with anti‐TNF therapy and methotrexate for CD (http://clinicaltrials.gov Identifier: NCT02764762).
Ustekinumab
Efficacy
Ustekinumab is a fully human monoclonal antibody targeting the common p40 subunit of IL‐12 and IL‐23. Through the inhibition of IL‐23, ustekinumab limits the differentiation of naïve T cells to TH17 cells. While clinical efficacy was well documented in the CERTIFI and UNITI trials, a more rapid clinical response at induction was observed when ustekinumab is administered intravenously (in comparison with subcutaneous administration) and at higher doses. Patients are then maintained subcutaneously at a lower dose, which offers the benefit of convenience to the patients.
There is as yet no direct head‐to‐head comparison with anti‐TNF therapies. However, a significant clinical response has been observed both for biologic naïve and experienced patients, as summarized in Table 3.
Table 3.
Efficacy data of ustekinumab for Crohn's disease in registration trials and postmarketing cohort studies
| Study | Design | Patients | Anti‐TNF experienced | Efficacy |
|---|---|---|---|---|
| Registration trials | ||||
| Sandborn et al. (CERTIFI)91 | RCT (phase 2b) Induction: IV UST 1 mg/kg vs 3 mg/kg vs 6 mg/kg vs placebo Maintenance: SC UST 90 mg at week 8 and 16 |
CD: 526 | TNF failure: 99.7% | Clinical response at week 6: |
| 1 mg/kg: 36.6% (P = 0.02) 3 mg/kg: 34.1% (P = 0.06) 6 mg/kg: 39.7% (P = 0.005) Placebo: 23.5% | ||||
| Clinical response at week 22: | ||||
| 69.4% vs 42.5% (placebo) | ||||
| Clinical remission at week 22: | ||||
| 41.7% vs 27.4% (placebo) | ||||
| Feagan et al. (UNITI‐1, UNITI‐2, and IM‐UNITI)43 | RCT (phase 3) Induction UNITI‐1 (patients failing anti‐TNF therapy) IV UST 130 mg vs 6 mg/kg vs placebo |
CD: 741 | TNF failure: 99.2% | Clinical response at week 6: |
| IV UST 130 mg/kg: 34.4% IV UST 6 mg/kg: 33.7% Placebo: 21.5% | ||||
| UNITI‐2 (patients failing conventional therapy) IV UST 130 mg vs 6 mg/kg vs placebo |
CD: 628 | TNF failure: 32% | Clinical response at week 6: | |
| IV UST 130 mg/kg: 51.7% IV UST 6 mg/kg: 55.5% Placebo: 28.7% | ||||
| Maintenance (IM‐UNITI) SC UST 90 mg Q8W vs Q12W vs placebo |
CD: 397 | TNF failure: 60.5% | Clinical remission at week 44: | |
| SC UST Q8W: 53.1% (P = 0.005) SC UST Q12W: 48.8% (P = 0.04) Placebo: 35.9% | ||||
| Postmarketing cohort studies | ||||
| Harris et al.92 | Retrospective multicenter (US) | CD: 45 | TNF failure: 100% | At 3 months: |
| Clinical response:46% Clinical remission: 35% | ||||
| Khorrami et al.44 | Retrospective multicenter (Spain) | CD: 116 | TNF failure: 100% | Clinical response: |
| After induction: 83.6% At 6 months: 76.4% At 12 months: 63.6% | ||||
| Kopylov et al.93 | Retrospective multicenter (Montreal, Canada) | CD: 38 | TNF failure: 100% | Clinical response: |
| At 3 months: 73.7% At 6 months: 64.5% At 12 months: 47.4% | ||||
| Ma et al.42 | Retrospective multicenter (Alberta, Canada) | CD: 167 | TNF failure: 95.2% | Steroid‐free clinical response/remission: |
| At 3 months: 38.9%, 15.0% At 6 months: 60.3%, 25.2% At 12 months: 59.5%, 27.9% | ||||
| Endoscopic/radiographic response: | ||||
| At 6 months: 54.5% At 12 months: 55.8% | ||||
| Wils et al.63 | Retrospective multicenter (GETAID, France/Swiss) | CD: 122 | TNF failure: 100% | Clinical benefits †: |
| At 3 months: 65% | ||||
Defined as reductions in symptoms, biochemical markers, steroid free, and without surgery or immunosuppressant therapies.
UST, ustekinumab.
Mucosal healing
Endoscopic healing was reported in a post hoc analysis of UNITI trials.41 Colonoscopy was performed at baseline (week 0), week 8, and week 52 (IM‐UNITI week 44). At week 8, the endoscopic response, defined by ≥3 points reduction of SES‐CD, was significantly higher in the ustekinumab group compared with the placebo group, 47.7% versus 29.9%, P < 0.01. A sustained response in the ustekinumab group was observed at week 52, with 50% reduction in SES‐CD in 33.3% (q8w group) versus 17.0% (q12w group) versus 13.7% (placebo group), P = 0.01. A postmarketing cohort study from Canada reported an endoscopic response and remission of 55.4 and 27.2%, respectively, at median follow up of 46 weeks.42
Role in perianal CD
While up to 43% of patients in UNITI‐1 and 30% of patients in UNITI‐2 trials had perianal disease, the efficacy of ustekinumab in patients with perianal CD was not specifically addressed.43 Limited real‐world data have reported favorable efficacy, with a clinical improvement rate of 61–100%.44, 45 It is possible that this cohort of patients may benefit from a higher ustekinumab trough level, but more data will be required.42, 45
Safety
Ustekinumab is approved for use in CD in North America and Europe since 2016. While long‐term safety data are limited in CD, safety data are available on its use in psoriasis/psoriatic arthritis.46 Ustekinumab, which is used at a lower dose in psoriasis, appears to be safe, with no increased risk of AEs, SAEs, infection, and malignancy. Data from clinical trials (phase 2 CERTIFI, phase 3 UNITI) demonstrated a similarly favorable safety profile with no increased risk in comparison with placebo‐treated participants.
