Abstract
Background and purpose
We aimed to compare the ability of conventional Alberta Stroke Program Early Computed Tomography Score (ASPECTS), automated ASPECTS and ischemic core volume on CT perfusion (CTP) to predict clinical outcome in ischemic stroke due to large vessel occlusion up to 18 hours after symptom onset.
Methods:
We selected acute ischemic stroke patients from the Computed Tomography Perfusion to Predict Response to Recanalization in ischemic Stroke Project (CRISP) study with successful reperfusion (modified treatment in cerebral ischemia score 2b or 3). We used e-ASPECTS software to calculate automated ASPECTS and RAPID software to estimate ischemic core volumes. We studied associations between these imaging characteristics and good outcome (modified Rankin Scale [mRS] 0–2) or poor outcome (mRS 4–6) in univariable and multivariable analysis, after adjustment for relevant clinical confounders.
Results
We included 156 patients. Conventional and automated ASPECTS were not associated with good or poor outcome in univariable analysis (p = NS for all). Automated ASPECTS was associated with good outcome in multivariable analysis (p = 0.02), but not with poor outcome. Ischemic core volume was associated with good (p < 0.01) and poor outcome (p = 0.04) in univariable and multivariable analysis (p = 0.03 and p = 0.02 respectively). CTP predicted good outcome with an area under the curve (AUC) of 0.62 (95% CI 0.53–0.71) and optimal cut-off core volume of 15 ml.
Conclusion
Ischemic core volume assessed on CTP is a predictor of clinical outcome among patients in whom endovascular reperfusion is achieved up to 18 hours after symptom onset. In this population, conventional or automated ASPECTS did not predict outcome.
Keywords: Ischemic Stroke, ASPECTS, CT perfusion, Ischemic Core, Functional outcome, Endovascular Stroke Treatment, Thrombectomy, Recanalization, Cerebrovascular Procedures, Ischemic Stroke
Introduction
Randomized clinical trials (RCTs) in acute ischemic stroke studying the effect of endovascular therapy have defined different baseline imaging inclusion criteria to select patients. In some trials, Alberta Stroke Program Early CT score (ASPECTS) ratings were obtained and in others CT perfusion (CTP) was performed to select patients with small core volumes and salvageable tissue. The aim of this baseline neuroimaging assessment was to identify those patients more likely to benefit from endovascular treatment. Mechanical thrombectomy is also beneficial beyond the 6 h time window in patients selected based on baseline perfusion imaging (1–3). In two RCTs endovascular treatment of patients with a beneficial profile, defined as a small ischemic core volume on CTP and either severe clinical symptoms (clinical-core mismatch) or a large perfusion deficit (perfusion mismatch), resulted in improved clinical outcomes three months after endovascular therapy (2, 3).
The Alberta Stroke Program Early CT score (ASPECTS) is a standardized semi-quantitative CT grading system used to quantify early ischemic signs in patients with acute anterior circulation ischemic stroke in 10 regions of the brain parenchyma. ASPECTS is rated on a scale from 10 (no early signs of ischemia) to 0 (early ischemic changes in all 10 regions). Low ASPECTS rating on non-contrast CT has been associated with poor outcome after reperfusion and was an exclusion criterion in several randomized clinical trials on endovascular therapy (4–8). CTP and ASPECTS correlated with good outcome in acute ischemic stroke patients who underwent endovascular treatment, although patients selected based on perfusion mismatch profile had better clinical outcomes after reperfusion, and CTP more consistently predicts the acute ischemic lesion volume (9, 10). The ASPECTS assessment is subject to substantial variability between and within raters (11). Automated “e-ASPECTS” eliminates interrater variability and may thus be a more reliable tool to predict functional outcome after endovascular treatment for ischemic stroke (12). The performance of ASPECTS to select acute ischemic stroke patients for endovascular therapy in the extended treatment time window is unclear.