Infection
Four cases of opportunistic infections were reported during the UNITI trials: one case of listeria meningitis (patient received concomitant ustekinumab and oral prednisone 30 mg daily), two cases of esophageal candidiasis (one patient who received ustekinumab 90 mg every 8 weeks and one patient who received placebo and concomitant prednisone), and a case of pulmonary tuberculosis occurring 10 months after the administration of a single dose of ustekinumab 130 mg intravenously.43
Using phases 2 and 3 clinical trials data for ustekinumab across all indications (CD, psoriasis, and psoriatic arthritis), the overall incidence rates of active tuberculosis was low compared with patients treated with anti‐TNF agents—0.02 per 100 patient years; (95% CI: 0.00, 0.06) versus 0.28 (95% CI: 0.21, 0.37), respectively.47 With isoniazid prophylaxis, ustekinumab treatment appeared to be safe with no latent TB reactivation in a systematic review of five clinical trials (3177 patients).48
Malignancy
The 5‐year safety data of 3117 ustekinumab‐treated psoriasis patients reported 47 cases of non‐melanoma skin cancer (NMSC).46 The risk of NMSC with ustekinumab is, however, not increased in comparison with the placebo‐treated cohort, 0.48 versus 0.52 per 100 patient years, respectively. Furthermore, there is no reversal of the BCC and SCC ratio (4:1), as expected in the general population.49 Over the 5‐year follow up, there was no time‐ or dose‐dependent (45 mg vs 90 mg) difference observed.46 Despite the use of higher doses for the CD cohort, no increased risk of NMSC was noted in comparison with the placebo cohort in the registration trials.43 No concern for other malignancies (excluding NMSC) was raised.46
Major adverse cardiovascular events
Concern regarding the association of ustekinumab with major adverse cardiovascular events was first raised in psoriasis trials. This included briakinumab, which has a similar mode of action. Meta‐analyses of RCTs in the psoriasis cohorts have so far reported conflicting conclusions.50 Nonetheless, 5‐year safety data of ustekinumab in psoriasis did not show any dose‐ or duration‐dependent relationship.46 As psoriasis is independently related to adverse cardiovascular events, it is unclear if this could be a concern for CD.51 No association has so far been reported.
Safety of ustekinumab in special populations
Children
Use of ustekinumab in pediatric patients with CD was only limited to case reports.52 Larger cohort studies will be required to validate the efficacy and side effect profile in the pediatric population.
Elderly
Use of ustekinumab in elderly patients (65 years and above), a total 46 patients, with psoriasis was reported in two retrospective studies.53, 54 No increased risk of AE was reported at the end of 1 year. No data for the CD cohort are yet available.
Pregnancy
Prior to the implementation of the Pregnancy and Lactation Labelling Rule (PLLR), ustekinumab was labeled as FDA pregnancy category B. Human data have so far been restricted to the patient registry, case reports, and observational studies. Animal studies with cynomolgus macaques receiving doses of up to 45 mg/kg twice weekly did not report an increased risk of teratogenicity or developmental delay up to 6 months of age.55 The current dermatology literature has not reported an increased risk of miscarriage or congenital malformation.56 Case reports for its use in CD have observed conflicting pregnancy outcomes.57, 58, 59 As most of the patients had active disease during pregnancy, it is unclear if the adverse outcomes were related to the active disease state rather than the medication. More data from prospective pregnancy registry will be needed.
Perioperative settings
Perioperative safety outcomes of patients exposed to ustekinumab were assessed in two retrospective multicenter cohort studies.60, 61 Ustekinumab use was not observed, in both studies, to be associated with the increased risk of postoperative surgical site infection when compared with anti‐TNF‐treated patients. This is in spite of a higher use of concomitant immunosuppressants in the ustekinumab group in the study by Shim et al. The study by Lightner et al., however, noted an increased risk of reoperation in the ustekinumab cohort. It is unclear if the observations were limited by the small sample number. While perioperative ustekinumab exposure appears to be safe, more studies are required. This will hopefully guide the decision on the role of an elective drug holiday prior to elective operation.
Other challenges
Immunogenicity
Immunogenicity with ustekinumab is remarkably low. Antibodies to ustekinumab were reported in 0.7% of patients by week 36 in the CERTIFI trial and in 2.3% by 1 year in the maintenance IM‐UNITI trial.43 This is much lower compared with anti‐TNF agents, such as infliximab (0–83%), adalimumab (0–54%), and golimumab (0–19%).62 Long‐term immunogenicity data for ustekinumab are eagerly awaited.
Combination therapy
In comparison with anti‐TNF therapies, the benefit of combination therapy in ustekinumab is less clear. The synergistic effect of a concomitant immunomodulator was observed in the GETAID cohort but not in others.42, 44, 63 In a prospective study of 62 patients, there was no significant difference in the ustekinumab trough level between those with and without concomitant immunomodulator use.64 Given this observation and the overall low immunogenicity associated with ustekinumab, it is likely that the use of a concomitant immunomodulator is less important.
The use of ustekinumab in combination with another biologics (anti‐TNF agent or vedolizumab) in refractory CD has been described with favorable outcomes.65, 66 Evidence to date is, however, very limited, particularly for long‐term safety.