In this study, we compared the accuracy of conventional and automated ASPECTS and CTP core volume to predict good and poor outcome after successful endovascular reperfusion of acute anterior circulation ischemic stroke patients up to 18h after stroke onset.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study design and patient selection
We retrospectively analyzed data from the Computed Tomography Perfusion to Predict Response to Recanalization in ischemic Stroke Project (CRISP; Stanford, USA) (1). Detailed study protocol and design has been previously described (1). CRISP is a multi-center prospective cohort study that included 201 ischemic stroke patients with a large vessel occlusion in the anterior circulation who were scheduled to undergo endovascular therapy within 18 hours from symptom onset. All patients underwent non-contrast CT, CT angiography (CTA) and CTP imaging prior to endovascular therapy. Endovascular therapy was initiated within 90 minutes following baseline imaging. Baseline imaging profile was not a selection criterion for CRISP although investigators were not blinded for CTP core volumes and mismatch profile. Informed consent was obtained from all patients or their proxy if the patient was unable to consent. The institutional review board at each study site approved the study.
For this study we limited the analysis to patients with successful reperfusion, defined as a modified Treatment In Cerebral Infarction (mTICI) score of 2b or 3.
Imaging and outcome analysis
Four raters (1 neurologist in training, 1 vascular neurologist and 2 interventional neuroradiologists) independently assessed the non-contrast CT blinded to all clinical data except for affected hemisphere. Conventional Alberta Stroke Program Early Computed Tomography Score (ASPECTS) was rated using narrow window and level settings centered between 35–45 HU width and 35–45 HU level, as specified on http://www.aspectsinstroke.com. Four raters achieved one common ASPECT score per patient through consensus during a joint reading session where regions of disagreement between at least 2 raters were reviewed.
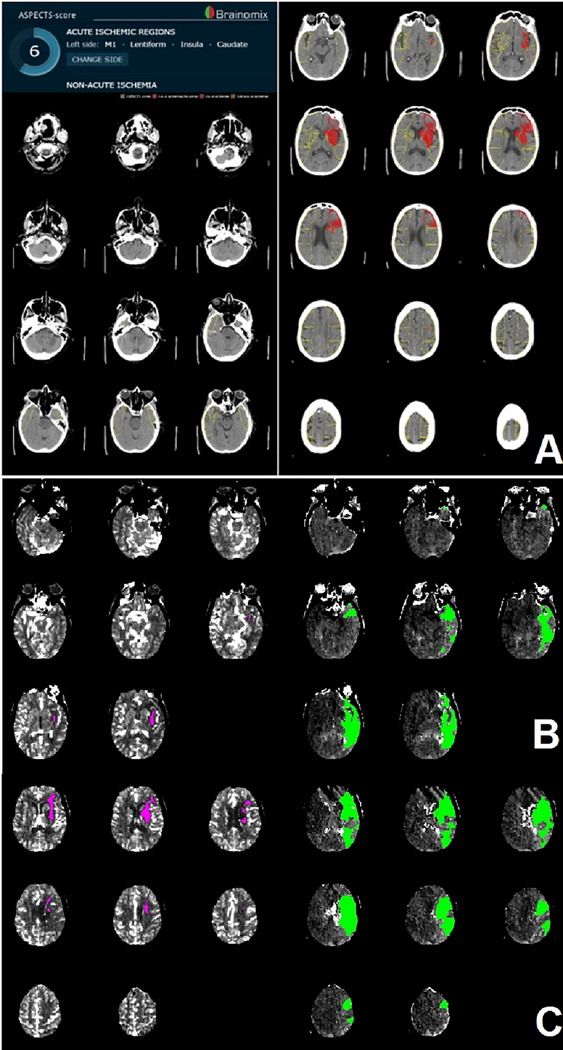
We performed automated ASPECTS analysis using validated e-ASPECTS software (Brainomix, Oxford, UK) after indicating lesion side for each scan (12). The automated software uses a machine-learning algorithm to calculate ASPECTS on full 3D non-contrast CT DICOM images (Figure 1A).