Choice of biologics
For more than a decade, the only biologics licensed for use in IBD patients were the anti‐TNF agents. It was not possible for patients to be switched out of class even when they are obviously not responding to anti‐TNF agents. This has changed dramatically since 2014, with vedolizumab and ustekinumab joining the ranks of biologics approved for use in IBD.
These new agents appear to be associated with a lower risk of patients developing cancers and a lower risk of infective complications. They also appear to be less immunogenic than the older anti‐TNF agents. However, we have less real‐world data on how different subsets of IBD patients will respond, for example, pregnant patients, patients with pouchitis, patients with perianal CD, and patients with the extraintestinal manifestations. With the advent of biosimilars, the newer agents are also more expensive than the anti‐TNF agents.
To date, there are no published data on the direct head‐to‐head comparison between various biologics therapies. Clinical trials of UC comparing vedolizumab and adalimumab (NCT02497469) and etrolizumab and infliximab (NCT02136069) are currently under way. Currently, available comparative safety and efficacy data between various biologics therapies in both UC and CD are derived solely from network meta‐analyses. The overall safety profile appears to be comparable, although vedolizumab may theoretically be the safest. For treatment of UC, there was no significant difference in mucosal healing between vedolizumab and anti‐TNF agents.67 For infliximab‐experienced UC, vedolizumab was found to be superior to adalimumab in achieving mucosal healing during maintenance, OR 6.72, (95% CI 1.36–41.0), although no difference was found during induction.68 For treatment of CD, adalimumab and combination infliximab with azathioprine are more effective than vedolizumab in achieving clinical remission, OR 2.4 (95% CrI, 1.2–4.6).69
Rather than one‐size‐fits‐all, the choice of biologics should be tailored to the individual patient profile. The strength and limitation of each agent, given the inherent differences in the pharmacokinetics and pharmacodynamics properties should be considered in conjunction with the heterogeneous disease profile in each patient (Figs 1, 2). A patient with CD with concomitant extraintestinal manifestation or psoriasis, for example, may benefit from anti‐TNF therapies or ustekinumab rather than vedolizumab. On the other hand, vedolizumab may be preferable in a patient with a history of non‐GI malignancy or other comorbidities, such as hepatitis B or C or pulmonary tuberculosis infection. Vedolizumab and ustekinumab have lower immunogenicity than anti‐TNF agents and could be a better option if monotherapy is considered.62
Figure 1.
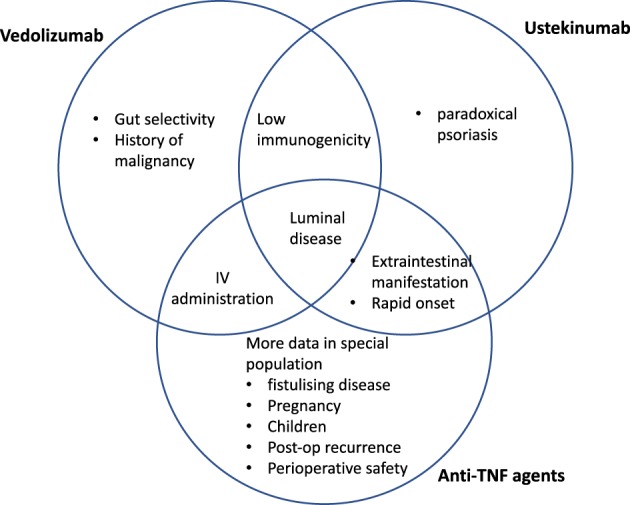
Strength of various agents in Crohn's disease (CD).
Figure 2.
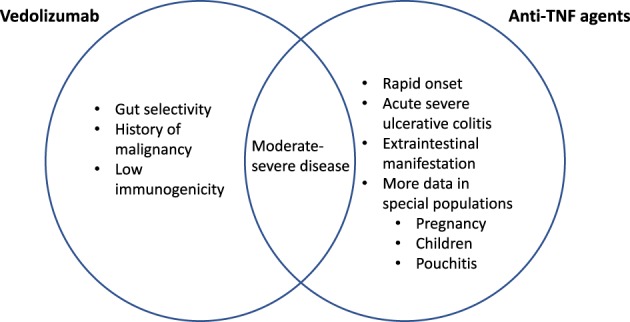
Strength of various agents in ulcerative colitis (UC).
Data to guide subsequent choices of biologics, especially in CD, are currently limited. While registration data observed a relatively inferior response with vedolizumab in comparison with ustekinumab in anti‐TNF‐experienced patients, no definite conclusion could be drawn given the heterogeneity in study design. Clear evidence from direct head‐to‐head comparisons is currently lacking. Indirect comparisons by separate groups did not observe any significant difference between ustekinumab and vedolizumab in achieving clinical remission in anti‐TNF‐experienced CD.70, 71, 72 The efficacy of ustekinumab and vedolizumab in patients with fistulizing CD was reported in a recent meta‐analysis, with pooled RR 1.77 (95% CI, 0.93–3.37; P = 0.08) and RR 2.54 (95% CI, 0.63–10.29; P = 0.19), respectively.73 Interestingly, while treatment response is known to be poorer in biologic‐experienced patients, there may be a potential role of using the etiology of withdrawal to guide subsequent choices of biologic. In a recent meta‐analysis, treatment response of a second biologic, particularly with ustekinumab, is poorer in anti‐TNF therapy primary nonresponder, in comparison with secondary nonresponder (RR, 0.64 [0.52–0.80]). There was no difference in treatment response to vedolizumab for both anti‐TNF primary nonresponder and secondary nonresponder (RR, 1.16 [0.85–1.58]).74
Predictor of response
Clinicians have tried to predict which patients will respond to biologics since the advent of biologics. Clinical characteristics, drug factors, biochemical markers, endoscopic factors, and genetic and serological markers have been proposed as predictors of response. These have recently been reviewed by Ding et al. with regard to anti‐TNF agents.75
Clinical predictors of response to vedolizumab and ustekinumab have also been studied. Patients with severe, complicated disease phenotype (in CD) or previous anti‐TNF therapy exposure were less likely to respond. Other poor prognostic factors in CD include smoking, history of intestinal resection, ileocolonic disease, and low trough level of both biologics.76 Genetic polymorphism contributes to the variation in clinical presentation and the response to ustekinumab.75, 77 HLA‐cw6 (C*06:02) expression, a major psoriasis‐susceptible gene, was found to be useful in predicting response to ustekinumab in the psoriasis cohort.77 In a phase 2a study, CD patients who received MEDI2070, a selective IL 23/p19 inhibitor, and who responded had a greater reduction in IL 22. In particular, the benefit was greater for those with a higher baseline IL 22 level.78 For vedolizumab, molecular imaging of mucosal α4β7 integrin expression with FITC‐labeled vedolizumab was useful in predicting response to vedolizumab in CD in a small case series.79 While validation with more data is required, these exploratory observations are encouraging and may allow personalized treatment in the future.
Conclusions
The availability of novel therapeutics modalities for IBD is encouraging. Vedolizumab and ustekinumab complement anti‐TNF agents and provide us with alternatives for patients who have failed anti‐TNFs. While more real‐world data is awaited, the safety and efficacy profiles of these two novel agents in the registration trials have been encouraging. In selected patient subsets, they could be considered first‐line biologics over anti‐TNF agents.
Declaration of conflict of interest: HHS: Advisory board member for Ferring and Janssen. PWC: Advisory board member for Janssen. SWC: Advisory board member for Janssen, Takeda, Abbvie, Ferring, and Shire. BJS: Advisory board member for Janssen. SCK: Nil. KLL: Consultant for Takeda, JnJ, and Abbvie.
Funding: None declared.
Writing assistance: None declared.
References
- 1. Bouguen G, Peyrin‐Biroulet L. Surgery for adult Crohn's disease: what is the actual risk? Gut. 2011; 60: 1178–81. 10.1136/gut.2010.234617. [DOI] [PubMed] [Google Scholar]
- 2. Targan S, Hanauer S, van Deventer S et al A short‐term study of chimeric monoclonal antibody cA2 to tumor necrosis factor a for Crohn's disease. N. Engl. J. Med. 1997; 337: 1029–35. [DOI] [PubMed] [Google Scholar]
- 3. Khanna R, Bressler B, Levesque BG et al Early combined immunosuppression for the management of Crohn's disease (REACT): a cluster randomised controlled trial. Lancet. 2015; 386: 1825–34. 10.1016/S0140-6736(15)00068-9. [DOI] [PubMed] [Google Scholar]
- 4. Khanna R, Sattin BD, Afif W et al Review article: a clinician's guide for therapeutic drug monitoring of infliximab in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013; 38: 447–59. 10.1111/apt.12407. [DOI] [PubMed] [Google Scholar]
- 5. Sandborn WJ, Feagan BG, Rutgeerts P et al Vedolizumab as induction and maintenance therapy for Crohn's disease. N. Engl. J. Med. 2013; 369: 711–21. 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 6. Rosario M, French JL, Dirks NL et al Exposure–efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn's disease. J. Crohns Colitis. 2017; 11: 921–9. 10.1093/ecco-jcc/jjx021. [DOI] [PubMed] [Google Scholar]
- 7. Colombel J‐F, Loftus EV, Siegel CA et al Sa1270 efficacy of vedolizumab with concomitant corticosteroid or immunomodulator use in patients with Crohn's disease from GEMINI 2. Gastroenterology. 2015; 148: S‐277 10.1016/S0016-5085(15)30910-0. [DOI] [Google Scholar]
- 8. Sands BE, Sandborn WJ, Van Assche G et al Vedolizumab as induction and maintenance therapy for Crohn's disease in patients naïve to or who have failed tumor necrosis factor antagonist therapy. Inflamm. Bowel Dis. 2017; 23: 97–106. 10.1097/MIB.0000000000000979. [DOI] [PubMed] [Google Scholar]