Figure 1. Example of automated CT imaging outputs.
Automated e-ASPECTS output (A). Regions with early ischemic changes are superimposed and marked red on the non-contrast CT of the brain. In this patient, the left caudate, lentiform nucleus, insula and M1 region show early ischemic changes. Resulting e-ASPECTS is 6. Automated RAPID CT perfusion output in 2 imaging slabs (B). Core (pink) and penumbra (green) volume are superimposed on the non-contrast CT. Estimated core volume is 6 ml. The penumbra volume is estimated at 116 ml.
We used CTP core volumes registered in the CRISP study. Ischemic core volumes were calculated with RAPID software (iSchemaView, Menlo Park, CA). A relative cerebral blood ow (rCBF) threshold of < 30% was used to define the ischemic core. Critically hypoperfused tissue was identified as the brain volume with a time-to-maximum (Tmax) of more than 6 seconds (Figure 1B). Stanford’s core imaging laboratory manually reviewed all RAPID software outputs and removed artifacts if needed. In addition, the core imaging laboratory analyzed the digital subtraction angiography images to score the degree of reperfusion.
The primary outcome was the modified Rankin Scale (mRS) score at 90 days. Trained CRISP investigators assessed the mRS during 30 and 90-day follow-up clinic visits. Patients unable to return to clinic were visited at their residence by study coordinators or received mRS assessment by telephone. Investigators who assessed the mRS at 90 days were not blinded from imaging data (1). Good outcome was defined as functional independence (mRS 0–2) and poor outcome as severe disability, dependency or death (mRS 4–6). We performed a sensitivity analysis for excellent outcome (mRS 0–1). We used symptomatic intracranial hemorrhage (sICH) as a safety endpoint. sICH was defined as any intracranial hemorrhage associated with at least 4 points worsening on the NIHSS (1).
Statistical analysis
We used SPSS statistics (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp) and R (R Core Team, version 3.3.3, Released 2017) for all statistical analyses. We tested the distribution of clinical and imaging variables using a Shapiro-Wilk test for normality. We compared clinical and imaging variables between groups using a chi square test (for categorical variables), a Mann-Whitney U test (for non-categorical variables without normal distribution) or a student-t test (for non-categorical variables with normal distribution). We applied Bonferroni adjusted p-values with an alpha of 0.05 to account for multiple comparisons in the good vs. poor outcome subgroup comparison.
We used Spearman’s rho intraclass correlation coefficient (ICC; two way mixed effects model with absolute agreement and single measures) and a scatter plot to assess the correlation between conventional and automated ASPECTS and baseline ischemic core volume as predicted by CTP.
We tested associations between clinical outcome and conventional ASPECT, automated ASPECTS, CTP core volume, age, sex, premorbid mRS, National Institutes of Health Stroke Scale (NIHSS) score, hypertension, hypercholesterolemia, diabetes or atrial fibrillation, history of previous stroke and history of myocardial ischemia in univariable analyses. We entered variables into the multivariable logistic regression model if they were significant at the p < 0.1 level in univariable analysis with either good or poor functional outcome as a dependent variable and used the variance ination factor to identify multicollinearity. We retained variables with p values < 0.05 level in the final adjusted model. We determined the association of conventional ASPECTS, automated ASPECTS and CTP core volume with good outcome (vs. others) and poor outcome (vs. others) using logistic regression with and without adjustment for these predictive clinical or imaging variables. Optimum cut-off values, where applicable, were calculated using the Youden index. We used area under the receiver operating characteristics curve (AUC) on imaging variables that were significantly associated with the outcome variable in univariable analysis, to assess accuracy of each imaging modality to predict good and poor outcome (13).
We calculated the ICC to evaluate inter-rater agreement for conventional ASPECTS. Results of the ICC were interpreted by the proposed standards of Cicchetti: ICC < 0.40 (poor); 0.41 – 0.59 (fair); 0.60 – 0.74 (good); 0.75 – 1.00 (excellent) (14).