- 9. Feagan BG, Rutgeerts P, Sands BE et al Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013; 369: 699–710. 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 10. Vermeire S, Loftus EV, Colombel J‐F et al Long‐term efficacy of vedolizumab for Crohn's Disease. J Crohn's Colitis. 2017; 11: 412–24. 10.1093/ecco-jcc/jjw176. [DOI] [PubMed] [Google Scholar]
- 11. Loftus EV, Colombel J‐F, Feagan BG et al Long‐term efficacy of vedolizumab for ulcerative colitis. J. Crohns Colitis. 2017; 11: 400–11. 10.1093/ecco-jcc/jjw177. [DOI] [PubMed] [Google Scholar]
- 12. Dulai PS, Singh S, Jiang X et al The real‐world effectiveness and safety of vedolizumab for moderate–severe Crohn's disease: results from the US VICTORY consortium. Am. J. Gastroenterol. 2016; 111: 1147–55. 10.1038/ajg.2016.236. [DOI] [PubMed] [Google Scholar]
- 13. Rosario M, Dirks NL, Milch C et al A review of the clinical pharmacokinetics, pharmacodynamics, and immunogenicity of vedolizumab. Clin. Pharmacokinet. 2017; 56: 1–15. 10.1007/s40262-017-0546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Panaccione R, Ghosh S, Middleton S et al Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014; 146: 392–400.e3. 10.1053/j.gastro.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 15. Colombel JF, Sandborn WJ, Reinisch W et al Infliximab, azathioprine, or combination therapy for Crohn's disease. N. Engl. J. Med. 2010; 15: 1383–95. 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 16. Colombel J‐F, Loftus EV, Siegel CA et al Sa1271 efficacy of vedolizumab with concomitant corticosteroid or immunomodulator use in patients with ulcerative colitis from GEMINI 1. Gastroenterology. 2015; 148: S‐277‐8 10.1016/S0016-5085(15)30910-0. [DOI] [Google Scholar]
- 17. Allegretti JR, Barnes EL, Stevens B et al Predictors of clinical response and remission at 1 year among a multicenter cohort of patients with inflammatory bowel disease treated with vedolizumab. Dig. Dis. Sci. 2017; 62: 1590–6. 10.1007/s10620-017-4549-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williet N, Boschetti G, Fovet M et al Association between low trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin. Gastroenterol. Hepatol. 2017. Feb; 15: 1750–1757.e3. 10.1016/j.cgh.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 19. Ungar B, Kopylov U, Yavzori M et al Association of vedolizumab level, anti‐drug antibodies, and α4β7 occupancy with response in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2017; 6 Dec; 16: 697–705.e7. 10.1016/j.cgh.2017.11.050. [DOI] [PubMed] [Google Scholar]
- 20. Feagan BG, Schwartz DA, Danese S et al Vedolizumab for the treatment of fistulizing crohn's disease: an exploratory analysis of data from GEMINI 2. Gastroenterology. 2015; 1: S274 10.1016/S0016-5085(15)30901-X. [DOI] [Google Scholar]
- 21. Tadbiri S, Grimaud J, Peyrin‐Biroulet L et al Efficacy of vedolizumab on extraintestinal manifestation in patients with inflammatory bowel diseases: a post‐hoc analysis of the observ‐IBD cohort of the getaid. Gastroenterology. 2017; 152: S396. [Google Scholar]
- 22. Tse CS, Loftus EV, Raffals LH, Gossard A, Lightner AL. Efficacy of vedolizumab in reducing biliary inflammation in primary sclerosing cholangitis (PSC) in individuals with inflammatory bowel disease. Gastroenterology. 2017; 152(5 Suppl. 1): S402–3. [Google Scholar]
- 23. Christensen B, Micic D, Gibson PR et al Vedolizumab is safe and effective for IBD, but has no effect on liver biochemistry in patients with concurrent PSC. Gastroenterology. 2017; 152(5 Suppl. 1): S405. [Google Scholar]
- 24. Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017; 46: 3–15. 10.1111/apt.14075. [DOI] [PubMed] [Google Scholar]
- 25. Colombel J‐F, Sands BE, Rutgeerts P et al The safety of vedolizumab for ulcerative colitis and Crohn's disease. Gut. 2017; 66: 839–51. 10.1136/gutjnl-2015-311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stuve O, Marra CM, Bar‐Or A et al Altered CD4+/CD8+ T‐cell ratios in cerebrospinal fluid of natalizumab‐treated patients with multiple sclerosis. Arch. Neurol. 2006; 63: 1383–7. Retrieved Jan 8, 2018 from http://www.ncbi.nlm.nih.gov/pubmed/18852339. [DOI] [PubMed] [Google Scholar]
- 27. Milch C, Wyant T, Xu J et al Vedolizumab, a monoclonal antibody to the gut homing a4b7 integrin, does not affect cerebrospinal fluid T‐lymphocyte immunophenotype. J. Neuroimmunol. 2013; 264: 123–6. 10.1016/j.jneuroim.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 28. Bonovas S, Fiorino G, Allocca M et al Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta‐analysis. Clin. Gastroenterol. Hepatol. 2016; 14: 1385–1397.e10. 10.1016/j.cgh.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 29. Conrad MA, Stein RE, Maxwell EC et al Vedolizumab therapy in severe pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2016; 22: 2425–31. 10.1097/MIB.0000000000000918. [DOI] [PubMed] [Google Scholar]
- 30. Ledder O, Assa A, Levine A et al Vedolizumab in paediatric inflammatory bowel disease: a retrospective multi‐centre experience from the paediatric IBD Porto Group of ESPGHAN. J. Crohn's Colitis. 2017;Jun 9; 11: 1230–7. 10.1093/ecco-jcc/jjx082. [DOI] [PubMed] [Google Scholar]
- 31. Yajnik V, Khan N, Dubinsky M et al Efficacy and safety of vedolizumab in ulcerative colitis and Crohn's disease patients stratified by age. Adv. Ther. 2017; 34: 542–59. 10.1007/s12325-016-0467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Navaneethan U, Edminister T, Zhu X, Kommaraju K, Glover S. Vedolizumab is safe and effective in elderly patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2017; 23: E17 10.1097/MIB.0000000000001071. [DOI] [PubMed] [Google Scholar]
- 33. Chakraborti TK. Pharmacology Review(s) of Vedolizumab (Entyvio, MLN0002). 2013. Food and Drug Administration Center For Drug Evaluation and Research. Retrieved Jan 8, 2018 from URL: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125476Orig1s000PharmR.pdf.
- 34. Mahadevan U, Sunters H, Vermeire S et al Vedolizumab exposure in pregnancy: outcomes from clinical studies in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017; 7 Feb; 45: 941–50. 10.1111/apt.13960. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35. Lightner AL, Raffals LE, Mathis KL et al Postoperative outcomes in vedolizumab‐treated patients undergoing abdominal operations for inflammatory bowel disease. J. Crohns Colitis. 2016; 19 Aug; 11: 185–90. 10.1093/ecco-jcc/jjw147. [DOI] [PubMed] [Google Scholar]
- 36. Yamada A, Komaki Y, Patel N et al Risk of postoperative complications among inflammatory bowel disease patients treated preoperatively with vedolizumab. Am. J. Gastroenterol. 2017. (Jan); 112: 1423–9. 10.1038/ajg.2017.201. [DOI] [PubMed] [Google Scholar]
- 37. Lightner AL, Tse CS, Potter DD, Moir C. Postoperative outcomes in vedolizumab‐treated pediatric patients undergoing abdominal operations for inflammatory bowel disease. J. Pediatr. Surg. 2017. (Oct 9). 10.1016/j.jpedsurg.2017.09.019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38. Dietrich N, Baer F, Dignass A, Bokemeyer B, Fellermann K, Buening J. P579 Combined therapy of tacrolimus and vedolizumab in severe refractory ulcerative colitis. J. Crohns Colitis. 2016; 10(Suppl. 1): s394. [Google Scholar]
- 39. Buer L, Høivik ML, Medhus AW, Moum B. P514 Combination treatment with vedolizumab and anti‐TNF‐α in inflammatory bowel disease: safety data. J. Crohns Colitis. 2017; 11(Suppl. 1): S341–2. [Google Scholar]
- 40. Fischer S, Rath T, Geppert CI et al Long‐term combination therapy with anti‐TNF plus vedolizumab induces and maintains remission in therapy‐refractory ulcerative colitis. Am. J. Gastroenterol. 2017; 112: 1621–3. 10.1038/ajg.2017.242. [DOI] [PubMed] [Google Scholar]
- 41. Sandborn W, Gasink C, Chan D et al PD‐012 endoscopic healing in the ustekinumab phase 3 UNITI/IMUNITI Crohn's disease program and relationship of clinical outcomes to baseline ulceration status. Inflamm. Bowel Dis. 2017; 23 (suppl_1): S9. 10.1097/01.MIB.0000512536.69855.de. [DOI] [Google Scholar]
- 42. Ma C, Fedorak RN, Kaplan GG et al Clinical, endoscopic and radiographic outcomes with ustekinumab in medically‐refractory Crohn's disease: real world experience from a multicentre cohort. Aliment. Pharmacol. Ther. 2017; 45: 1232–43. 10.1111/apt.14016. [DOI] [PubMed] [Google Scholar]
- 43. Feagan BG, Sandborn WJ, Gasink C et al Ustekinumab as induction and maintenance therapy for Crohn's disease. N. Engl. J. Med. 2016; 375: 1946–60. 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 44. Khorrami S, Ginard D, Marín‐Jiménez I et al Ustekinumab for the treatment of refractory Crohn's disease. Inflamm. Bowel Dis. 2016; 22: 1662–9. 10.1097/MIB.0000000000000842. [DOI] [PubMed] [Google Scholar]
- 45. Battat R, Bessissow T, Strohl M et al P626 ustekinumab for the treatment of perianal fistulas in patients with Crohn's disease. J. Crohns Colitis. 2017; 11(Suppl. 1): S400–1. [Google Scholar]
- 46. Papp KA, Griffiths CEM, Gordon K et al Long‐term safety of ustekinumab in patients with moderate‐to‐severe psoriasis: final results from 5 years of follow‐up. Br. J. Dermatol. 2013; 168: 844–54. 10.1111/bjd.12214. [DOI] [PubMed] [Google Scholar]
- 47. Loftus EV, Sloan S, Ramachandran P, Yang Z, Guo C‐Y, Gasink C. Comparison of rates of active tuberculosis infection in the phase 2 and 3 clinical trial programs for anti‐IL12/23 and anti‐TNFS. Gastroenterology. 2017; 152: S596 10.1016/S0016-5085(17)32134-0. [DOI] [Google Scholar]
- 48. Tsai TF, Ho V, Song M et al The safety of ustekinumab treatment in patients with moderate‐to‐severe psoriasis and latent tuberculosis infection. Br. J. Dermatol. 2012; 167: 1145–52. 10.1111/j.1365-2133.2012.11142.x. [DOI] [PubMed] [Google Scholar]
- 49. Janssen Biotech Inc. Stelara® (Ustekinumab) product information, 2017.