Results
Of the 201 patients enrolled in the CRISP study, we included 170 in whom reperfusion was achieved. Fourteen patients were excluded based on insufficient imaging quality. A total of 156 patients were included in the analysis (Table 1). Median admission NIHSS was 17 (IQR 13–21) and 71 (45.5%) of patients received intravenous thrombolysis before mechanical thrombectomy.
Table 1.
Population characteristics
| Characteristic | Participants (n = 156) |
|---|---|
| Median age (IQR) | 68 (57 – 77) |
| Male sex, No. (%) | 87 (55.7%) |
| Premorbid mRS score | |
| 0, No. (%) | 135 (86.5%) |
| 1, No. (%) | 20 (12.8%) |
| 2, No. (%) | 1 (0.6%) |
| Hypertension, No. (%) | 102 (65.4%) |
| Hyperlipidemia, No. (%) | 68 (43.6%) |
| Diabetes, No. (%) | 35 (22.4%) |
| History of stroke, No. (%) | 16 (10.3%) * |
| History of myocardial ischemia, No. (%) | 11 (7.1%) * |
| History of atrial fibrillation, No. (%) | 53 (34%) |
| Median admission NIHSS score (IQR) | 17 (13–21) |
| Treated with intravenous thrombolysis, No. (%) | 71 (45.5%) |
Abbreviations: IQR = interquartile range; No.: number; mRS = modified Rankin Scale; NIHSS = National Institutes of Health Stroke Scale
Data available on 155/156 participants
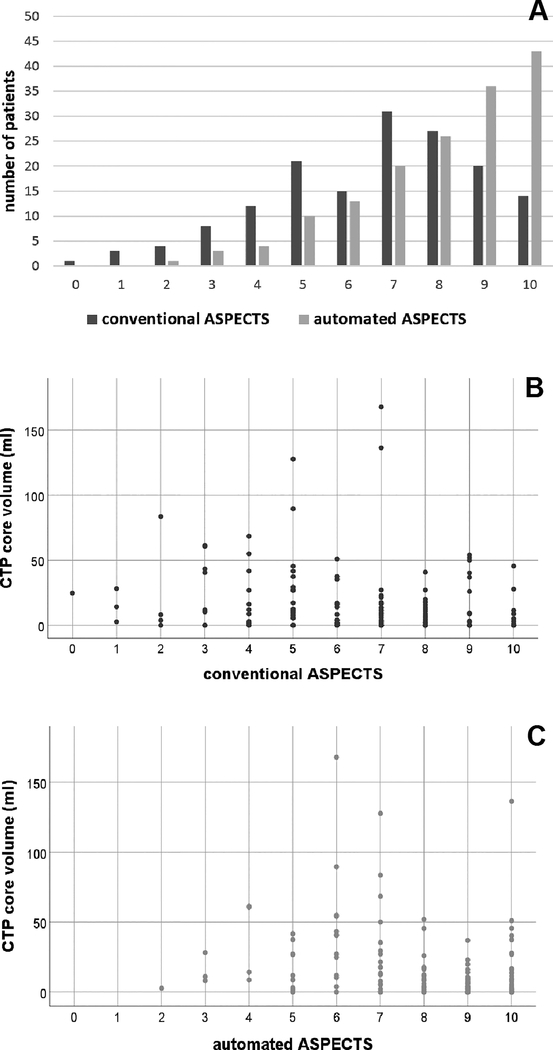
Imaging and procedural characteristics are described in Table 2. Median conventional ASPECTS was 7 (IQR 5–8) and the median automated ASPECTS was 9 (IQR 7–10) (Figure 2A). On the conventional ASPECTS scale, 41% had a score of less than 7 and 17.9% had a score of less than 5. For the automated ASPECTS, these percentages were 19.2% and 5.1%. respectively. The agreement between conventional and automated ASPECTS was fair (rs = 0.52; p < 0.001; ICC = 0.47, 95%CI 0.12 – 0.67). The interrater agreement for conventional ASPECTS was fair (ICC 0.46, 95%CI 0.37–0.54). The region with the highest degree of agreement was the caudate nucleus (ICC 0.45, 95%CI 0.37–0.53) and the region with the least agreement was the internal capsule (ICC 0.05, 95%CI −0.01–0.13; supplemental table I). Absolute agreement was low: all four raters agreed on the total ASPECTS in 3.8% of cases. In 19% of cases, at least three of four raters agreed and in 55% of cases at least two raters agreed on the total ASPECTS score (Supplemental figure I). The median volume of the ischemic core lesion was 6 ml (IQR 0 – 18). In 12 patients (7.7%) the ischemic core volume was more than 50 ml and in 5 patients (3.2%) the volume was more than 70ml. There was a weak correlation between CTP core volume and both conventional ASPECTS (rs = −0.23, p = 0.004, Figure 2B) and automated ASPECTS (rs = −0.36, p < 0.0001, Figure 2C).
Table 2.
Imaging and procedural characteristics
| Characteristicc | Participants (n = 156) |
|---|---|
| MedianconventionalASPECTS,(IQR)í | 7(5–8) |
| ASPECTS 10, n<%) | 14(9.0) |
| ASPECTS 9, n<%) | 20(12.8) |
| ASPECTS S, n<%) | 27(17.3) |
| ASPECTS 7, n(%) | 31(19.9) |
| ASPECTS 6, n(%) | 15 (9.6) |
| ASPECTS 5, n(%) | 21(13.5) |
| ASPECTS 4, n(%) | 12(7.7) |
| ASPECTS 3, n(%) | 8(5.1) |
| ASPECTS 2, n(%) | 4(2.6) |
| ASPECTS 1, n(%) | 3(1.9) |
| ASPECTS 0, n(%) | 1(0.6) |
| Medianautomated-ASPECTS,(IQR) | 9(7–10) |
| ASPECTS 10, n(%) | 43 (27,6) |
| ASPECTS 9, n(%) | 36(23,1) |
| ASPECTS 8, n(%) | 26(16,7) |
| ASPECTS 7, n(%) | 20(12,8) |
| ASPECTS 6, n(%) | 13(8,3) |
| ASPECTS 5, n(%) | 10(6,4) |
| ASPECTS 4, n(%) | 4(2,6) |
| ASPECTS 3, n(%) | 3(1,9) |
| ASPECTS 2, n(%) | 1(0,6) |
| ASPECTS 1, n(%) | 0(0) |
| ASPECTS 0, n(%) | 0(0) |
| Median CT p erfusion ischemic core volume in ml, (IQR) | 6(0–18) |
| Median CT p erfusion Trnax > 6 s lesion volume in ml, (IQR) | 131 (78–177) |
| Median symptom onset to start of CT perfusion in h, (IQR) | 4.4(2.6–6.9) |
| Median symptom onset to procedure completion time in h, (IQR) | 6.8 (5.2–9.4) |
| mTICI score, n (%) | |
| 2b | 79 (50.6%) |
| 3a | 77(49.4%) |
| sICH, n (%) | 8(5) |
Abbreviations: IQR = interquartile range; n = number; mTICI = modified Treatment In Cerebral Infarction scale; sICH = symptomatic intracranial hemorrhage
Figure 2. ASPECTS ratings and ischemic core volumes.
Histogram of conventional (blue) and automated (red) ASPECT scores (A). The X-axis shows the conventional or automated ASPECTS score. The Y-axis shows the number of patients that achieved that ASPECTS score. Scatter plot of conventional ASPECTS scores (x-axis) and baseline CTP core volume (y-axis) (rs = −0.23, p = 0.004) (B). Scatter plot of automated ASPECTS scores (x-axis) and baseline CTP core volumes (y-axis) (rs = − 0.36, p < 0.0001) (C). Abbreviations: ASPECTS = Alberta Stroke Program Early Computed Tomography Score; CTP = Perfusion Computed Tomography.