- 50. Tzellos T, Kyrgidis A, Trigoni A, Zouboulis CC. Association of ustekinumab and briakinumab with major adverse cardiovascular events: an appraisal of meta‐analyses and industry sponsored pooled analyses to date. Dermatoendocrinol. 2012; 4: 320–3. 10.4161/derm.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol. Clin. 2015; 33: 41–55. [DOI] [PubMed] [Google Scholar]
- 52. Bishop C, Simon H, Suskind D, Lee D, Wahbeh G. Ustekinumab in pediatric Crohn disease patients. J. Pediatr. Gastroenterol. Nutr. 2016; 63: 348–51. 10.1097/MPG.0000000000001146. [DOI] [PubMed] [Google Scholar]
- 53. Hayashi M, Umezawa Y, Fukuchi O, Ito T, Saeki H, Nakagawa H. Efficacy and safety of ustekinumab treatment in elderly patients with psoriasis. J. Dermatol. 2014; 41: 974–80. 10.1111/1346-8138.12653. [DOI] [PubMed] [Google Scholar]
- 54. Megna M, Napolitano M, Balato N et al Efficacy and safety of ustekinumab in a group of 22 elderly patients with psoriasis over a 2‐year period. Clin. Exp. Dermatol. 2016; 41: 564–6. [DOI] [PubMed] [Google Scholar]
- 55. Martin PL, Sachs C, Imai N et al Development in the cynomolgus macaque following administration of ustekinumab, a human anti‐il‐12/23p40 monoclonal antibody, during pregnancy and lactation. Birth Defects Res. B Dev. Reprod. Toxicol. 2010; 89: 351–63. 10.1002/bdrb.20250. [DOI] [PubMed] [Google Scholar]
- 56. Götestam Skorpen C, Hoeltzenbein M, Tincani A et al The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann. Rheum. Dis. 2016; 75: 795–810. 10.1136/annrheumdis-2015-208840. [DOI] [PubMed] [Google Scholar]
- 57. Galli‐Novak E, Mook S‐C, Büning J et al Successful pregnancy outcome under prolonged ustekinumab treatment in a patient with Crohn's disease and paradoxical psoriasis. J. Eur. Acad. Dermatol. Venereol. 2016; 30: e191–2. 10.1111/jdv.13499. [DOI] [PubMed] [Google Scholar]
- 58. Cortes X, Borrás‐Blasco J, Antequera B et al Ustekinumab therapy for Crohn's disease during pregnancy: a case report and review of the literature. J. Clin. Pharm. Ther. 2017; 42: 234–6. 10.1111/jcpt.12492. [DOI] [PubMed] [Google Scholar]
- 59. Venturin C, Nancey S, Danion P et al Fetal death in utero and miscarriage in a patient with Crohn's disease under therapy with ustekinumab: case‐report and review of the literature. BMC Gastroenterol. 2017; 17: 80 10.1186/s12876-017-0633-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lightner AL, McKenna NP, Tse CS et al Postoperative outcomes in ustekinumab‐treated patients undergoing abdominal operations for Crohn's disease. J Crohn's Colitis. 2017. (Dec 6); 12: 402–7. 10.1093/ecco-jcc/jjx163. [DOI] [PubMed] [Google Scholar]
- 61. Shim HH, Ma C, Al‐Farhan H et al Preoperative ustekinumab treatment is not associated with increased postoperative complications in Crohn's disease: a canadian multicentre case–control cohort study. Gastroenterology. 2017; 152: S577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Strand V, Balsa A, Al‐Saleh J et al Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs. 2017; 31: 299–316. 10.1007/s40259-017-0231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wils P, Bouhnik Y, Michetti P et al Subcutaneous ustekinumab provides clinical benefit for two‐thirds of patients with Crohn's disease refractory to anti‐tumor necrosis factor agents. Clin. Gastroenterol. Hepatol. 2016; 14: 242–50. 10.1016/j.cgh.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 64. Battat R, Kopylov U, Bessissow T et al Association between ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn's disease. Clin. Gastroenterol. Hepatol. 2017; 15: 1427–1434.e2. 10.1016/j.cgh.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 65. Huff‐Hardy K, Bedair M, Vazquez R, Burstein E. Efficacy of combination vedolizumab and ustekinumab for refractory Crohn's disease. Inflamm. Bowel Dis. 2017; 23: E49 10.1097/MIB.0000000000001232. [DOI] [PubMed] [Google Scholar]
- 66. Yzet C, Dupas J‐L, Fumery M. Ustekinumab and anti‐TNF combination therapy in patients with inflammatory bowel disease. Am. J. Gastroenterol. 2016; 111: 748–9. 10.1038/ajg.2016.66. [DOI] [PubMed] [Google Scholar]
- 67. Cholapranee A, Hazlewood GS, Kaplan GG, Peyrin‐Biroulet L, Ananthakrishnan AN. Systematic review with meta‐analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn's disease and ulcerative colitis controlled trials. Aliment. Pharmacol. Ther. 2017; 45: 1291–302. 10.1111/apt.14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vickers AD, Ainsworth C, Mody R et al Systematic review with network meta‐analysis: comparative efficacy of biologics in the treatment of moderately to severely active ulcerative colitis. PLoS One. 2016; 11: e0165435 10.1371/journal.pone.0165435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hazlewood GS, Rezaie A, Borman M et al Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn's disease: a network meta‐analysis. Gastroenterology. 2015; 148: 344–54. 10.1053/j.gastro.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 70. Hather G, Curtis R, Minda K, Zouraq IA, Khalid JM. P305 Indirect comparison of two novel biologics for the treatment of Crohn's disease: network‐meta analysis of ustekinumab vs vedolizumab. J. Crohns Colitis. 2017; 11(Suppl. 1): S232–3. [Google Scholar]
- 71. Kawalec P, Moćko P. An indirect comparison of ustekinumab and vedolizumab in the therapy of TNF‐failure Crohn's disease patients. J. Comp. Eff. Res. 2017; 7: 101–11. 10.2217/cer-2017-0041. [DOI] [PubMed] [Google Scholar]