Ninety-two patients (58.9%) achieved a good outcome and 39 patients (25%) had a poor outcome 90 days after stroke. Patients with good outcome had a lower median NIHSS (15 [IQR 11–19]) compared to patients without good outcome (19 [IQR 16–22]; p < 0.0001) (Supplemental Table II). When compared to patients without poor outcome, patients with poor outcome were older (median 76 [IQR 70–81] vs. 63 [IQR 54–75]; p < 0.0001), had higher admission NIHSS scores (median 20 [IQR 16–23] vs. 15 [IQR 11–20]; p = 0.001), more frequently developed sICH (3.8% vs. 1.3%, p = 0.001) and more often had a history of diabetes (43.6% vs. 15.4%; p < 0.0001) (Supplemental Table III).
Eight patients (5%) developed sICH, of whom 3 patients died. Only 1 patient with sICH achieved a good outcome.
In univariable analyses, there was no association between good outcome and conventional ASPECTS (OR 1.09, 95%CI 0.95–1.26) or automated ASPECTS (OR 1.17, 95%CI 0.99–1.39) but there was an association between good outcome and CTP core volume (OR 0.98, 95%CI 0.96–0.99; p < 0.01) (Supplemental Table IV). After adjusting for clinical variables associated with outcome in univariable analysis (age, premorbid mRS, admission NIHSS, hypertension, hypercholesterolemia, diabetes and prior stroke), a relationship between good outcome and automated ASPECTS was revealed (adjusted OR 1.30, 95%CI 1.06–1.60; p = 0.01) and the association between good outcome and CTP core volume remained (adjusted OR 0.98, 95%CI 0.96–1.00; p = 0.03) (Supplemental Table VI). The AUC for predicting good outcome based on core volume was 0.62 (95% CI 0.53–0.71) (Figure 3), and the optimal cut-off for predicting good outcome was <15 ml (sensitivity 0.79; specificity 0.39; positive predictive value 0.65; negative predictive value 0.57; Youden’s J = 0.18).
Figure 3. Receiver-operating-characteristics curve for ischemic core volume and good functional outcome.
The x-axis shows the specificity and the Y-axis shows the sensitivity of a particular ischemic core volume for prediction of good functional outcome (AUC = 0.62). Abbreviations: AUC = area under the curve.
In the sensitivity univariable analysis, only ischemic core volume was associated with excellent outcome (0R 0.98, 95%CI 0.97–1.00; p = 0.05). Conventional ASPECTS (OR 1.12, 95%CI 0.97 – 1.29) and automated ASPECTS (OR 1.05, 95%CI 0.88 – 1.25) were not associated with excellent outcome. Of the clinical covariables, age (OR 0.98, 95%CI 0;96–1.00; p = 0.046), admission NIHSS (OR 0.88, 95%CI 0.83 – 9.4; p < 0.0001), hypertension (OR 0.49, 95%CI 0.25–0.96; p = 0.04) and diabetes (OR 0.37, 95%CI 0;16–0.86; p = 0.02) were associated with excellent outcome in univariable analysis. In multivariable analysis, none of the imaging variables were associated with excellent outcome (adjusted OR for conventional ASPECTS 1.15 [95%CI 0.97 – 1.36], automated ASPECTS 1.08 [95%CI 0.89 – 1.32] and CTP core volume 0.99 [95%CI 0.97 – 1.01]).
Similarly, we did not find an association between conventional ASPECTS and poor outcome (OR 1.05, 95%CI 0.89 – 1.23) and automated ASPECTS and poor outcome (OR 0.97, 95%CI 0.80 – 1.17) (Supplemental Table V). CTP core volume was associated with poor outcome in univariable analysis (OR 1.01, 95%CI 1.00–1.03; p = 0.04) and remained a significant predictor in multivariable analysis after adjusting for age, premorbid mRS, NIHSS, hypertension, hypercholesterolemia, diabetes and previous stroke (adjusted OR 1.02, 95%CI 1.00–1.04; p = 0.02) (Supplemental Table VII). The AUC for predicting poor outcome based on CTP core volume was 0.53 (95% CI 0.42–0.64).
Discussion
In this study, we evaluate the association between conventional ASPECTS, automated ASPECTS, or CTP core volume and good or poor outcome in acute ischemic stroke patients presenting within 18 hours from presumed symptom onset in whom reperfusion with endovascular therapy was achieved. Conventional ASPECTS was not associated with clinical outcomes, whereas CTP core volume was associated with both good and poor outcome. Automated ASPECTS was associated with good outcome after adjusting for relevant clinical confounders. Although similar results were obtained in the sensitivity analysis for excellent outcome, none of the imaging variables were associated with excellent outcome after adjustment for confounders.
In a meta-analysis of five randomized clinical trials on endovascular therapy for acute ischemic stroke in the early time window no interaction between the treatment benefit and ASPECTS was identified (p for interaction = 0.29). The OR did not differ between patients with ASPECTS 6–8 or 9–10 (common OR for improvement of at least 1 point on the mRS on shift analysis of 2.34 [95%CI 1.68 – 3.26] and 2.66 [95%CI 1.61 – 4.40] respectively), but few patients with very low ASPECTS scores (0-5) were included and thus the analysis was underpowered to detect treatment effect in this subgroup (common OR 1.24 [95%CI 0.62–2.49]) (15). In this study, we did not find an association between conventional ASPECTS and functional outcome, despite a substantial proportion of patients with lower ASPECTS scores and in spite of the fact that early ischemic changes on non-contrast CT become more apparent over time (16). Automated ASPECTS scores were notably higher compared to conventional ratings and correlation between automated ASPECTS and baseline ischemic core volume predicted by CTP was slightly better than conventional ASPECTS, suggesting an overestimating of early ischemic changes by conventional ASPECTS in this population. Therefore, like the HERMES meta-analysis, this study may be underpowered to detect an association between very low ASPECTS scores and functional outcome after successful endovascular therapy (15).
This study confirms the low inter-rater agreement for ASPECTS and our inter-rater reliability was even slightly lower than previously reported (11). Although we did not compare to a ground truth, we did not find an association between rater experience or training and accuracy for prediction of outcome. Using automated ASPECTS eliminates interrater variability, but automated ASPECTS added little value compared to conventional ASPECTS for prediction of outcome in this study. To our knowledge, only one other study compared conventional and automated ASPECTS for prediction of outcome after endovascular therapy within an early time window after stroke onset (median onset-to-treatment: 160 minutes) (17). In this study, an association between poor outcome and automated ASPECTS as well as the conventional ASPECTS in only one out of three raters was found.
Imaging based selection with CTP can identify patients likely to benefit from endovascular therapy in the extended time window (1). Two RCTs showed benefit of extended window endovascular therapy (up to 16 to 24 hours) after symptom onset or last-seen-well in patients with a favorable mismatch profile, i.e. a small ischemic core and severe clinical symptoms or a substantial volume of salvageable tissue (2, 3). In the RCTs that only included patients within 6h after (known) symptom onset, the rate of good outcome in the endovascular treated arms was also higher in trials that selected patients with mismatch profiles, most commonly based on CTP (5, 18). In our study, core volume was an independent predictor of good, but not excellent, outcome. Predictive accuracy, however, was low, which suggests other clinical variables modify reperfusion treatment effect. In addition, accuracy for identification of those patients that would not benefit from reperfusion was insufficient. Therefore, this study does not provide evidence to exclude patients solely based on the size of the predicted ischemic core.