- 72. Biemans V, van der Woude C, van der Meulen – de Jong A et al DOP052 Vedolizumab vs. ustekinumab for Crohn's disease: comparative effectiveness in a real‐life observational cohort study (ICC case series). J. Crohns Colitis. 2018; 12(Suppl. 1): S066–7. [Google Scholar]
- 73. Lee MJ, Parker CE, Taylor SR et al Efficacy of medical therapies for fistulizing Crohn's disease: systematic review and meta‐analysis. Clin. Gastroenterol. Hepatol. 2018. (Mar). pii: S1542‐3565(18)30098‐3. 10.1016/j.cgh.2018.01.030. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 74. Singh S, George J, Boland BS, Vande Casteele N, Sandborn WJ. Primary non‐response to tumor necrosis factor antagonists is associated with inferior response to second‐line biologics in patients with inflammatory bowel diseases: a systematic review and meta‐analysis. J. Crohns Colitis. 2018; 12: 635–43. 10.1093/ecco-jcc/jjy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti‐TNF therapy in Crohn's disease—algorithm for practical management. Aliment. Pharmacol. Ther. 2016; 43: 30–51. 10.1111/apt.13445. [DOI] [PubMed] [Google Scholar]
- 76. Barré A, Colombel JF, Ungaro R. Review article: predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2018; 47: 896–905. 10.1111/apt.14550. [DOI] [PubMed] [Google Scholar]
- 77. Chiu HY, Wang TS, Chan CC, Cheng YP, Lin SJ, Tsai TF. Human leucocyte antigen‐Cw6 as a predictor for clinical response to ustekinumab, an interleukin‐12/23 blocker, in Chinese patients with psoriasis: a retrospective analysis. Br. J. Dermatol. 2014; 171: 1181–8. 10.1111/bjd.13056. [DOI] [PubMed] [Google Scholar]
- 78. Sands BE, Chen J, Feagan BG et al Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn's disease: a phase 2a study. Gastroenterology. 2017; 153: 77–86.e6. 10.1053/j.gastro.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 79. Rath T, Bojarski C, Neurath MF, Atreya R. Molecular imaging of mucosal α4β7 integrin expression with the fluorescent anti‐adhesion antibody vedolizumab in Crohn's disease. Gastrointest. Endosc. 2017; 86: 406–8. 10.1016/j.gie.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 80. Sands BE, Feagan BG, Rutgeerts P et al Effects of vedolizumab induction therapy for patients with Crohn's disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014; 147: 618–627.e3. 10.1053/j.gastro.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 81. Noman M, Ferrante M, Bisschops R et al Vedolizumab induces long term mucosal healing in patients with Crohn's disease and ulcerative colitis. J. Crohns Colitis. 2017; 111: 1147–55. 10.1093/ecco-jcc/jjx048. [DOI] [PubMed] [Google Scholar]
- 82. Amiot A, Grimaud J‐C, Peyrin‐Biroulet L et al Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2016; 14: 1593–1601.e2. 10.1016/j.cgh.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 83. Amiot A, Serrero M, Peyrin‐Biroulet L et al One‐year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multicentre cohort study. Aliment. Pharmacol. Ther. 2017; 46: 310–21. 10.1111/apt.14167. [DOI] [PubMed] [Google Scholar]
- 84. Baumgart DC, Bokemeyer B, Drabik A, Stallmach A, Schreiber S, the Vedolizumab Germany Consortium . Vedolizumab induction therapy for inflammatory bowel disease in clinical practice—A nationwide consecutive German cohort study. Aliment. Pharmacol. Ther. 2016; 43: 1090–102. 10.1111/apt.13594. [DOI] [PubMed] [Google Scholar]
- 85. Eriksson C, Marsal J, Bergemalm D et al Long‐term effectiveness of vedolizumab in inflammatory bowel disease: a national study based on the Swedish National Quality Registry for Inflammatory Bowel Disease (SWIBREG). Scand. J. Gastroenterol. 2017; 52: 722–9. 10.1080/00365521.2017.1304987. [DOI] [PubMed] [Google Scholar]
- 86. Kopylov U, Ron Y, Avni‐Biron I et al Efficacy and safety of vedolizumab for induction of remission in inflammatory bowel disease—the Israeli real‐world experience. Inflamm. Bowel Dis. 2017; 23: 404–8. 10.1097/MIB.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 87. Shelton E, Allegretti J, Stevens B et al Efficacy of vedolizumab as induction therapy in refractory IBD patients: a multicenter cohort. Inflamm. Bowel Dis. 2016; 21: 2879–85. 10.1097/MIB.0000000000000561.Efficacy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Singh N, Rabizadeh S, Jossen J et al Multi‐center experience of vedolizumab effectiveness in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2016; 22: 2121–6. 10.1097/MIB.0000000000000865. [DOI] [PubMed] [Google Scholar]
- 89. Stallmach A, Langbein C, Atreya R et al Vedolizumab provides clinical benefit over 1 year in patients with active inflammatory bowel disease—a prospective multicenter observational study. Aliment. Pharmacol. Ther. 2016; 44: 1199–212. 10.1111/apt.13813. [DOI] [PubMed] [Google Scholar]
- 90. Vivio EE, Kanuri N, Gilbertsen JJ et al Vedolizumab effectiveness and safety over the first year of use in an IBD clinical practice. J. Crohns Colitis. 2016; 10: 402–9. 10.1093/ecco-jcc/jjv226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sandborn WJ, Gasink C, Gao LL et al Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N. Engl. J. Med. 2012; 367: 1519–28. 10.1056/NEJMoa1203572. [DOI] [PubMed] [Google Scholar]
- 92. Harris KA, Horst S, Gadani A et al Patients with refractory Crohn's disease successfully treated with ustekinumab. Inflamm. Bowel Dis. 2016; 22: 397–401. 10.1097/MIB.0000000000000624. [DOI] [PubMed] [Google Scholar]
- 93. Kopylov U, Afif W, Cohen A et al Subcutaneous ustekinumab for the treatment of anti‐TNF resistant Crohn's disease—the McGill experience. J. Crohns Colitis. 2014; 8: 1516–22. 10.1016/j.crohns.2014.06.005. [DOI] [PubMed] [Google Scholar]