This study has several limitations. First, the ischemic core volumes were small in most patients and core volumes above 50 ml were rare (less than 10% of patients). The small percentage of patients with large cores might explain the lack of an association between CTP core volumes and poor outcome. Second, we did not explore other methods for selection of clinical covariables in the multivariable model (e.g. Aikaike information criterion or Bayesian information criterion), which may yield a better model fit.
In conclusion, ischemic core volume as identified by CTP is associated with functional outcomes in patients with successful reperfusion following endovascular therapy up to 18 h after symptom onset. Conventional and automated ASPECTS were not associated with functional outcome in that time window.
Supplementary Material
Acknowledgements
The authors thank the CRISP investigators and patients who participated in the study.
Source of funding
The CT Perfusion to predict Response in Ischemic Stroke Project (CRISP) was funded by a grant from the National Institute of Neurological Disorders and Stroke (principal investigator, M.G.L.).
Jelle Demeestere is funded by a clinical research and education board (KOOR) grant from Leuven University Hospitals. Robin Lemmens is a Senior Clinical Investigator of Fonds Wetenschappelijk onderzoek (FWO) anders.
Footnotes
Conict of interest
Sören Christensen and Gregory Albers have equity interest in and are consultants for iSchemaView. Gregory Albers is a consultant for Medtronic.
Social media handles: Twitter @UZLeuven; @StanfordHealth
References
- 1.Lansberg MG, Christensen S, Kemp S, Mlynash M, Mishra N, Federau C, et al. Computed tomographic perfusion to Predict Response to Recanalization in ischemic stroke. Ann Neurol. 2017;81:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 3.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 6.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 7.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Menon BK, Coutts SB, Hill MD, Demchuk AM, Penumbra Pivotal Stroke Trial Investigators ClSP, and the Seaman MR Research Center. Effect of baseline CT scan appearance and time to recanalization on clinical outcomes in endovascular thrombectomy of acute ischemic strokes. Stroke. 2011;42:93–97. [DOI] [PubMed] [Google Scholar]
- 9.Haussen DC, Dehkharghani S, Rangaraju S, Rebello LC, Bouslama M, Grossberg JA, et al. Automated CT Perfusion Ischemic Core Volume and Noncontrast CT ASPECTS (Alberta Stroke Program Early CT Score): Correlation and Clinical Outcome Prediction in Large Vessel Stroke. Stroke. 2016;47:2318–2322. [DOI] [PubMed] [Google Scholar]
- 10.Demeestere J, Garcia-Esperon C, Garcia-Bermejo P, Ombelet F, McElduff P, Bivard A, et al. Evaluation of hyperacute infarct volume using ASPECTS and brain CT perfusion core volume. Neurology. 2017;88:2248–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farzin B, Fahed R, Guilbert F, Poppe AY, Daneault N, Durocher AP, et al. Early CT changes in patients admitted for thrombectomy: Intrarater and interrater agreement. Neurology. 2016;87:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagel S, Sinha D, Day D, Reith W, Chapot R, Papanagiotou P, et al. e-ASPECTS software is non-inferior to neuroradiologists in applying the ASPECT score to computed tomography scans of acute ischemic stroke patients. Int J Stroke. 2017;12:615–622. [DOI] [PubMed] [Google Scholar]
- 13.Hanley JA. Receiver operating characteristic (ROC) methodology: the state of the art. Crit Rev Diagn Imaging. 1989; 29:307–335. [PubMed] [Google Scholar]
- 14.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6:284–290. [Google Scholar]
- 15.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 16.Bal S, Bhatia R, Menon BK, Shobha N, Puetz V, Dzialowski I, et al. Time dependence of reliability of noncontrast computed tomography in comparison to computed tomography angiography source image in acute ischemic stroke. Int J Stroke. 2015;10:55–60. [DOI] [PubMed] [Google Scholar]
- 17.Pfaff J, Herweh C, Schieber S, Schönenberger S, Bösel J, Ringleb PA, et al. e-ASPECTS Correlates with and Is Predictive of Outcome after Mechanical Thrombectomy. Am J Neuroradiol. 2017;38:1594–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.